Biochar amendments increase soil organic carbon storage and decrease global warming potentials of soil CH4 and N2O under N addition in a subtropical Moso bamboo plantation
Qun Li, Kunki Cui, Jinhu Lv, Juno Zhng, Chnghui Peng, Yongfu Li,Zhikng Gu, Xinzhng Song,*
a State Key Laboratory of Subtropical Silviculture, Zhejiang A&F University, Hangzhou, 311300, PR China
b Institute of Environment Sciences, Department of Biology Sciences, University of Quebec at Montreal, Case Postale 8888, Succursale Centre-Ville, Montreal, H3C3P8,Canada
c Huzhou Research Center of Ecological Forestry and Protection, Huzhou, 313000, PR China
Keywords:Biochar application Nitrogen addition Greenhouse gas Global warming potential Plantation
ABSTRACT
1. Introduction
Increasing greenhouse gas (GHG) emissions, in particular carbon dioxide (CO2), methane (CH4), and nitrous oxide (N2O), are the major drivers of global climate change (IPCC, 2013). Although CH4and N2O emissions are considerably lower than those of CO2,the global warming potentials(GWP)of CH4and N2O are 25 and 298 times(100-year horizon) greater than that of CO2, respectively (Tian et al., 2015). In turn,GHG fluxes are influenced by global change factors(Montzka et al.,2011;Voigt et al., 2017), including nitrogen (N) deposition (Liu and Greaver,2009;Song et al.,2020).
Atmospheric N deposition, which mainly occurs due to excessive artificial fertilizer use and high levels of fossil fuel combustion(Stevens,2019), has increased three- to five-fold over the last century(Lamarque et al., 2005) and is predicted to continue increasing (Galloway et al.,2008;Liu et al.,2013).Forests,which cover 30.8%of the total land area globally,are significant sources or sinks of GHG and,thus,play a key role in regulating global climate change (Krause et al., 2013; Xiang et al.,2021;Wang et al.,2021;Xia et al.,2021).However,reports on the effects of N deposition on forest soil GHG emissions have been contradictory due to analysis of different forest types, as well as differing N levels and sampling time points (Liu and Greaver, 2009; Krause et al., 2013;Gomez-Casanovas et al., 2016; Deng et al., 2020). Furthermore, most studies have exclusively observed the fluxes of one or two GHGs(Cusack et al.,2016;Pajares and Bohannan,2016).As such,our understanding of the effects elicited by N deposition on forest GHG flux remains limited as few studies have concomitantly measured fluxes of all three major GHGs under elevated N deposition conditions at a single experimental site(Tian et al.,2019;Song et al.,2020).
The decrease in soil pH,which is induced by N input(Tian and Niu,2015; Chen et al., 2019), can affect soil CO2emissions by altering the physiology of soil microbes and vegetation (Oulehle et al., 2011; Chen et al.,2016;Deng et al.,2020).Low soil pH increases N2O emissions by increasing the abundance and activity of denitrifying bacteria(Tian et al.,2019; Song et al., 2020) while suppressing CH4uptake by inhibiting bacterial methanotroph activity(Bradford et al.,2001).Furthermore,the increase in available N content (AN) following N addition can improve soil nitrification or denitrification, subsequently increasing N2O emissions(Fu et al.,2015),while suppressing CH4consumption by increasing competition with methane monooxygenase (Bodelier and Laanbroek,2004). Therefore, increasing soil pH and decreasing soil AN represent potential effective strategies for mitigating soil GHG emissions driven by N input in forests.
Biochar is produced via biomass combustion under high-temperature and low-oxygen conditions (Lehmann, 2007; Khan et al., 2013).Considering the alkaline property of biochar(Song et al.,2016b;Li et al.,2018b),its application can increase soil pH in forests(Wang et al.,2014;Wrobel-Tobiszewska et al., 2016). Additionally, biochar has high porosity and a large adsorption capacity,implying that it can adsorb soil NH4+-N and NO3--N (Lu et al., 2019;Song et al., 2019). Biochar application can also affect soil properties, thus influencing GHG flux in forest soils(Song et al.,2016b;Li et al.,2018b).Such as,biochar amendments can decrease soil CO2and N2O emissions in pine (Pinus) forests (Sun et al., 2014) and Moso bamboo (Phyllostachys edulis) plantations (Song et al., 2019; Ge et al., 2020), and increase CH4uptake in a subtropical Chinese chestnut (Castanea mollissima) plantation (Lu et al., 2019).Moreover, short-term studies have reported that the combination of biochar amendment and N addition significantly reduces soil CO2emissions in a Moso bamboo plantation(Ge et al.,2020),however,increases CO2emissions in a Torreya grandis orchard (Zhang et al., 2019). Meanwhile, the effects of biochar amendments on soil GHG emissions in subtropical plantations receiving continuous N input have not yet been characterized.Hence related studies are needed to comprehensively,and accurately,assess the effects of N deposition and biochar amendment on plantation soil C storage,thus,providing a scientific basis for improving plantation carbon neutrality under increasing N deposition.
Moso bamboo plantations, which are widely distributed in the tropical and subtropical regions of East and Southeast Asia, cover 4.68 million ha in subtropical China(i.e.,73%of the total bamboo forests of China) and, as such, represent an important plantation resource in southern China (Li et al., 2019; Li and Feng, 2019). Moso bamboo plantations exhibit rapid carbon(C)sequestration and,thus,have a high potential to mitigate global warming, owing to their high growth rates and regeneration properties (Song et al., 2016a, 2017a). However, the region with the largest distribution of Moso bamboo plantations in China has been experiencing significant N deposition(30 kg N·ha-1·yr-1)over recent decades (Liu et al., 2013; Jia et al., 2014) and is predicted to remain high (Reay et al., 2008; Song et al., 2015). Our previous study found that short-term (i.e., four years) N addition to Moso bamboo plantations significantly decreases soil pH and soil CH4uptake, while significantly increasing soil CO2and N2O emissions (Song et al., 2020;Zhang et al., 2021). However, the responses of soil GHG emissions to long-term N addition, biochar amendment alone or in combination, remains unclear in Moso bamboo plantations.
In the present study, we established a biochar amendment field experiment in Moso bamboo plantations subjected to simulated N deposition for seven years—beginning 2013. The primary study hypotheses include: (1) long-term N addition increases soil CO2and N2O emissions, while decreasing CH4uptake; (2) biochar amendment decreases soil CO2and N2O emissions,however increases soil CH4uptake;(3)biochar amendment can mitigate the promoting effects of N addition on GHG emissions by increasing soil pH.
2. Materials and methods
2.1. Study site
The field experiment was located in Qingshan Township,Lin'an District (30°14′ N, 119°42′ E), Hangzhou City, Zhejiang Province, China.The area is characterized by a subtropical monsoonal climate, with approximate annual averages of 1,847 sunshine hours and 230 frost-free days. The mean annual temperature and precipitation at the study site are 15.6°C and 1,420 mm, respectively. The soil type is classified as a ferrisol derived from granite(Song et al.,2015).
The native evergreen broadleaf forests in this study site were converted to Moso bamboo plantations in the late 1970s and the plantations have been managed by digging bamboo shoots and thinning mature bamboo biennially.The understory vegetation is sparse,with a cover of approximately 5% and total herbal biomass of 14.6 kg·ha-1. The initial stand and soil characteristics prior to N and biochar addition are presented in Table S1.
2.2. Experimental design
In November 2012,12 plots of four treatments with three replicates were established in the Moso bamboo plantation.Each plot was 20 m×20 m and was bounded by a 20-m wide buffer zone. According to the local atmospheric N deposition rate(30 kg N·ha-1·yr-1)with an average NH4+-N/NO3--N ratio of 1.28(Jia et al.,2014;Song et al.,2017b),N was added as ammonium nitrate(NH4NO3)solution at four N levels:control(ambient + 0 kg N·ha-1·yr-1), N30, N60, and N90 (ambient + 30,ambient + 60, and ambient + 90 kg N·ha-1·yr-1, respectively). From January 2013 to December 2019, nitrogen addition rates were evenly split into twelve monthly applications each year and the required NH4NO3quantities were dissolved in 10 L of water and applied evenly onto the forest floor of each plot once per month using an electric backpack sprayer. Each control plot was treated with an equal volume(10 L)of water,without NH4NO3addition,to control for the effects of the added water. In September 2014, after 21 months of simulated N addition, each plot was divided into one subplot of 10 m × 20 m and two subplots of 10 m×10 m(Fig.S1);adjacent subplots were isolated with a plastic panel(depth of 1 m).
Referring to previous studies,biochar was applied once to each plot with three application rates:BC0,BC20,and BC40(0,20,and 40 t·ha-1,respectively) (Lei et al., 2018; Zhang et al., 2019). After biochar application, the topsoil supporting bamboo stems (0–30 cm) was plowed among bamboo stems using hoes to maintain thorough mixing in the corresponding plots. Plowing was also performed in BC0 plots.Subsequently,static chambers were randomly installed in each subplot.Thereafter, all plots received N addition monthly, as described previously herein.Biochar was produced by pyrolyzing Moso bamboo chips at 600°C in an oxygen-limited environment by the Yaoshi Charcoal Industry Company(Hangzhou,China),with a pH of 9.76, bulk density of 0.53 g·cm-3,cation exchange capacity of 14.9 cmol·kg-1,and C and N contents of 81.73% and 0.57%, respectively. All information pertaining to air temperature and rainfall during 2015–2019 is presented in Fig. S2.
2.3. Measurement of soil GHG fluxes
The soil GHG fluxes were measured using the widely employed static chamber-gas chromatography technique (Tang et al., 2006; Song et al.,2020).Each static chamber system comprised two parts made of opaque polyvinyl chloride panels as follows:a square base box(0.4 m×0.4 m×0.1 m)with a U-shaped groove(50 mm×50 mm,width×depth),and an opaque chamber box (0.4 m × 0.4 m × 0.4 m). Each base box was inserted directly 0.1 m into the soil in September 2014. From January 2015 to December 2019,gas samples were collected once per month on a clear day between 9:00 and 10:00 a.m. This time frame was selected as the rate of soil GHG flux at this time corresponds closely with the forest's daily means (Zhang et al., 2015; Song et al., 2020). To collect gas samples, the following approach was used: first, the vegetation inside the base box was removed by cutting and a small fan was installed inside at the top of each chamber to generate turbulence in the chamber.Second,the chamber box was placed in the groove of the base box. Third, the groove was filled with water to act as an air seal. Finally, gas samples were collected at 0, 10, 20, and 30 min after chamber closure using a 60-mL plastic syringe attached to a three-way stopcock. Subsequently,the gas samples were immediately injected into evacuated bags made of polymer film and aluminum foil (Desen Inc., Dalian, China). After gas sampling, the opaque chamber box was removed to maintain the base box under ambient conditions. The GHGs concentrations of each gas sample were analyzed within 2 days of gas sampling using gas chromatography(GC-2014;Shimadzu Corporation,Kyoto,Japan).
2.4. Measurement of soil properties
Soil samples of 0–20 cm depth were collected in the growing (July)and non-growing (December) seasons of 2015–2018 and in January,April, July, and October of 2019; they were sourced from random locations within each subplot using a soil auger with 3.5-cm diameter.Prior to the measurement,soil samples were sieved through a 2-mm sieve and divided into two portions.One portion was stored at 4°C for use in the analysis of soil microbial biomass C (MBC), NH4+-N, and NO3--N, while the other was air-dried in the laboratory for use in the determination of soil physicochemical properties.In December 2019,five soil cores were obtained randomly from each plot at two depths, 0–20 and 20–40 cm,and mixed.The cutting ring method was used to measure the bulk density of soil at the two depths.
Soil MBC was determined using the chloroform fumigation extraction method (Vance et al., 1987), and the K2SO4-extracted C was measured using a total organic C analyzer (TOC-VCPH; Shimadzu Corporation,Kyoto, Japan), with 0.45 used as a correction factor for calculating soil MBC.Each soil sample was extracted with 2 mol·L-1KCl solution,while NH4+-N and NO3--N concentrations were determined for each extract with an intermittent chemical analyzer(Smartchem140,AMS Alliance,Rome,Italy). Soil pH was estimated using a pH meter (FE20; Mettler Toledo,Switzerland)with a soil-to-water ratio of 1:2.5.Soil organic C(SOC)and total N concentrations were determined using an elemental analyzer(ElementarVario EL III,Germany).Available N(AN)was measured using the hot alkaline permanganate method(Lu,2000).
2.5. Data calculations
The soil CO2,CH4,and N2O fluxes were obtained using the following formula(Tang et al.,2006):



where SOC denotes SOC storage at a depth of 0–40 cm, i represents the soil layer (0–20 and 20–40 cm), and Diand Birepresent SOC concentration and bulk density at depth i, respectively.
2.6. Statistical analyses
Statistical analyses were performed using IBM SPSS Statistics 22.0(IBM Corp.,Armonk,NY,USA).Repeated measures analysis of variance(ANOVA) was used to assess N addition (N), biochar amendment (BC),time in year dynamic (TD), and their interactive effects on annual soil GHG fluxes. Two-way ANOVA was used to assess the effects of N addition,biochar amendment,and their interaction on mean annual soil GHG fluxes, annual GWP,and SOC storage. One-way ANOVA combined with the least significant difference test was used to determine the statistical significance of differences in soil properties, SOC storage, annual soil GHG fluxes, and GWP among treatments. Prior to ANOVA, the assumptions of normality and homogeneity associated with the variance of all the data were assessed, and data were log-transformed if the homogeneity of the variance was not met. The relationship between soil properties and soil GHG fluxes was investigated using Pearson correlation coefficients. A structural equation model (SEM) was constructed to investigate relationships among soil properties,MBC,and soil GHG fluxes using AMOS 22.0(AMOS IBM,USA).
3. Results
3.1. Soil properties
Compared to the control treatment, N addition alone significantly increased mean annual soil AN (20.3%–30.2%) and NH4+-N (18.9%–32.8%) concentration, while decreasing mean annual soil pH (2.4%–6.4%)(Fig.S3).The mean annual MBC concentration in the N30 and N60 treatments was significantly higher (46.1%–72.9%) than that of the control (Fig. S3f). Biochar amendment significantly decreased mean annual soil AN (11.5%–22.8%), NH4+-N (21.0%–33.2%), and MBC concentration (3.8%–20.5%) compared to the control treatment while increasing the mean annual soil pH (2.0%–2.9%). The combination of biochar amendment and N addition treatments significantly decreased mean annual soil AN(17.5%–30.4%),NH4+-N(18.5%–32.5%),and MBC concentration(15.0%–31.6%)compared to those obtained using only N addition;however,it significantly increased mean annual soil pH(2.2%–4.4%,P <0.05).
3.2. Soil CO2 emissions
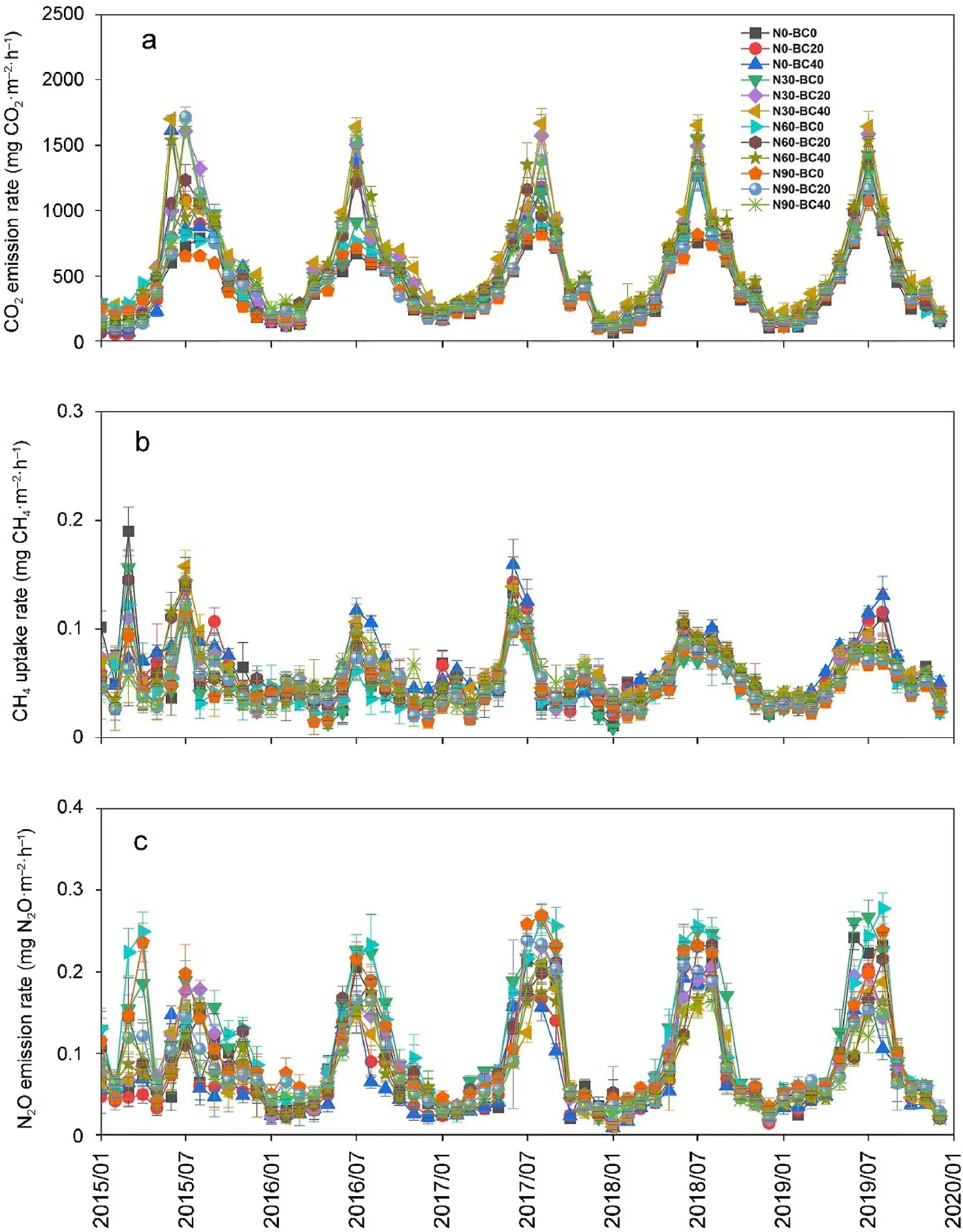
Fig.1. Monthly soil CO2 emission(a),CH4 uptake(b),and N2O emission(c)rates under different nitrogen addition and biochar amendment treatment conditions in the Moso bamboo plantation from January 2015 to December 2019 (mean ± SD, n = 3).
Soil CO2emission rates followed a clear seasonal pattern in all treatments, with the maximum and minimum emissions observed in summer and winter,respectively (Fig. 1a). In the control treatment,the soil CO2emission rate ranged from 53.37 to 1,138.03 mg CO2·m-2·h-1(average 403.24 ± 18.36 mg CO2·m-2·h-1). The highest mean annual soil CO2emission(54,603.27±1,649.45 kg CO2·ha-1)was recorded in the N30-BC40 treatment, which was 1.54 times higher than that of the control treatment (P <0.05). Compared to the control treatment, N addition(N30 and N60)and biochar amendment significantly increased annual CO2emissions each year,however,the promoting effect elicited by N addition and biochar amendment decreased with time(Figs.1a and S4). The combination of N and biochar addition significantly increased annual CO2emissions each year relative to N addition alone(Figs.1a and S4).Compared to that in the control,the mean annual soil CO2emissions in the N30 and N60 treatments were significantly higher by 17.0% and 25.4%,respectively(Fig.2a),whereas that in the N90 treatment did not differ significantly.Mean annual soil CO2emissions during the combined use of biochar amendment and N addition treatments(N0,N30,N60,and N90) were significantly higher (11.2%–34.4%) than those observed under N addition only(P <0.05);such promotion effects increased with an increased biochar amendment rate at identical N deposition rates.Pearson's correlation analysis, and SEM results, indicated that soil CO2emission rates were significantly and positively correlated with soil pH,MBC,and SOC concentrations,however,were negatively correlated with soil C/N ratio(Fig.3a;Table S2).Biochar amendment and N addition had significant effects on annual soil CO2emissions (P <0.001); however,time had no significant effect on annual soil CO2emissions(P=0.401).Soil annual CO2emissions were significantly affected by the two-way(N× BC, N × TD, and BC × TD) and three-way interactions among N addition,biochar amendment, and time(P <0.001,Table 1).
3.3. Soil CH4 uptake
Soil CH4uptake rate in the control treatment was in the 0.009–0.212 mg CH4·m-2·h-1range,with an average uptake rate of 0.0561± 0.012 mg CH4·m-2·h-1(Fig.1b).Compared to that of the control treatment,N addition significantly decreased annual CH4uptake in 2018–2019(Figs.1b and S5).Under the control and N addition treatments,biochar amendment significantly increased annual CH4uptake in 2016–2019,however, this promoting effect decreased with time (Figs. 1b and S5).Compared to that of the control treatment, the mean annual soil CH4uptake was 15.9%higher for that of the B40 treatment and 12.4%–16.8%lower for the N addition(P <0.05,Fig.2b).The combination of biochar amendment and N deposition treatments (N30, N60, and N90) significantly increased the mean annual soil CH4uptake by 9.7%–23.5%relative to that obtained with N addition alone (P <0.05). Significant positive correlations with soil CH4uptake were observed for both soil pH and MBC. Moreover, soil CH4uptake was significantly and negatively correlated with soil NH4+-N concentration and soil C/N ratio (Fig. 3b;Table S2). Repeated measures ANOVA further revealed that N addition,biochar amendment,time,and N×TD significantly affected soil annual CH4uptake(P <0.01,Table 1).
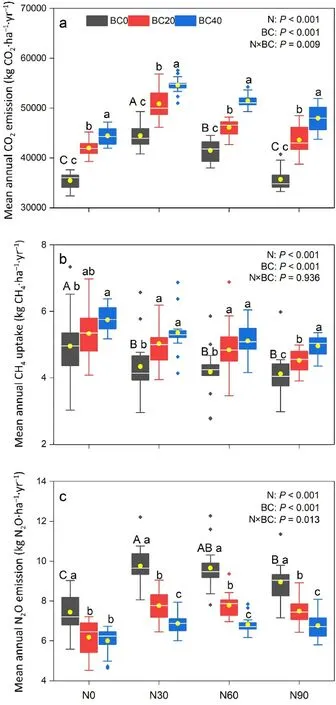
Fig.2. Mean annual soil CO2 emission(a),CH4 uptake(b),and N2O emission(c)under different nitrogen addition and biochar amendment treatment conditions in the Moso bamboo plantation.Rhombus indicates the individual values of each dataset. The length of the line: maximum and minimum values; box: upper and lower quartiles; horizontal line within the box: median; yellow dot: mean.Different capital letters indicate significant differences among N addition rates for BC0 treatment.Different lower-case letters indicate significant differences among biochar amendment rates at identical N addition rates.P-values from the two-way ANOVA indicate significant differences among N addition (N) and biochar amendment(BC)treatments.(For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
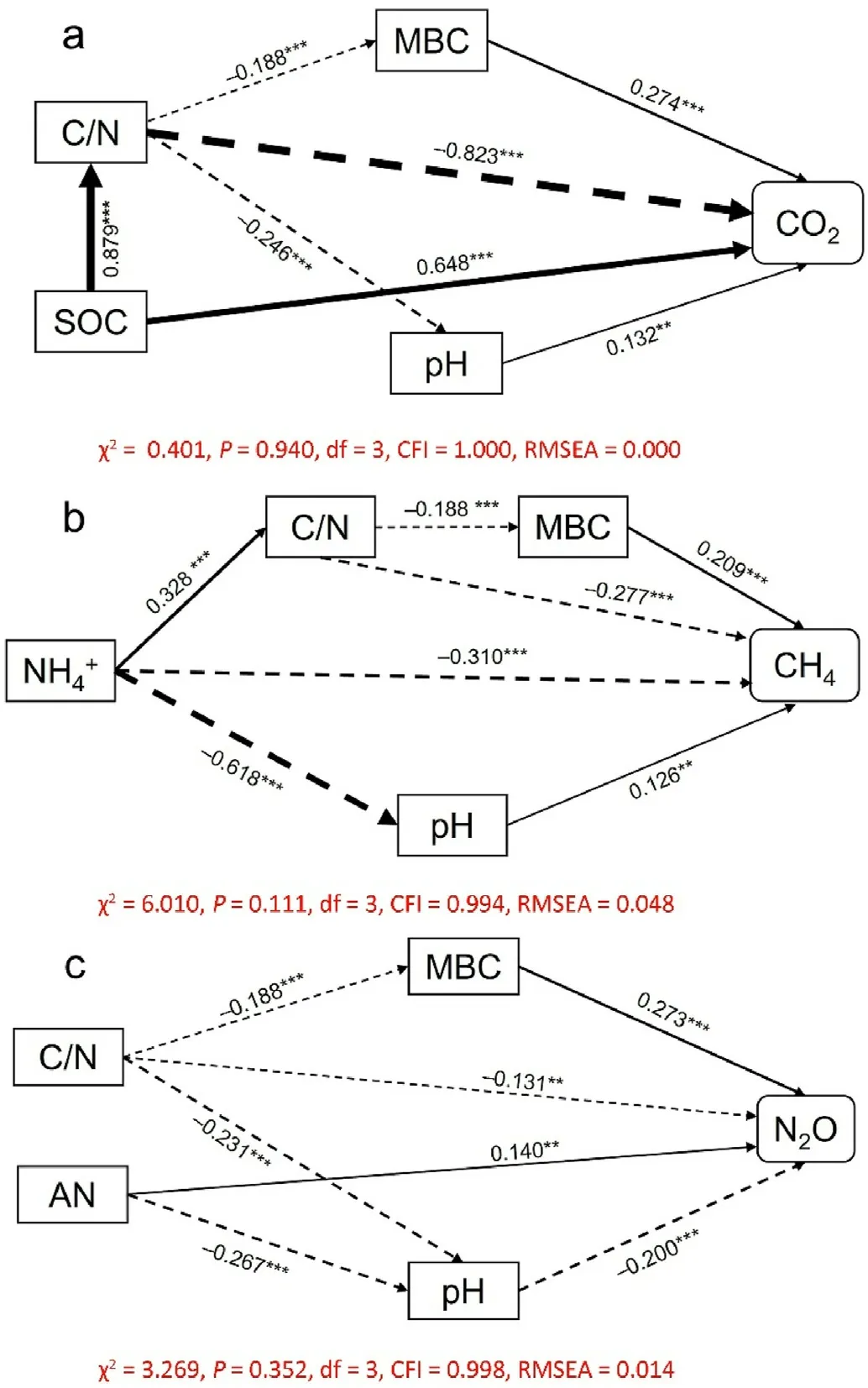
Fig. 3. Structural equation model results for the correlations of soil CO2 emission (a), CH4 uptake (b), and N2O emission (c) rates with soil properties.Numbers adjacent to arrows are standardized path coefficients. Arrow widths increase with the path coefficient value. Solid and dashed arrows indicate positive and negative relationships, respectively. *,P <0.05; **,P <0.01;***,P <0.001. AN, available N; C/N, C/N ratio; CFI, comparative fit index; MBC,microbial biomass C; RMSEA, root mean square error approximation; TN,total N.
3.4. Soil N2O emissions
The soil N2O emission rate from the control treatment was 0.011–0.247 mg N2O·m-2·h-1(average: 0.085 ± 0.007 mg N2O·m-2·h-1; Fig. 1c). High seasonal variation was observed, with higher emissions in summer compared to winter, regardless of the treatment (Fig. 1c). Compared to the control treatment, N30 and N60 treatments significantly increased annual N2O emissions each year and biochar amendment significantly decreased annual N2O emissions each year (Figs. 1c and S6). The promoting effect elicited by N addition,and the inhibitory effect of biochar amendment, decreased with time.Compared to N addition alone, combined addition of N and biochar significantly reduced annual N2O emissions each year. Moreover, N addition significantly increased mean annual soil N2O emissions by 20.3%–31.1%, whereas biochar amendment significantly decreased mean annual soil N2O emissions by 16.9%–19.3%compared to those of the control treatment(P <0.05,Fig.2c).Additionally,the combination of biochar amendment and N addition treatments (N30, N60, and N90)significantly reduced mean annual soil N2O emission by 16.4%–35.9%relative to those obtained with N addition alone (P <0.05). Pearson's correlation analysis and SEM analysis explained the variation in N2O emissions through the positive relationship between MBC and AN concentrations,as well as the negative effects of pH and C/N ratios(Fig.3c;Table S2). Biochar amendment(P <0.001) and N addition(P <0.001)significantly affected annual soil N2O emissions across the 5-year study period (Table 1), and annual soil N2O emissions were significantly affected by N×BC and N× TD interactions(P <0.001,Table 1).
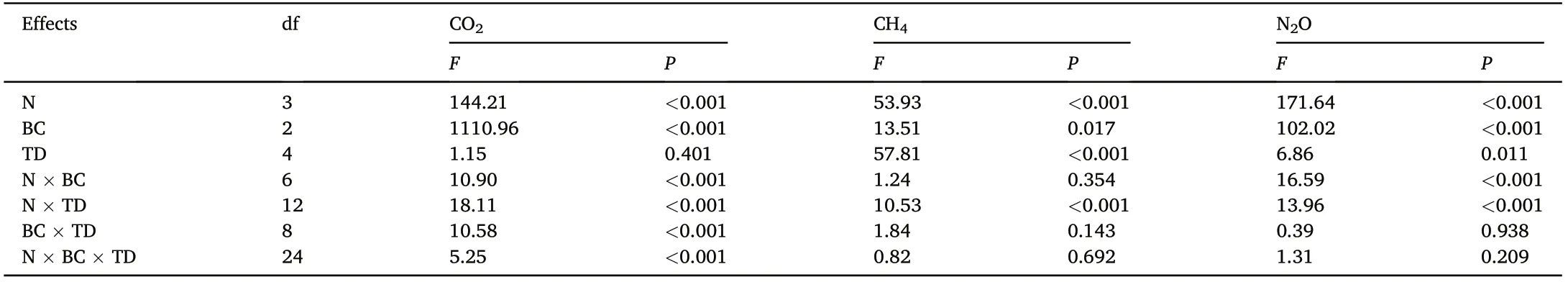
Table 1 Results(F and P values)of repeated measurements analysis of variance:effects of N deposition(N,4 levels),biochar amendment(BC,3 levels),and time in year dynamic(TD, 5 years)on annual soil CO2, CH4,and N2O fluxes in the Moso bamboo plantation.
3.5. Mean annual GWP and SOC storage
The mean annual GWP was 2.09 t CO2-equivalent·ha-1·yr-1in the control treatment (Fig. 4a). Comparatively, the mean annual GWP for treatments N30,N60,and N90 increased significantly,by 44.0%,32.5%,and 22.5%, respectively. Meanwhile, biochar amendment significantly decreased the mean annual GWP by 18.4%–21.4%,as compared to that of the control (Fig. 4a). The combination of biochar amendment and N addition (N30, N60, and N90) significantly decreased the mean annual GWP by 27.4%–36.5%, 20.7%–31.4%, and 17.4%–26.2%, respectively,relative to that observed with N addition alone(P <0.05).Annual GWP was significantly affected by both treatments independently, as well as via interactions among N addition and biochar amendment (P <0.05,Fig.4a).
Compared to the control, the N60 treatment significantly decreased SOC storage by 6.5%, whereas biochar amendment significantly increased SOC storage by 7.1%–13.4% (Fig. 4b). The combination of biochar amendment and N addition significantly increased SOC storage by 10.8%–34.2% relative to those obtained with N addition alone (P <0.05). N addition and biochar amendment alone, as well as in combination significantly affected SOC storage(P <0.05,Fig.4b).
4. Discussion
4.1. Effects of long-term N addition on soil GHGs
Soil CO2emissions had a strong seasonal pattern under all treatments in the Moso bamboo plantation (Fig. 1a), which was consistent with previous study results in similar subtropical forest soils (Li et al., 2019;Zhang et al.,2021),and may be due to temperatures altering the biomass and activity of microbes and root (Fig. S2; Warner et al., 2017; Deng et al., 2020). Moreover, long-term low and moderate N input (≤60 kg N·ha-1·yr-1) significantly promoted soil CO2emissions,which supports our first hypothesis.However,other studies have shown that long-term N addition reduces soil CO2emissions in Chinese fir (Cunninghamia lanceolata) forests (Fan et al., 2014; Wang et al., 2016) and a tropical monsoon evergreen broadleaf forest (Tian et al., 2019). These discrepancies are likely attributed to the initial soil N conditions. That is, in N-limited ecosystems,N addition can enhance soil respiration(Hyv¨onen et al.,2007); however,a contrasting effect is observed in N-rich ecosystems(Cusack et al.,2011).Indeed,our previous research revealed leaf N:P ratios <14 and N:P resorption efficiency ratios >1 in the Moso bamboo plantation(Song et al.,2016a;Li et al.,2021),indicating that our study site was N-limited and,thus N addition promoted soil respiration in this study. Moreover, the promotion effects of long-term N addition on soil CO2emissions in the present study might be attributed to the increase in soil respiration, which is induced via enhancement of plant growth and microbe activity following N addition(Li et al.,2019;Wang et al.,2019).In our previous studies,N addition enhanced the photosynthetic rates of Moso bamboo (Zhang et al., 2017b) and leaf litterfall (Zhang et al.,2017a),decomposition rates of both leaf litter(Song et al.,2015)and fine roots(Song et al.,2017b), and MBC(Li et al.,2016). Collectively,these factors might partially lead to an increase in soil CO2emissions resulting from N addition. Additionally, high N addition (90 kg N·ha-1·yr-1) did not significantly increase soil CO2emissions in the present study. Similarly, Tian et al. (2019) reported that N addition (50 and 100 kg N·ha-1·yr-1) does not affect soil CO2emissions in a tropical monsoon evergreen broadleaf forest. These results verified that the direction and magnitude of N effects on soil CO2emissions are strongly dependent on N addition rates(Fan et al., 2014;Tian et al.,2019;Deng et al., 2020).
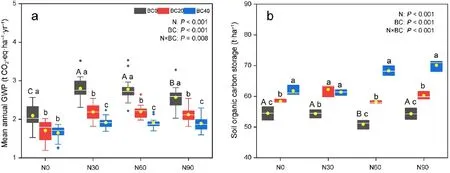
Fig. 4. Global warming potential (GWP) of mean annual soil CH4 uptake, and N2O emission (a) and soil organic carbon storage(b) under different nitrogen addition and biochar amendment treatment conditions in the Moso bamboo plantation. Rhombus indicates the individual values of each dataset.The length of the line: maximum and minimum values; box: upper and lower quartiles;horizontal line within the box: median; yellow dot: mean. Different capital letters indicate significant differences among N addition rates for BC0 treatment.Different lower-case letters indicate significant differences among biochar amendment rates at identical N addition rates. BC, biochar amendment. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
In the present study, soil CH4uptake was significantly reduced by long-term N addition, thereby supporting our first hypothesis and concurring with the results of a recent study in the tropical monsoon evergreen broadleaf forest(Tian et al.,2019).Generally,the decrease in soil CH4uptake after N addition was likely caused by a reduction in the activities of both methanogenic archaea and methanotrophic bacteria(Kim et al.,2015).In N addition plots,pH(<4.4,Fig.S3)was lower than the optimal pH (4.6–6.8) for methanotrophic activity (Benstead and King, 2001; Bradford et al., 2001), thus suppressing CH4consumption.Moreover,the observed significant positive correlation between soil CH4uptake and soil pH values(Fig.3b;Table S2)supports the notion that low pH inhibits CH4consumption.Additionally,the SEM results showed that soil NH4+-N concentrations significantly affected CH4uptake,which was consistent with the findings of several studies (Aronson and Helliker,2010; Feng et al., 2020; Song et al., 2020). This is likely explained by competition between CH4and high NH4+-N concentrations in the soil for methane monooxygenase, which in turn inhibits CH4oxidation and decreases CH4uptake (Bodelier and Laanbroek, 2004). Furthermore,nitrite-N (NO2--N) toxicity, resulting from increased nitrification of NH4+-N, might also inhibit methanotrophic activity (Schnell and King,1994),thus hindering CH4oxidation.Our previous study at the same site also showed that N addition significantly reduced methanotroph abundance and diversity, further indicating that N deposition decreases CH4oxidation(Song et al.,2020).
In the present study,we observed that long-term N addition enhanced soil N2O emissions, which supports our first hypothesis and previous findings in a subtropical secondary forest dominated by Masson pine(Pinus massoniana) (Xie et al., 2018) and a tropical monsoon evergreen broadleaf forest (Tian et al., 2019). In the present study, AN concentration was higher in the N addition treatments than in the control treatments and was significantly and positively correlated with soil N2O emissions(Figs.3c and S3;Table S2),indicating that N addition increases soil N2O emissions by increasing soil AN concentrations. That is, N enrichment in N-limited areas increases N availability for nitrifying and denitrifying bacteria, leading to an increase in soil N2O emissions (Fu et al., 2015; Wu et al., 2017; Song et al., 2019). Additionally, previous studies have demonstrated that a decrease in soil pH can significantly increase N2O production by enhancing denitrification (Cheng et al.,2015;Tian et al.,2019),which is attributed to the increasing abundance of denitrifying bacteria at low pH(Tian et al.,2019;Song et al.,2020).In the present study,the SEM results showed that soil pH was significantly and negatively correlated with N2O emissions(Fig.3c;Table S2),which is consistent with previously published results (Cheng et al.,2015;Tian et al.,2019;Song et al.,2020).In fact,our previous study at the same site found that N input significantly increases the number of denitrifying bacteria(norB),which might partially influence the increase in soil N2O emissions following N addition(Song et al., 2020).
N addition significantly increased GWP,which was attributed to the increase in soil N2O emissions and the decrease in soil CH4uptake following N addition. Furthermore, the present study, as well as our previous study at the same site,found the promoting effect of N addition on soil CO2and GWP persisted over the seven-year study period(Table 1;Figs. S4–6), indicating that the effects of short-term and long-term N addition on soil GHG fluxes were similar in the Moso bamboo plantation.Moreover,N addition reduced SOC storage in this study(Fig.4b),which was similar to the finding of Deng et al. (2018) and Lu et al. (2011). N addition may have increased soil respiration and decreased soil CH4uptake (Fig. 2a), causing accelerated C loss (Deng et al., 2018) and resulting in reduced C accumulation. In addition, our previous study reported that N addition increases dissolved organic carbon leaching loss(Lei et al., 2018), leading to C leaching to deeper soils and loss through surface runoff(Lu et al., 2011).
4.2. Effects of biochar amendment on soil GHGs
Biochar amendment significantly promoted soil CO2emissions in the Moso bamboo plantation,which is consistent with previous findings in a temperate forest(Mitchell et al.,2015),a Douglas-fir forest(Pseudotsuga menziesii) (Hawthorne et al., 2017), and a Torreya grandis plantation(Zhang et al.,2019).A primary reason for this is the reduced aluminum toxicity resulting from increased soil pH under biochar amendment(Shi et al.,2020),which further increases soil microbial diversity and activity(Mitchell et al.,2015;Song et al.,2016b;Li et al.,2018b),plant growth and root biomass (Lehmann et al., 2011; Song et al., 2016b) and,consequently,soil respiration and soil CO2emissions.The present study,as well as our previous studies, the document that biochar amendment increases soil pH, bacterial diversity (Li et al., 2018a), and cellulase activity (Peng et al., 2019), all of which could promote microbial respiration. Moreover, biochar amendment enhanced the photosynthetic rates of Moso bamboo(Liu et al.,2019); thus, more C was transported below ground, which could also promote root respiration (Davidson et al.,2004;Janssens et al.,2010).Indeed,Li et al.(2018c)found that biochar amendment increased soil autotrophic respiration in a Moso bamboo plantation. In contrast, Ge et al. (2020) showed that low biochar application rates (5 t·ha-1) significantly decreased soil respiration rate in a Moso bamboo plantation.The discrepancy between these results may be attributed to the different rates of biochar amendment,as demonstrated by a meta-analysis showing that low biochar application rates(≤10 t·ha-1) decrease CO2emissions, whereas high application rates(>10 t·ha-1)significantly increase CO2emissions(Song et al.,2016b).
In the present study,biochar amendment significantly increased soil CH4uptake, which supported our second hypothesis, and previous findings from an intensively managed Chinese chestnut (Castanea mollissima)plantation(Xiao et al.,2018;Lu et al.,2019).This result might be attributed to the increase in soil pH caused by biochar application(Fig. S3), which favors the growth of methanotrophs (Inubushi et al.,2005; Jeffery et al., 2016) and promotes CH4uptake. Biochar amendments decrease soil NH4+-N concentrations by adsorbing NH4+-N(Li et al.,2018b; Song et al., 2019) and increase methanotrophic activity, thus increasing CH4oxidation. In addition, the decrease in soil NH4+-N was attributed to the increase in soil pH values that enhance ammonia volatilization (Cheng et al., 2015). Herein this relationship was confirmed by SEM analysis, as soil CH4uptake was increased by decreasing soil NH4+-N concentration and increasing soil pH under biochar amendment (Figs. 3b and S3). Additionally, a previous study reported that biochar amendment can decrease CH4uptake (Hawthorne et al., 2017), while others did not observe significant results (Sackett et al.,2015;Lin et al.,2017).These discrepancies were dependent on the type and application rate of biochar,soil type,and timing of the biochar application(Song et al.,2016b;Li et al.,2018b).
We also observed that biochar application decreased soil N2O emissions,which supports our second hypothesis.Similarly,previous studies have reported that biochar amendment inhibits soil N2O emissions in spruce (Picea) forests (Malghani et al., 2013), pine forests (Sun et al.,2014),and subtropical Chinese chestnut plantations(Lu et al.,2019).A potential explanation for this phenomena is that biochar has a strong sorption capacity,thereby decreasing N availability for denitrifying and nitrifying bacteria, consequently inhibiting nitrification and denitrification processes(Harter et al.,2014;Lu et al.,2019).This notion has been further corroborated in the present study as soil AN concentrations were lower in the biochar amendment treatments than in the control group(Fig.S3c)and soil AN was significantly and positively correlated with soil N2O emissions(Fig. 3c; Table S2). In addition,high soil pH inhibits the N2O/(N2O+N2)product ratio(McMillan et al.,2016),which decreases soil N2O emissions.This finding is consistent with our findings,as soil pH was significantly and negatively correlated with N2O emission (Fig. 3c;Table S2). A previous study observed that low biochar amendments (5 and 15 t·ha-1) decreased soil N2O emissions by decreasing labile N concentrations, N-cycling enzyme activities, and nitrification/denitrification rates in Moso bamboo plantations (Song et al., 2019). Therefore,biochar amendment(≤40 t·ha-1)may be an effective approach for mitigating soil N2O emissions in Moso bamboo plantations.
Biochar amendment significantly decreased GWP, which was attributed to the increase in soil CH4uptake and the decline in soil N2O emissions. In addition, biochar amendment influenced annual soil GHG flux across the 5-year study period(Figs. S4–6), indicating that the promoting effects of biochar amendment on soil GHG flux could persist for an extended period of time. Moreover, biochar amendment positively affected SOC storage(Fig.4b),which was similar to the finding of Wang et al. (2014), who observed that biochar application increased SOC storage in a Chinese chestnut plantation significantly.This was attributed to the stability of biochar, causing it to decompose slowly in soil environments, thus contributing to the recalcitrant soil C pool (Li et al.,2018b).Moreover,biochar amendment could promote plant productivity and litter biomass formation,which contributes to increased SOC storage(Li et al.,2018b).
4.3. Effects of biochar amendment and N deposition on soil GHGs
The combination of biochar amendment and N addition significantly increased soil CO2emissions as compared to those observed by adding only N, suggesting that biochar application intensifies the promoting effect of N addition on soil CO2emissions in the Moso bamboo plantation,which contradicted our third hypothesis.Similar results have been observed in laboratory studies (Sigua et al., 2016; Senbayram et al.,2019)and a Torreya grandis plantation(Zhang et al.,2019).One possible explanation is that the biochar amendment significantly enhances soil pH and alleviates the negative effect of N addition-induced soil acidification on microbial activity, thus promotes soil respiration. In our previous studies at the same site,bacterial diversity(Li et al.,2018a)and cellulase activity (Peng et al., 2019) under combined biochar and N addition treatments were significantly higher than those observed under N addition treatments alone, which directly supports the view that biochar amendment increases soil CO2emissions by increasing soil pH. In previous studies, combined biochar and N addition significantly enhanced the carboxylation efficiency rates of Moso bamboo (Liu et al., 2019).Thus, belowground C allocation increased, which could stimulate root respiration.However,Ge et al.(2020)reported that biochar amendment(5 and 20 t·ha-1) + N addition (50 kg·ha-1) treatments significantly decrease CO2emissions, as compared to those only treated with N addition in a Moso bamboo plantation. This could be attributed to the different addition times, addition rates, and methods (spraying, broadcast application, and groove fertilization)of biochar and N addition.
Under N addition treatments, biochar amendment significantly increased soil CH4uptake, however decreased N2O emissions, which supports the third hypothesis and indicates that biochar amendment offsets the inhibition effects of N addition on soil CH4uptake rates and the promoting effects of N addition on N2O emissions in the Moso bamboo plantation. Previous studies have also observed similar results(Xiao et al.,2018;Shaukat et al.,2019).For example,Xiao et al.(2018)showed that biochar+N addition treatments significantly decrease soil CH4emissions as compared with those observed under N addition treatments alone in acidic soils.Similarly,Shaukat et al.(2019)observed that soil N2O emissions under biochar+N addition treatments declined,as compared with those under N addition treatments alone in paddy rice soil.Hence,we postulate that biochar amendments increase soil pH and decrease soil NH4+-N, NO3--N, and AN concentrations under N addition conditions,which then promote CH4uptake and decrease N2O emissions.Additionally, previous studies have demonstrated that biochar amendment could significantly change microbial community structure and diversity under N addition conditions, and ultimately alter soil CH4and N2O fluxes(Li et al.,2018a,b).
In the present study,combining different biochar amendment and N addition treatments significantly decreased GWP and increased SOC storage,indicating that biochar amendment offsets the promotion effects of N deposition on soil GWP and the negative effects on SOC storage.Furthermore,the effects of biochar amendment on soil GHG fluxes could persist for an extended period of time under N addition.Taken together,these results enhance our understanding of the effects of biochar amendment on soil GWP and SOC storage in subtropical Moso bamboo plantations,particularly under increasing atmospheric N deposition.
5. Conclusion
Dynamic measurements of soil GHG fluxes under long-term N addition and biochar amendment treatments demonstrated that Moso bamboo plantation soil served as a source of CO2and N2O,and a sink for CH4, regardless of treatment. More specifically, long-term N addition promoted soil CO2and N2O emissions, inhibited soil CH4uptake, and increased soil GHG emissions in Moso bamboo plantations. Meanwhile,biochar amendment reduced soil N2O emissions, while increasing soil CO2emissions, CH4uptake, and the soil C pool. In the combined treatment,biochar amendment intensified the promoting effects of N addition on soil CO2emissions; however, it offset the promoting effects of N addition on N2O emissions and its inhibitory effects on CH4uptake and soil C pool,owing to the increase in soil pH and decrease in soil NH4+-N concentration. Overall, these results demonstrate that biochar application could be applied as a global climate change mitigation strategy,particularly under scenarios of N addition. Further research should endeavor to study the effect of biochar amendment on soil microbes and their gene functions related to C cycling under N addition conditions,which would facilitate an improved understanding regarding the mechanisms by which biochar influences GHGs fluxes in plantation soils.
Authors’contributions
Quan Li: Methodology, Formal analysis, Investigation, Resources,Data curation, Writing-original draft, Funding acquisition. Kunkai Cui:Formal analysis, Investigation, Writing-original draft, Visualization.Jianhua Lv: Investigation, Writing-review & editing. Junbo Zhang:Investigation,Writing-review&editing.Changhui Peng:Writing–review& editing. Yongfu Li: Writing-review & editing. Zhikang Gu: Writingreview & editing. Xinzhang Song: Conceptualization, Methodology,Validation,Writing-review &editing,Supervision,Funding acquisition.
Funding
This study was sponsored by the National Natural Science Foundation of China, China (Grant Nos. 31470529, 32125027) and Zhejiang A&F University Research and Development Fund, China (Nos. 2022LFR006,2021LFR060).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We sincerely thank Honghao Gu, Zhaofeng Lei, Qiang Yang, and Yaoxiong Wang for help in the field and laboratory.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://do i.org/10.1016/j.fecs.2022.100054.
- Forest Ecosystems的其它文章
- Prediction of the global potential geographical distribution of Hylurgus ligniperda using a maximum entropy model
- The 2/3 scaling of twig nitrogen to phosphorus in woody plants
- Monitoring the abundance of saproxylic red-listed species in a managed beech forest by landsat temporal metrics
- Trade-offs among fine-root phosphorus-acquisition strategies of 15 tropical woody species
- Structure complexity is the primary driver of functional diversity in the temperate forests of northeastern China
- Consistent response of nematode communities to management of coniferous plantations

