Diversity of click beetles in managed nonnative coniferous and native beech stands:Consequences of changes in the structural and species composition of tree stands in Central Europe
V′clv Zumr,Oto Nkl′dl,JiˇríRemeˇs,Terez Brestovnsk′,V′clv Zumr
a Czech University of Life Sciences Prague,Faculty of Forestry and Wood Sciences,Kamýck′a 129,Praha 6,Suchdol,165 00,Czech Republic
b The Silva Tarouca Research Institute for Landscape and Ornamental Gardening,Kvˇetnov′e n′amˇestí391,Průhonice,252 43,Czech Republic
c SC57222591848,Czech Republic
Keywords:Species richness Forest management Saproxylic beetles Nonsaproxylic beetles Dead wood Elateridae Canopy openness
A B S T R A C T The natural composition of forests has undergone significant changes over recent centuries.A closer-to-natural tree species composition has long been perceived as key to a high biodiversity.We investigated the impact on communities of click beetles(Elateridae)caused by changes in the tree species composition of spruce monocultures compared to reference sites of recently unmanaged natural beech forests.To collect data,passive interception traps were distributed within managed spruce stands of different age classes and natural beech forests of various developmental stages.The beetle species richness was slightly but not significantly higher in the beech forests.The saproxylic species group was significantly more common in the spruce stands,whereas the group of nonsaproxylic species was significantly more abundant in the beech stands.In the commercial stands,the significantly highest species richness was in the clearings(0–10-year-old stands),and at this forest age class,the vast majority of the beetle species occurred in the spruce stands.In the developmental stages of the natural forest,a slightly higher beetle richness was found at the disintegration stage.The study results suggested that different tree species compositions and stand structures affect the communities of click beetles and substantially change their species composition and thus their response to external influences.Therefore,management of stands using diverse silvicultural systems is recommended for creating diverse ecological niches in forests.
1.Introduction
The majority of forests in Europe have changed over recent centuries due to forest management(Hannah et al.,1995).Only a fraction of one percent of native forests remains in Europe(Sabatini et al.,2018).Most of the forest area in Europe has been converted to simple stands of spruce(Picea abiesK.)and pine(Pinus sylvestrisL.)(Hahn and Fanta,2001).In these monocultures,a sharp decline in biodiversity has been observed(Brockerhoff et al.,2008),especially in insects(Seibold et al.,2015a;L′opez-Bedoya et al.,2021),wood-inhabiting fungi(Müller et al.,2007;Blaser et al.,2013),birds,and bats(Roberge et al.,2008;Ettwein et al.,2020).A soil degradation effect has also been documented in these stands(Podr′azskýand Remeˇs,2005,2010).These simple stands are very sensitive to changes in external natural conditions.In particular,they are highly susceptible to heavy winds and insect outbreaks(Hl′asny et al.,2021),which accelerate significantly with increasing air temperatures(Park Williams et al.,2013).Therefore,alternative forest management models are being sought to achieve higher biodiversity(e.g.,Roth et al.,2019;Gustaffson et al.,2020),better soil conditions,and higher stand stability(e.g.,Brang et al.,2014;Remeˇs,2018).Indeed,the way forests are managed influences the most important attributes of forest biodiversity.The most important attributes include deadwood(Christensen et al.,2005;Klepzig et al.,2012),the species composition of stands(Oxbrough et al.,2016;Hor′ak et al.,2019),the degree of insolation or canopy closure(Lachat et al.,2012,2016),and the abundance of microhabitats(Winter and M¨oller,2008).Deadwood is crucial because it contributes enormously to forest biodiversity(Dudley and Vallauri,2005;Graf et al.,2022).In particular,standing deadwood hosts more saproxylic beetle species than fallen deadwood(Sverdrup-Thygeson,2001;Bouget et al.,2012).Saproxylic beetles are the most studied forest organisms(Seibold et al.,2015b),as they are considered bioindicators of the state of the forest environment(Zumr and Remeˇs,2020)and are dependent on deadwood of any development stage or type(Speight,1989;Jaworski et al.,2019).
Click beetles(Elateridae)are primarily associated with forest and forest-steppe environments(Laibner,2000;Zbuzek,2017).They develop in forest soil,soil litter,and deadwood microhabitats(Laibner,2000).The family Elateridae includes many of the most threatened species that are at risk due to current intensive forest management(C′alix et al.,2018).Landscape management is why more than 60% of the species in this family are at risk in the Czech Republic(Zbuzek,2017),while Europe-wide,25%of click beetles are in the Critically Endangered(CR),Endangered(EN),Vulnerable(VU),Near Threatened(NT)categories,and 8%are in those categories without NT(Nieto and Alexander,2010).Almost half of the species of the family Elateridae living in Europe are endemics(Nieto and Alexander,2010).The vast majority of the species included on the Red List of Threatened Species are saproxylic click beetles associated with deadwood in any form(Laibner,2000).Their flight activity is primarily related to warm sunny days(Laibner,2000),and they are relatively easy to survey using flight interception traps(Hor′ak and R′ebl,2013).A large group of rare species of click beetles are cavity specialists that rarely leave their habitats(e.g.,Gouix et al.,2015;Mertlik,2017,2019).The ecology of many click beetles has not been adequately investigated(Johnson,2002;Nieto and Alexander,2010),and hence,new monitoring methods are emerging,e.g.,using pheromones inElater ferrugineusLinnaeus,1758(Harvey et al.,2017a;b).The most endangered saproxylic beetles are known to have poor dispersal activity(Brunet and Isacsson,2009).The family Elateridae is important to study because it is widely variable ecologically,abundant in species and specimens(Thomas et al.,2009),and tends to be the most frequently captured group of beetles in entomological studies(Parisi et al.,2016).This fulfils the prerequisites for the family Elateridae to serve as bioindicators of the state of the local environment.
In our study,we investigated the impact of intensive forest management on the biodiversity of Elateridae compared to that in unmanaged stands,which can be considered natural for their given habitat conditions.We posed the following research hypotheses:1)Forest management with its influence on the overall structure and the nature of stands has no effect on the biodiversity of click beetles;2)there is a significant difference in click beetle richness between stands of allochthonous spruce and stands with autochthonous tree species composition;and 3)the age structure of the stand affects the diversity of the captured model group.We have also addressed the research question of what other variables significantly support the biodiversity of click beetles.
2.Materials and methods
2.1.Study areas
The study was performed in the forests of Central Europe in the Czech Republic(Table 1).Two geographically similar forest study sites were selected at elevations ranging from 400 to 700 m a.s.l.,with pure beech stands forming their climax geobiocoenoses(Zlatník,1976).One site was not substantially altered by forest management.It was part of a complex of protected natural beech stands where no management has been carried out over the last 20–70 years.The other study site represented standard Central European commercial forest stands that have been converted in the past from natural forests(in this case,from beech stands to spruce-dominated ones).
The site in the Voltuˇs sampling area represents a standard Central European commercial forest.Twenty traps were placed in 0–181-yearold stands that were classified into 5 classes,and each age class had 4 replicated samples.To assess the influence of the developmental stage of natural beech forests,the Jevany sampling area was selected,where stands of three developmental stages(Fig.1)were singled out:A)an optimum stage,which had the largest stand stock,a lower number of trees,horizontal closure,and a structure similar to that of commercial stands,B)a disintegration stage,which had canopy openings,dying trees,a decreasing stand stock,and a substantial accumulation of deadwood,and C)a growing up stage,which had a large number of trees per area,a predominance of young individuals,significant self-reduction of young stands,and vertical canopy closure(Korpeˇl,1982;1995;Saniga et al.,2019).Ten traps were placed at each developmental stage site.The individual traps were more than 50 m apart,only in one case the distance was 25 m,but the vast majority were located much farther away in other localities.The monitored plots were purposefully delineated inside of forest complexes to avoid data biasing by edge effects.
2.2.Study groups and collection methodology
This study targeted the family Elateridae,which is considered one of the most threatened groups of beetles due to the intensive management of their habitat(C′alix et al.,2018).This group was further divided into saproxylic and nonsaproxylic species according to Laibner(2000),Schmidl and Buβler(2004).The division by habitat and trophic relationships was made according to Laibner(2000)and Mertlik(2014).The taxonomy of the species corresponded to the concept of Zicha O.(2021)BioLib(http://www.biolib.cz).The species were further classified according to the Red List of Endangered Species of the Czech Republic,Invertebrates(Hejda et al.,2017).Flight interception traps were used for the study:20 in commercial stands and 30 in natural beech stands,for a total of 50 traps.Data were collected for a year at each separately site in Voltuˇs(2017)and Jevany(2021).Sampling units(traps)were picked up at two-to three--week intervals during April–September.Each trap consisted of a roof,plexiglass barrier,funnel,and collection container.The roof of the trap was made of a plastic dish that was 45 cm in diameter.Underneath the roof,there were two plexiglass panes perpendicular to one another,forming a 40 cm×50 cm(width×height)barrier.The traps were placed at a height of 1.5 m above the ground to have the best chance of capturing the species(Ruchin and Egorov,2021).The preservative was a salt or propylene glycol solution(1:1.5)with a drop of degreasing agent as detergent.
2.3.Study variables
All selected variables(Table 2)were quantified in circles with radii of 20 m(1,257 m2).The response of species composition to the environmental variables is the best at a 20-m radius(Loskotov′a and Hor′ak,2016).The canopy closure was determined using hemispherical photographs taken with a fisheye lens over each trap.The analysis was performed using the Gap Light Analyser software.The ground cover for each buffer was measured as the proportion of plants and bare soil as litter and upper humus horizons in the stand.In addition,we quantified the amount of fallen deadwood>7 cm in diameter(measured as length×basal area)and standing deadwood(tree torsos with a minimum height of 1.3 m),the volumes of which were determined as the height times the basal area(π×r2).For each radius,the volume of dead wood was measured and then converted to hectares.Microhabitats were surveyed according to the methodology of Winter and M¨oller(2008).

Table 1Description of study sites.
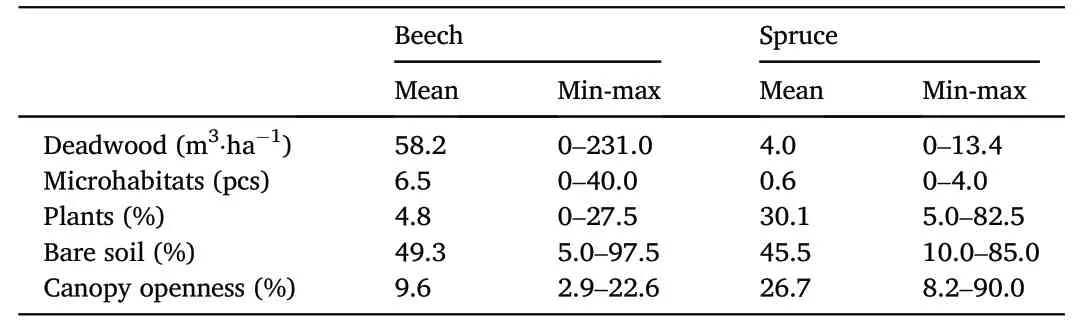
Table 2Mean values of measured variables by study site.

Fig.1.Depiction of growth and developmental stages of stands.The top pictures show commercial stands from clearing to mature stages.The bottom pictures show the developmental stages of natural beech forests.
2.4.Statistical analysis
We used a gamma diversity estimation method for the two study sites.Rarefaction-extrapolation approach that estimated the rate of increase in the number of species per number of samples,was used similarly to Seibold et al.(2018).We used the Inext programme(Chao et al.,2016)to analyse the species richness and diversity curves.Sample based rarefaction was used to generate the species richness curve,where the estimated richness profile(200 bootstraps,incidence data)was calculated.Diversity indices are based on Chao et al.(2014)with Hill numbers:q=0(species richness)andq=1(exponential of Shannon's entropy index).Hill numbers offer some distinct advantages over other diversity indices(Chao et al.,2014).Parameterqcharacterizes the degree of dependence on rarely occurring species.If it is zero,we get an index that gives the total number of species present,thus the weight of rare species is the largest.Whenqequals one,we obtain an index that represents the number of all commonly occurring taxa and is characterized by the exponential of the Shannon's entropy index.
We used nonmetric multidimensional scaling(NMDS,Bray–Curtis distance)to plot differences in communities within a site(beech vs.spruce).NMDS is a robust ordination approach that attempts to represent pairwise(dis)similarity between objects as faithfully as possible in a lowdimensional space(Buttigieg and Ramette,2014).Significance was tested using distance-based RDA(Legendre and Anderson,1999)calculated with the CANOCO 5 software.
Differences in click beetle richness(all,saproxylic,and nonsaproxylic)between natural beech and commercial spruce stands were evaluated using the generalized linear model(GLM)with a Poisson distribution(Log link function).Differences between age classes were evaluated using a post hoc Tukey HSD in a general linear model.The influence of individual environmental variables on the click beetle richness was assessed using a generalized linear model(Poisson distribution).All the variables we obtained were entered into the GLM model(Table 2),and best modelling subset(according to the lowest Akaike information criterion(AIC)value was separately used to determine the significant variables for the two stand types(natural beech and spruce stands).Statistical analyses were performed in the Statistica 13 software(StatSoft,Inc.).An alpha value of 0.05 was calibrated for all statistical computations.
Site-specific preferences of the species were tested using a unimodal constrained canonical correspondence analysis(CCA)method with log transformation.Constrained linear redundancy RDA(log)analysis with all canonical axis tests was used to determine the species preference for the examined stand ages and stages.All ordination analyses were computed with 4,499 unrestricted permutations.Calculations were performed in the CANOCO 5 multivariate statistical analysis software using ordination methods(Lepˇs andˇSmilauer,2010;ˇSmilauer and Lepˇs,2014).
3.Results
A total of 3,165 click beetles were captured during the study.They belonged to 29 species,12 of which were saproxylic and 17 were nonsaproxylic(Checklist species in Table 6).In the commercial spruce stands,1,475 individuals of 18 species were captured.In the natural beech forests,1,690 individuals of 24 click beetle species were captured.Click beetle communities were significantly different between sites(F=13.1,p<0.001)(Fig.2).
3.1.Species diversity
Most click beetle species preferred the beech forest(Fig.3).A significant difference in alfa diversity was not confirmed(Table 3).Saproxylic species were more abundant in commercial spruce stands in contrast to non-saproxylic species,which were more abundant in beech stands(Table 3).The overall species(gamma)diversity was slightly higher in the natural beech stands than in the spruce stands while there was no difference for Shannon diversity(Fig.4).The number of saproxylic species did not differ between sites in terms of Hill numbers(q=0)(Fig.5).This contrasted with nonsaproxylic species,which were more abundant in beech stands than in spruce stands(Fig.5).
3.2.Age structure
Spruce stands:the species richness of managed stands was highest in the clearings,to which almost only the saproxylic species responded,while no significant differences were found for nonsaproxylic species(Table 4,Fig.6).Fifteen species(80%)of the click beetle found in the managed spruce stands were in the youngest growth stages of the forest(clearings and plantations up to 10 years old;Figs.6 and 7).The post hoc test showed that there were no differences in beetle preferences for stand ages from 40 years,indicating a mature stand build-up and a microheterogeneous homogenization at the site(Fig.6).
Beech stands:there were no significant differences in the all beetle richness in the beech stands by GLM(Table 4).The disintegration phase was in tendency richer in saproxylic species than the growing up phase.Similarly,individual species favoured the optimal phase during its slow transition to the disintegration phase and subsequently the disintegration phase itself(Figs.6 and 7).Again,the nonsaproxylic species showed no changes according to environmental conditions(Table 4,Fig.6).
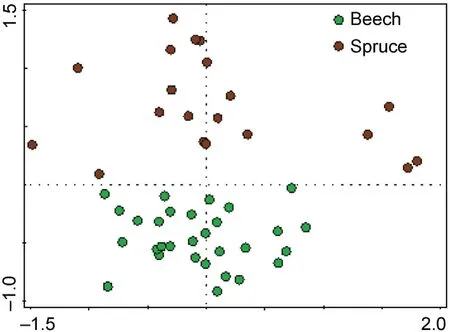
Fig.2.Nonmetric multidimensional scaling(NMDS)shows differences in beetle communities within the studied managed and unmanaged localities.

Fig.3.Species preference(CCA)for study sites by dominant tree species represented(F=11.7,p<0.001).Explained variation by axes:axis 1(19.60%);axis 2(17.58%).
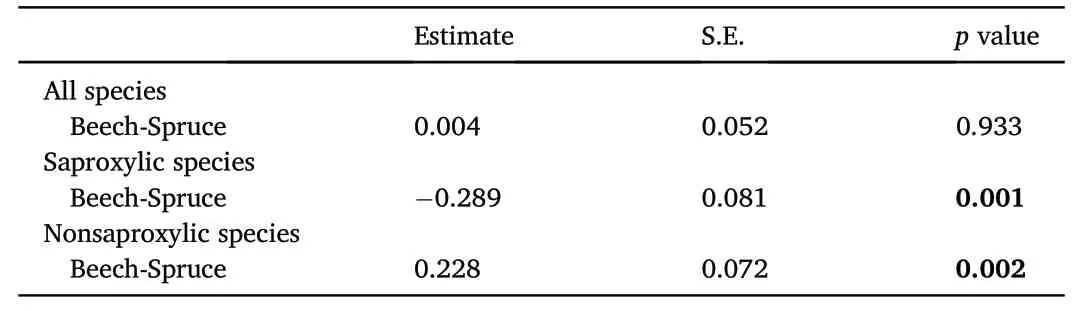
Table 3Results of the comparison of the number of species within the model groups by site.A sigma-restricted generalized linear model with a Poisson distribution was used.Significant differences are marked(p<0.05).Estimate with positive value represents higher numbers of species in the study site.S.E.,Standard Error.
3.3.Environmental variables
For all species richness only one variable was found to be important,canopy closure(openness).The most important variable in spruce stands is canopy openness(Table 5).The species diversity of click beetles,both of saproxylic and nonsaproxylic species,showed a demonstrable increase as the canopy opened(Table 5).
In the beech stands,the situation was similar,even with the lesser importance of the canopy openness,although it remained marginally significant for all species richness(Table 5).In the beech stands,no significant effect was found on the number of saproxylic and nonsaproxylic species(Table 5).

Table 4Species richness of click beetles in the study locality(overparameterized generalized linear model,Poisson distribution).S.E.,Standard Error.
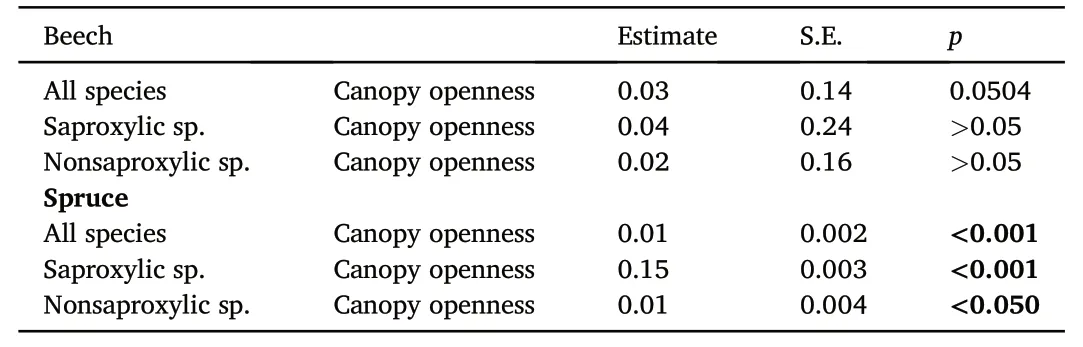
Table 5The results of the model for the captured numbers of all detecting species.The stepwise generalized linear model with a Poisson distribution based on the lowest AIC value was used for detecting the most important variable.Positive significance variable are marked.
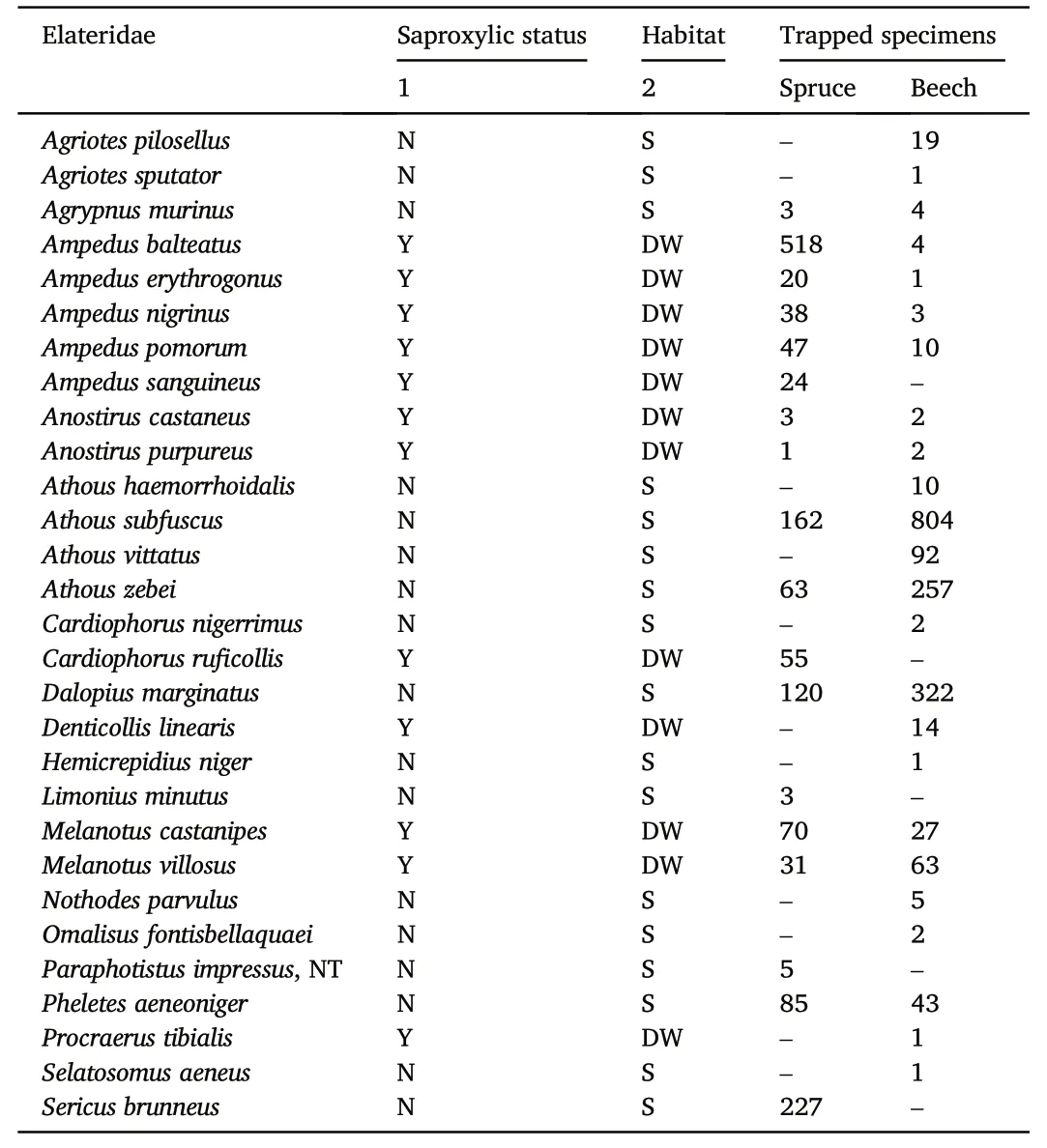
Table 6List of observed species and their numbers,broken down by site.
4.Discussion
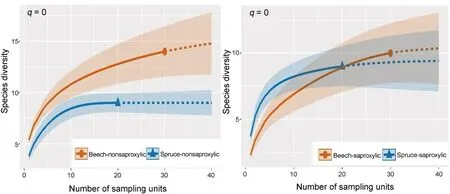
Fig.5.Sample-size-based rarefaction and extrapolation sampling gamma diversity curve within sites showing Hill's numbers q=0(Species richness)of model groups of saproxylic and nonsaproxylic species by locality.Coloured shaded areas are the 95% confidence intervals.Solid symbols represent the total number of study samples.
Our study showed that forest management is an important factor influencing the species richness of click beetles in forest stands.Forest management affects the overall species composition while altering the occurrence of the species themselves,which respond differently to altered natural conditions as observed in our study.In particular,the importance of insolation(reduced canopy closure)for click beetle species was detected.Click beetles are sensitive to changes in the whole ecosystem.The study sites and their dominant species(Picea abiesandFagus sylvatica)correspond to the most abundant tree species in Central Europe.Therefore,we suggest that our results may contribute to addressing the issue of biodiversity loss in the region's commercial forests.These two tree species are good indicators of the degree of anthropogenic influence on forest ecosystems in Central Europe,as spruce has been(and still is)the most commercially exploited tree species in the region,and spruce stands were most often established on sites that were previously occupied by native beech stands(Leidinger et al.,2021).
When comparing the study sites(both types of stands),a higher overall species richness of click beetles in the natural beech stands was documented,yet the difference was statistically insignificant.In other studies,natural and seminatural stand composition has been shown to be important for beetle diversity(e.g.L′opez-Bedoya et al.,2021).However,though these studies were conducted over other years and it is difficult to compare them to each other,after ten years of research on the distribution of saproxylic species in the forest,Martikainen and Kaila(2004)found that the dynamics of common species barely changed,suggesting that the samples in this case could be compared.In our study,we captured only common species that were indicative of the local environment.

Fig.6.Species richness progression by site and developmental stage.Black indicates all species,including saproxylic species(brown)and nonsaproxylic species(green)combined.Letters above the error bars show Post-Hoc Tukey HSD method comparisons.Error bars are confidence interval 95%.(For interpretation of the references to colour in this figure legend,the reader is referred to the Web version of this article.)
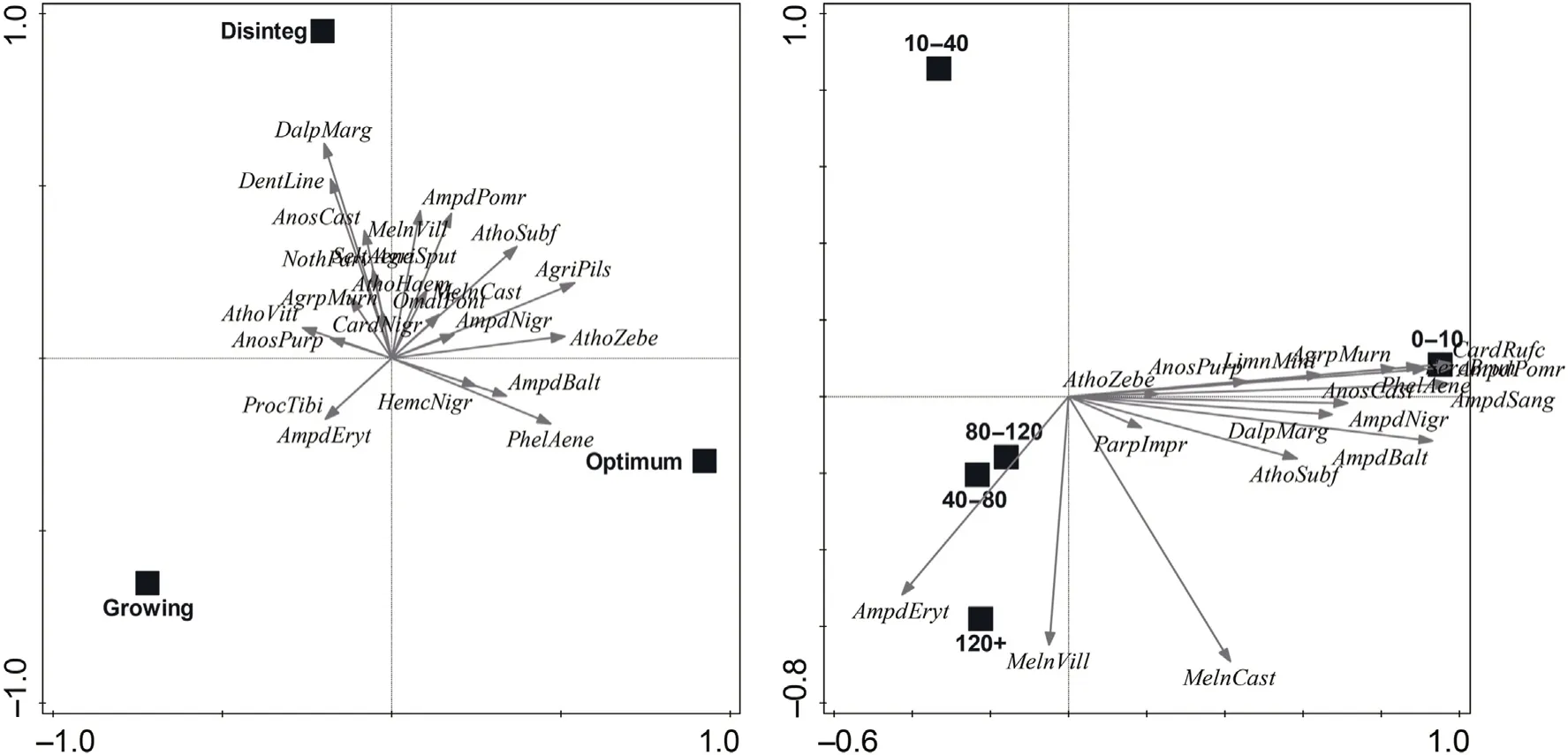
Fig.7.Species preferences for natural and commercial stands by RDA.The left diagram shows species preferences for developmental stages of natural beech stands(F=3.7,p=0.0009).Explained variation by axes:axis 1(13.15%);axis 2(8.33%).The diagram on the right shows species preferences for different age categories of commercial spruce stands(F=11.0,p=0.0002).Explained variation by axes:axis 1(67.94%);axis 2(4.52%).
In the saproxylic click beetle group,a slightly larger number of species was observed in the spruce stands than in the beech stands.Species richness and diversity of saproxylic species in spruce stands was evident,especially in the first years after deforestation when the area was exposed and the new stand was just becoming established.This was consistent with the theory that click beetles generally prefer sunny habitats(Hor′ak and R′ebl,2013;Mladenovi′c et al.,2018).Another reason may be that we monitored common saproxylic species.For these species insolation of the forest is particularly important(Bouget et al.,2013;Proch′azka and Schlaghamerský,2019),which was consistent with our findings.Nevertheless,significant increases in saproxylic biodiversity,and especially for threatened species,is mainly related to the amount of dead wood(Gossner et al.,2013;Seibold et al.,2016).A low proportion of saproxylic click beetles was detected in natural beech forests,which did not support the assumption that the higher volume of deadwood in natural and protected stands leads to a higher diversity of saproxylic click beetles compared to that in managed forests,as has been documented(Müller et al.,2008).Possible reason for this discrepancy is the relatively compact canopy closure and its low variation within the succession of natural beech stands.In our study sites,even in the disintegration stage area,the canopy was not completely open and thus lacked prominent stand gaps.Lachat et al.(2016)showed that the abundance of saproxylic species was substantially higher in the stand gap than under the canopy of natural beech forests.This corresponds to the regeneration stage on the bare soil in the commercial stands.Thomas et al.(2009)observed that stand gaps of up to 0.2 ha do not have a significant effect on changes in the communities of click beetle species,which was consistent with our findings.
The stand age structure was of great importance for the diversity of click beetles.Their species richness was highest in the clearings of clearcut spruce stands.In these areas,species were recorded when they were no longer present during subsequent growth.The change in stand structure as a result of the growth and development of commercial spruce stands leads to the creation of a full canopy,and thus to a loss of microclimatic and microhabitat variability,leading to the disappearance of previously occurring species.A diverse mosaic of age classes is key to promoting a higher biodiversity in stands that are clear-cut.In these cases,even clear-cutting can be more valuable to overall biodiversity than selection management of forests with vertically closed canopies that have low development dynamics(Schall et al.,2018).Nevertheless,as climate change is underway,selection management of forests and similar close-to-natural silvicultural systems are increasingly promoted for example by Brang et al.(2014)and Bana′s et al.(2018)for improved adaptation to warmer climatic conditions(Hagerman and Pelai,2018).However,from the point of view of invertebrate diversity,it seems preferable to promote management systems that include early successional stages rather than silvicultural systems that homogenize the landscape and where early succession-stage species and many others will not occur(Warnaffe and Lebrun,2004).In this study,saproxylic species were found to be responsive to changes in stand structure at the study sites,while nonsaproxylic species were barely responsive,and species diversity was relatively even throughout stand development,as documented by Stenbacka et al.(2010)for example.Within the group of nonsaproxylic,soil-inhabiting click beetles,substantially greater differences in species diversity were detected when comparing the two sites.The soil properties of nonnative spruce commercial stands have been studied for many decades.The degradation effect and reduction in microbial activity of forest soils have been documented,for example,by Fabi′anek et al.(2009)and Podr′azskýand Remeˇs(2012).According to our research,the condition of the forest soil was probably the determining factor responsible for the increased diversity of nonsaproxylic click beetles in the natural beech forests compared to spruce stands.Soil degradation leads to species loss and changes in the species composition of epigeic beetle species(Elek et al.,2001;Oxbrough et al.,2016),and this appears to be true for nonsaproxylic,soil-inhabiting click beetles,as this group is influenced by soil chemistry and nutrients(Kula,2010).In terms of nutrient levels in the humus horizon,wireworms(click beetle larvae)appear to be more abundant in soils with excessive phosphorus and calcium contents,as well as those with optimal potassium levels(Kula,2010).This aspect is probably the determining factor for the higher diversity of soil-inhabiting click beetles in the natural deciduous beech stands than in the nonnatural spruce stands represented in our study.In deciduous and mixed stands,the nutrient base content in forest soils and humus horizons is usually several times higher(Poleno and Vacek,2011).Basic elements leaching from the soil lead to,among other things,the formation of podzolic soil types and soil moisture deficiency(Poleno and Vacek,2011).An analogy can be drawn from research on communities of the family Carabidae:Sroka and Finch(2006)found that high soil moisture was a key attribute for the diversity of ground beetles,which was confirmed by Kacprzyk et al.(2021),higher pH and carbon content also have an influence.A significantly higher species diversity of ground beetles has been demonstrated in cambisols than in podzols(Tyler,2008).Similarly,earthworms are virtually absent in spruce stand soil due to its acidity and aridity,while earthworm communities are the richest in broadleaved mixed stands(Schelfhout et al.,2017).
Evaluation of differences in soil properties from the study sites was not the focus of this study but appeared to be a very important factor in the overall click beetle diversity.According to the cited sources,moisture and a rather rudimentary environment are important for most soil fauna.Beech stands cover the soil with their canopy and maintain a suitably moist microclimate.On the other hand,dense canopy cover has been shown to be undesirable for saproxylic species diversity(Sebek et al.,2016;Hor′ak et al.,2018).However,the litterfall of beech leaves contributes to the maintenance of mull and mull-moder humus forms that are suitable for soil fauna.Another factor was the presence of deadwood,which was relatively abundant in the studied beech stands,especially those in the disintegration stage.Beechwood is a major releaser of N,Mg,Ca,K,and P nutrients to the soil(Dhiedt et al.,2019),and it serves as a reservoir and source of water(Harmon and Sexton,1995).Deadwood also increases soil microbial activity(Minnich et al.,2021).Soil-inhabiting click beetles respond positively to these effects(Kula,2010).In this context,the question arises as to whether soil-inhabiting click beetles can also be saproxylic species.If so,then they could be influenced by deadwood and be tertiary to quaternary saproxylic beetles.This would mean that more than 25% of forest biodiversity could be affected by deadwood(Dudley and Vallauri,2005;Graf et al.,2022).
5.Conclusion
This study showed the change in composition and distribution of click beetle species in commercial spruce and natural beech stands.The potential diversity of the beetles in terms of salient microhabitats and deadwood increased as the beech stands evolved.Gradually,this can mean that large amounts of deadwood can partially compensate for a lack of insolation(Lachat et al.,2012;Müller et al.,2015).With establishment of the next generation of forests through regeneration and new growth,complete shading will gradually prevail,and thus,the invertebrate biodiversity will be reduced.Conversely,commercial forests can benefit from repeated disruption of the canopy by silvicultural interventions,and if these are combined with deadwood enrichment,forest biodiversity can be greatly increased as recommended by various researchers(e.g.,Doerfler et al.,2020;Zumr et al.,2021).In addition,if mixed stands are created through forest regeneration,a high species diversity can be achieved without losing an important function of timber production.Saproxylic beetles will benefit from the alteration of forest developmental stages,and nonsaproxylic and soil-inhabiting species will benefit from the mixed stand composition and the beneficial effect on the soil of the mixture of tree species.This integrated silvicultural approach can become even more valuable than a conservative strategy,especially at mid-and lowland elevations,due to the mixing and rotation of diverse ecological niches and the dependence of thermophilic species on open habitats for example.Forest management can thus actively create and conserve biodiversity(Leidinger et al.,2020).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We are grateful to JitkaˇSiˇs′akov′a and native speaker Richard Lee Manore(USA),who proofread this paper.The final version was proofread by AJE(certified).We greatly appreciated the assistance of Jiˇrí Brestovanskýand JiˇríSynek,who helped us identify the beetles.We are also grateful to the NCA of the Czech Republic,the School Forest Enterprise in Kostelec nadˇCernými lesy,and KAISER s.r.o.forests,and their consent for research in forests under their administration.This research was funded by the Internal Grant Agency of the Faculty of Forestry and Wood Science,No.43120/1312/3106 and with the support of the Ministry of Agriculture of the Czech Republic,NAZV No.QK21020371.
Finally,I would very much like to thank my grandfather(Dr.V′aclav Zumr†,1940–2021),who inspired me to study entomology and forestry from an early age,and passed on to me his deep scientific knowledge.
- Forest Ecosystems的其它文章
- Multiple forest structural elements are needed to promote beetle biomass,diversity and abundance
- Environmental and canopy conditions regulate the forest floor evapotranspiration of larch plantations
- Allometry-based estimation of forest aboveground biomass combining LiDAR canopy height attributes and optical spectral indexes
- Examining approaches for modeling individual tree growth response to thinning in Norway spruce
- Influence of soil and elevation on roadside cryptogam diversity in the tropical Andes
- Patterns of species diversity and its determinants in a temperate deciduous broad-leaved forest

