Trade-offs among fine-root phosphorus-acquisition strategies of 15 tropical woody species
Yuxin Li, Lijuan Sun, Biao Zhu
Institute of Ecology,College of Urban and Environmental Sciences,and Key Laboratory for Earth Surface Processes of the Ministry of Education,Peking University,Beijing,100871, China
Keywords:Root functional traits Phosphorus acquisition Mycorrhizal fungi Colonization ratio Acid phosphatase
ABSTRACT
1. Introduction
Phosphorus (P) is an essential nutrient for plant development and reproduction (Raghothama, 1999) since it makes up some important biomacromolecules such as nucleic acid, phospholipid, and triphosadenine (Filippelli, 2008), and plays a critical role in basal biochemical processes like energy generation,controlling key enzyme reactions,and signal transduction (Theodorou and Plaxton, 1993; Vance et al., 2003).However, although the total amount of phosphorus in the soil might be high (Bieleski, 1973), phosphorus is still a major constraining macronutrient for plants(Schachtman et al.,1998;Johnston et al.,2014).The major species of soil phosphorus are unavailable for plants, such as organic (George et al., 2018) or mineral (Holford, 1997) forms. Plants could only uptake the inorganic phosphate (available phosphorus) by fine roots directly, but its diffusion in soil is slow (Lewis and Quirk,1967),and due to the adsorption by iron-aluminum oxides,the content of available phosphorus is relatively low in various natural and managed ecosystems worldwide (Schachtman et al., 1998), especially in tropical forests(Camenzind et al.,2018),where the strong process of weathering creates an environment of abundant iron-aluminum oxides. In tropical forests,the utilization of phosphorus is highly dependent on the activities of extracellular enzymes or organic acids in soil (Walker and Syers,1976).
To build up symbioses with mycorrhizal fungi is an alternative strategy for plants against P deficiency (Clark and Zeto, 2000). In the mycorrhizal symbiosis system, it is usually considered that the plants offer carbon and energy to the fungi (Jakobsen and Rosendahl, 1990),and employ the fungi to enhance P acquisition in various ways(Lambers et al., 2006). The fungal hyphae are far thinner and longer than fine roots,therefore the symbioses could provide considerable extra root area,extend to distant places to overcome the ‘P depletion zones’ near root surfaces (Lewis and Quirk, 1967), and explore the soil micro-pores and particles that roots could not reach (Clark and Zeto, 2000). Thus, the collaboration with mycorrhizal fungi is considered as complementary to foraging with morphologically efficient fine roots (Liu et al., 2015; Lyu et al.,2016).Apart from these morphological benefits,mycorrhizal fungi could also excrete enzymes and acids to mineralize organic phosphorus,and increase the content of available P for plants(Bolan,1991).There are also researches suggesting that the fungi-infected roots show a higher level of phosphorus-transporters(Grace et al.,2009),and the arbuscular mycorrhizal fungi(AMF)may have a positive effect on plants in the genetic level.
Under limited phosphorus availability,plants have developed diverse strategies to promote P acquisition of fine roots (Biddinger et al., 1998;Lambers et al., 2006). These strategies include accelerated root growth,changes of more efficient morphology,production of carboxylates(Rose et al.,2010),protons(Neumann and R¨omheld,1999),and release of acid phosphatase (Richardson et al., 2011). They can be classified as root-foraging, P-mining, and improving internal P-utilization efficiency strategies(Clark and Zeto,2000).
Overall, plants show trade-offs among these P-acquisition strategies.Under P deficiency, plants need to invest certain amount of carbon and energy to produce and keep these traits,and how they allocate resources and energy into different traits shows a trade-off of costs and benefits(Pearse et al.,2006). There is an increasing number of studies upon the interactions of different P-acquisition traits of roots.For example,thicker roots, such as Lupinus albus and Cicer arietinum, often show less active exudation of enzymes and organic acids, showing a trade-off pattern between foraging by efficient roots and P mining by organic acids (Lyu et al., 2016; Honvault et al., 2021). Those thinner and higher-branched fine roots are more morphologically efficient, and invest less to exudation of organic acids or mycorrhizal symbioses(Wen et al.,2019).These studies indicated a general mode of trade-offs between different P-acquisition strategies,but most of the patterns of these strategies need to be verified. Yet, the pattern was mostly verified among non-woody species (Wen et al., 2019; Honvault et al., 2021). The whole picture of phosphorus acquisition strategies is far from clear,especially for woody plants in(semi)natural ecosystems.
We targeted 15 woody species growing in a tropical common garden in Xishuangbanna,China.We explored the trade-offs of their abilities to release acid phosphatase (AP) from fine roots and the colonization by mycorrhizal fungi in response to their species-specific bulk soil P availability. This study aimed to show the interactions between two Pacquisition strategies, and the regulatory factors upon this trade-off. By exploring the relationship between acquiring P by root-originated Pdegrading enzyme, colonization of mycorrhizal fungi and their interactions, we aimed to testify the hypothesis that there is a trade-off between the strategy of AP exudation and AMF colonization, and to better understand the adaptation mechanisms of plant roots under P limitation.
2. Materials and methods
2.1. Study site and target species
This study was performed at Xishuangbanna Tropical Botanical Garden of the Chinese Academy of Sciences located in Mengla County,Yunnan Province,China(21°41′N,101°25′E).Climate here is a typical tropical monsoon type. Mean annual temperature is 21.6°C and mean annual precipitation is 1,476 mm. Soil here is Ferralic Cambisol (FAO Soil Taxonomy)with a mean pH of 5.1(Lin et al.,2017).The study site is an ancient riverbed with mainly hard rocks below 10 cm in soil depth,and fine roots are mainly distributed in the upper 10 cm of soil.
We sampled fifteen woody species in this study(Table 1).The plants have been monocultured since 1959, and all the planting conditions including soil type, topography, and planting density are the same. We selected three well-grown individuals for each species. Trees studied were all larger than 20 years old, and nearly no plants grew under the canopy.Thirteen out of the 15 species were classified as AM(arbuscular mycorrhizal) type, and the other two were EM (ectomycorrhizal) type.According to the APG-IV classification system of plants(The Angiosperm Phylogeny Group, 2016), Mesua ferrea (MF), Cassia siamea (CsS),Homalium cochinchinense(HC),Castanopsis indica(CI)and Erythrophleum fordii(EF)belong to four families,and these families are all members of the higher classification unit named Fabids, indicating a nearer phylogenetic relationship than the other species.
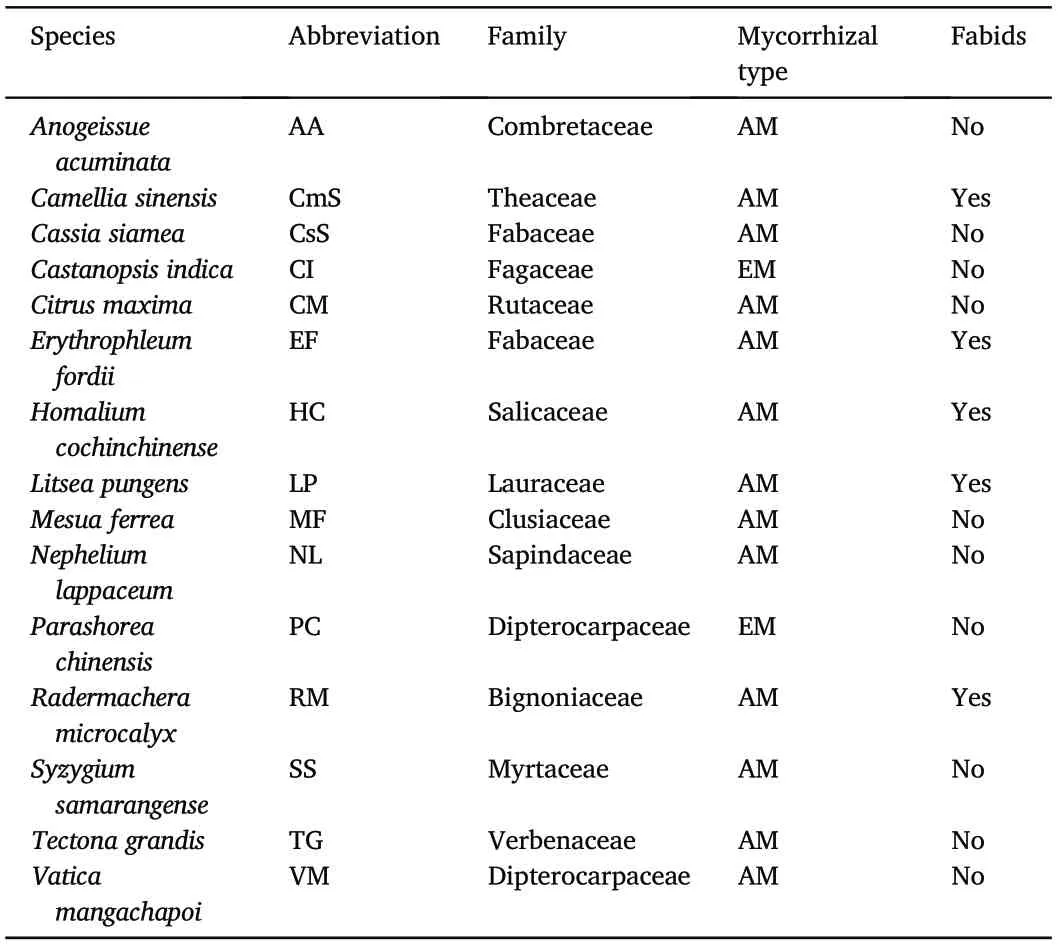
Table 1 Abbreviation, family, and mycorrhizal type of the 15 target species. The classification is based on the APG-IV system (The Angiosperm Phylogeny Group,2016). AM,arbuscular mycorrhizal type; EM,ectomycorrhizal type.
2.2. Soil sampling and laboratory measurements
Within the range of 1 m around the target tree stem, we randomly collected the upper 10 cm of soil using a 10-cm diameter stainless steel corer in April 2021.The fresh soils and roots were transformed to laboratory with cooler boxes and ice packs.They were stored at 4°C before further analyses.
For every sample, 5 g subsample in fresh weight was oven dried for 72 h at 105°C, and the water content was calculated using the weight loss data. The potential activities of three hydrolytic soil enzymes,including β-1,4-glucosidase(BG)related to C-cycling,β-1,4-N-acetylglucosaminidase (NAG) related to N-cycling, and acid phosphatase (AP)related to P-cycling,were measured on fresh soils based on the protocol by German et al.(2011).Soil available phosphorus was determined by a molybdate/ascorbic acid method after NaHCO3extraction.
We used the data of total C,N,P,and pH from soils sampled from the same site in December 2018. Total C and N concentrations were determined using an elemental analyzer (Vario EL III; Elementar, Hanau,Germany). A concentrated nitric acid–hydrofluoric acid–perchloric acid(HNO3–HF–HClO4; 6:2:1) mixture was used for soil digestion, using a microwave digestion system (CEM Mars 6; CEM Corp., Matthews, NC,USA). The total P concentration was then determined using inductively coupled plasma optical emission spectroscopy (iCAP 6000 Series;Thermo Scientific, Waltham, MA, USA). Soil pH was measured in suspension using a laboratory pH meter.
Following the method described by Guo et al.(2004),root sampling was conducted in laboratory in April 2021. We gently loosened the soil blocks, collected the roots and broken segments using fine forceps, and identified and discarded the dead roots. The collected live roots were placed in deionized water, and gently stirred to remove the adhering soils.Based on the classification method of McCormack et al.(2015),we dissected the roots,and absorptive roots were kept at 4°C for following experiments. We randomly took approximately 0.01 g of each sample,cultivated the root for 1 h at 25°C with 50 mL of 25 mmol·L-1sodium acetate buffer at the pH of 5.1,and then measured the potential activities of AP in the culture solution(Han et al.,2022).The roots after incubation were oven dried for 24 h at 65°C for moisture measurement. The units for AP activities of roots were nmol·(g-1dry weight)·h-1.
To estimate the root colonization by AMF,we cleared and stained the root sample based on the protocol put forward by Phillips and Hayman(1970) and improved by Koske and Gemma (1989). The roots were cleared with 10%KOH solution in a 90°C water bath for 60 min,washed with water, acidified with 2% HCl for 5 min, and stained with 0.05%Trypan blue (dissolved in 1:1 lactic acid-glycerol solution) for 30 min.Stained root samples were then put into 1:1 lactic acid-glycerol solution for at least 1 h to remove the adhering stain. For each individual plant sample, we randomly took 20 stained fragments from 6 to 7 absorptive roots, made the temporary specimens, and calculated the colonization ratio with a light microscope based on the improved method of Trouvelot et al.(1986).
For EM associated species,the roots were washed by deionized water,and observed under a 4×10 light microscope.We counted the colonized and uncolonized root tips of 20 fine roots, and calculated the average colonization ratio.
2.3. Statistical analysis
The fifteen species were naturally divided into two functional groups in the scatter diagram regarding the relationship between soil AP activity and root AP activity.Mycorrhizal type could not explain this difference,but all five Fabids species are all in the upper layer of the scatter diagram.Former studies have shown the differences of functional traits at a higher taxonomic level,Rosids and Asterids(Zhang et al.,2011),then we classified the species into two subgroups:Fabids and Others,to test whether the differences of functional traits still exist at a lower taxonomic level.Additionally,the Tectona grandis(TG)is not native species and could be classified into neither of the subgroups,then its data were not used in the following analysis.
All the statistical analysis were done in R(4.0.2,R Development Core Team). Two-way ANOVA was used to assess the effects of species variance, soil AP activity and their interaction on root AP activity. Linear regression was done to evaluate the correlation between soil AP activity and root AP activity within each subgroup.Quantile regression(Cade and Noon, 2003; Baird et al., 2021) was done to assess the correlation between the mycorrhizal colonization ratio and the AP activity of roots in the quantreg package.Phylogenetic independent contrast(Quader et al.,2004)was calculated in the phytools package,and the phylogenetic tree was done based on the APG-IV system on the platform of Phylomatic(http://phylodiversity.net/phylomatic/). Data were natural-log transformed to ensure the assumption of normality and homoscedasticity.
3. Results
3.1. Relationships between soil available P and soil AP, root AP, and mycorrhizal colonization
In our study site, the average content of soil available P was 0.23 mg·kg-1, showing an evident P-limitation. The results of two-way ANOVA were shown in Table 2. For bulk soil AP activity and root AP activity, only species had significant effect; and only bulk soil P availability had significant correlation with mycorrhizal colonization ratio.
3.2. Negative correlation between root AP activity and bulk soil AP activity
There was no general pattern between root AP activity and bulk soil AP activity.But we found positive correlations between them within the two sub-groups as Fabids and others according to the IPG-IV system(Fig. 1). A generalized linear model confirmed that the main effect of classification (P = 0.003) and the interaction effect between classification and bulk soil AP activity (P = 0.09) on root AP activity were both(marginally)significant(Table 2).At both species and individual levels,either of the subgroups (Fabids and others) shows a significant positive correlation between root AP activity and bulk soil AP activity(Fig.1).
3.3. Negative correlation between root AP activity and root AMF colonization ratio
Quantile regression showed a general negative correlation from the 0.10 to 0.90 quantile for AM species at the individual level. At 0.90 quantile,the negative correlation was significant(P=0.02).The slopes of different quantiles showed difference (P = 0.20) according to the result of Wald test(Fig.2),and a triangular distribution was supported.
Because we used different standards to measure AM and EM colonization,the average colonization ratio was higher in EM species(Table 3),thus the correlation between root AP activity and root mycorrhizal colonization ratio was insignificant when EM species were considered together,and EM species did not show any special pattern because only six individuals were studied. Moreover, phylogenetically independent contrast analysis showed no significant correlation (P = 0.20) between root AP activity and root AMF colonization ratio(Fig.3a).
4. Discussion
4.1. Plants make extra efforts to acquire soil P
In tropical forest ecosystems, phosphorus limitation is the main concern (Tanner et al., 1998), and available P is far from saturation. In our study site,the average content of soil available P was 0.23 mg·kg-1.The organic forms of P might take up half of the total soil P(Vance et al.,2003), and it need to be mineralized before utilization by plants. Synthesizing and releasing AP is considered an important strategy for organic P mineralization (Lambers et al., 2006), both for roots and soil microorganisms (Chen et al., 2002). In bulk soil without root's contribution, extracellular AP could be only from soil microorganisms, and takes the most important role to supply P to microorganisms(McGill and Cole, 1981). Then we could suppose that the activity of AP in bulk soil represents the ability of soil microorganisms to supply available P,which is also indicated by the significant positive correlation between soil P availability and soil AP activity.
Roots could not rely on soil AP only, and fine roots need to exudate extra AP for P acquisition (Chen et al., 2002; Han et al., 2022). Former studies showed that AP activity is inhibited by soluble inorganic P(McGill and Cole, 1981) and suggested that AP production responses to an inadequate supply of available P (DeForest and Moorhead, 2020).However,in our study,root AP activity shows a positive correlation with soil AP activity in Fig.1,indicating that root AP activity is not inhibited ata higher level of soil AP activity. In fact, neither of the taxonomical subgroups shows a significant positive correlation between root AP activity and soil AP activity.We suppose that AP from soil microorganisms is far from saturation to supply enough P for roots,and put no inhibition on root AP production.
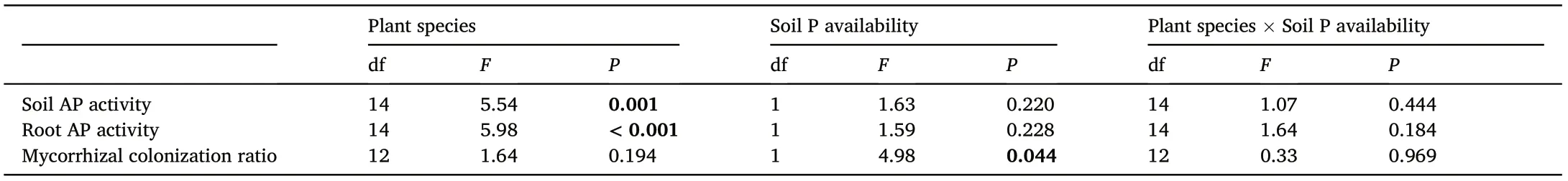
Table 2 Results of analysis of variance among plant species and soil phosphorus (P)availability. AP, acid phosphatase.

Fig.1. Linear regression between root AP activity and bulk soil AP activity in both subgroups of plant species(a:species level,each point means the average of three tree individuals of the same species; b:individual level, each point means a tree individual.). Different shapes represent different subgroups. The regression line and confidence interval were shown in the figure.
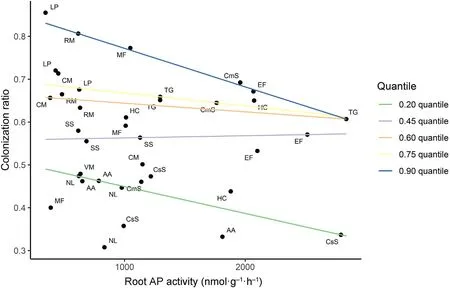
Fig. 2. Quantile regression lines at the 0.20, 0.45, 0.60, 0.75 and 0.90 quantiles, and the P values were 0.27, 0.94, 0.62, 0.35, and 0.01, respectively.
Apart from root AP production,plants employ other ways to acquire P.Efforts of plants into P-acquisition could be explained as two ways:‘do it by myself’ and ‘rely on mycorrhizal partner’ (Bergmann et al., 2020;Han et al.,2022).Root AP exudation is only one kind of the former,and the positive correlation between root AP activity and soil AP activity also indicates that other P-acquisition strategies might have trade-offs with the strategy of root AP exudation.
4.2. A trade-off between the strategy of AP exudation and AMF colonization
For plants,trade-offs among traits are common.Plants need to invest carbon and energy to support different functional traits, and balance of investments is necessary.Then,plants show a spectrum of allocation into different traits (Wright et al., 2004). For leaves, a global mode of trade-offs between allocation to structural tissues versus liquid phase processes,and trade-offs between leaf photosynthetic rates,construction costs,and leaf longevity have been verified(Shipley et al.,2006).
The theory of trade-offs could also apply to roots.Plants need to invest carbon and energy to support different P-acquisition strategies, such as more enzyme exudation or higher mycorrhizal colonization ratio (Helgason and Fitter, 2009; Allison et al., 2011), showing a spectrum of different traits,and this explained the triangular relationship of root AP activity and root AMF colonization ratio and the negative correlation of the upper limit in Fig.2.
Those points under the upper limit in Fig.2 show samples that do not allocate all resources and energy into these two strategies mentioned.To explain this phenomenon, we put forward two possible mechanisms.First,the fungi colonization might be dynamic.Fungi could change their investment into plants,and plants are always in a state of adjustment and adaptation. For example, the dynamics of mycorrhiza formation and spore number varied with the season(Fontenla et al.,1998).The points under the upper limit might represent plants that do not achieve the state of equilibrium, for example, in the process of gradually building up symbioses with mycorrhizal fungi. Second, plants develop diverse strategies of P acquisition, and this phenomenon may represent possible investments into other strategies and trade-offs not measured in our study.
Pioneer studies had reported trade-offs among different root Pacquisition strategies in no-woody species. Morphological traits representing efficient nutrient uptake, i.e., first-order root length and root branching, exhibited negative correlations with AMF colonization ratio(Wen et al., 2019), as well as exudation of organic acids and AP (Honvault et al.,2021).All these studies may indicate a more general mode of trade-offs between other morphological, physiological, and functionaltraits,and more extensive studies are needed to show the whole picture of belowground P-acquisition strategies of roots.

Table 3 Physicochemical properties and enzyme activities of soils under the woody plants at species level (mean and SD, n =3).

Fig.3. Phylogenetic independent contrasts(PICs)of 13 AM species.(a)Phylogenetic tree based on the APG-IV system(The Angiosperm Phylogeny Group,2016);(b)Negative correlation between root AP activity and AMF colonization (R2 = 0.03, P = 0.27). The horizontal and vertical axis represent values after removing phylogenetic signal with phylogenetic independence contrasts.
The separation of subgroups in Fig.1 indicated a possible influence of taxonomy or phylogeny. According to the hypothesis of phylogenetic niche conservatism (Losos, 2008), closely related species might share similar ecological niches and have similar functional traits,which could have some impacts on the trade-offs between different functional strategies.
Although AMF also make contribution to AP exudation (Koide and Kabir, 2000), the amount did not affect the general trade-off pattern between‘do it by myself’and‘rely on mycorrhizal partner’across target woody species in this study (Bergmann et al., 2020). It is because the contribution of higher colonization ratio does not change the whole downward trend of root AP activity(Fig.2).Instead,AMF species mainly rely on other morphological and functional traits to improve P acquisition and a multi-dimensional economics spectrum (McCormack and Iversen,2019;Han et al.,2022)is indicated.
5. Conclusion
Through the study on fine-root P-acquisition strategies against bulk soil P supply across 15 woody species, we verified a trade-off pattern between the P acquisition strategies of root AP exudation and AMF colonization. It indicated preference of plant investment to different Pacquisition strategies even under P limited situations.The root strategies were not a simple response to soil P availability and P-degrading activities.Although we found negative quantile regressions between root AP exudation and AMF colonization, other diverse morphological,physiological, and functional strategies are also possible to build up complex relationships. It should be noted that our results are based on one-time sampling only,and we thus call for further investigation on the temporal dynamics (particularly comparing the wet season and the dry season). Nevertheless, our findings show more information on root P acquisition, and help to understand the overall mechanism of root functioning in forest ecosystems.
Funding
This study was supported by funding from the National Natural Science Foundation of China(Nos.32001218, 42141006 and 31988102).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors appreciate the help from staff of the Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, especially Dr.Hui Chen and Mr.Huixu Zheng.We also appreciate the analytical support from Dr. Chengjun Ji, Dr.Yanhong Tang and Ms. Xiaofeng Ni of Peking University. We are also grateful to three anonymous reviewers for their constructive comments and suggestions which greatly improved the manuscript.
- Forest Ecosystems的其它文章
- Prediction of the global potential geographical distribution of Hylurgus ligniperda using a maximum entropy model
- Structure complexity is the primary driver of functional diversity in the temperate forests of northeastern China
- The 2/3 scaling of twig nitrogen to phosphorus in woody plants
- Monitoring the abundance of saproxylic red-listed species in a managed beech forest by landsat temporal metrics
- Evaluating and quantifying the effect of various spruce budworm intervention strategies on forest carbon dynamics in Atlantic Canada
- Consistent response of nematode communities to management of coniferous plantations

