The 2/3 scaling of twig nitrogen to phosphorus in woody plants
Zhiqiang Wang, Karl J. Niklas, Zqing Ma, Dhun Jiang, Jianming Dng
a Sichuan Zoige Alpine Wetland Ecosystem National Observation and Research Station, Southwest Minzu University, Chengdu, 610041, China
b Institute of Qinghai-Tibetan Plateau, Southwest Minzu University, Chengdu, 610041, China
c Plant Biology Section, School of Integrative Plant Science, Cornell University, Ithaca, NY, 14853, USA
d Center for Forest Ecosystem Studies and Qianyanzhou Ecological Station, Key Laboratory of Ecosystem Network Observation and Modeling, Institute of Geographic Sciences and Natural Resources Research, Chinese Academy of Sciences, Beijing, 100101, China
e Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu, 610041, China
f State Key Laboratory of Grassland Agro-Ecosystem, College of Ecology, Lanzhou University, Lanzhou, 730000, China
Keywords:Nitrogen Phosphorus Scaling exponents Twigs Woody plants
ABSTRACT
1. Introduction
Nitrogen (N) and phosphorus (P) are crucial elements in regulating plant physiological processes, growth, and development (Sterner and Elser,2002;Güsewell,2004).It is well known that coordinated variation of N and P concentrations in plant organs can be quantified via a stoichiometric scaling relationship characterized by equation N = βPα, or logN = logβ + αlogP when log-transformed, in which α and logβ represent the slope (i.e., the scaling exponent) and the Y-intercept (i.e., the normalization constant), respectively (Niklas, 1994; Niklas et al., 2005;Kerkhoff et al., 2006; Wang et al., 2020a). The scaling exponent intrinsically reflects the relative accumulation rate of N compared to P (Sardans and Pe~nuelas, 2015; Guo et al., 2020), and has been frequently applied to predict plant growth dynamic and ecosystem functioning(Wright et al.,2005; Niklas,2006;Elser et al., 2010). Thus,quantifying the N versus P scaling exponent in plant organs can advance our understanding of nutrients cycling across plants and ecosystem dynamics.
A uniform and constant scaling exponent of N to P is appealing because of its simplicity in empirical models. For example, using global datasets, two synthesis studies have proposed general 2/3 and 0.82 scaling “laws” for leaves (Reich et al., 2010) and for fine roots (Wang et al., 2019), respectively. However, several studies have also shown some statistically differences in the scaling exponent of leaves(Han et al.,2005;Niklas and Cobb,2005;McGroddy et al.,2004;Zhao et al.,2016a;Tian et al.,2018)and roots(Yuan et al.,2011;Wang et al.,2021a;Zhao et al., 2021) across major plant functional groups and biomes. For example,a study based on global pooled data reports that the leaf N and P scaling exponent varies considerably across different plant functional groups, latitudinal zones, and local sites, whereas metabolic requirements for P to sustain growth rates mainly account for differences in the numerical values of scaling exponent(Tian et al.,2018).Similarly,a recent study demonstrates that the N versus P scaling exponent of the absorptive fine roots of woody species differs significantly within different functional groups and climatic zones(Wang et al.,2021a).Soil relative P availability plays a crucial role in influencing the ability of fine roots to absorb N and P and thus N vs. P scaling exponents.
Twigs (here defined as a terminal segment of branches) bear leaves and flower buds(Wilson,1989;Xiang et al.,2009),which are responsible for light interception (and thus photosynthetic carbon gain) and reproduction.These organs therefore are the most metabolically active aerial compartments of the whole plant, and play important mechanical and hydraulic roles(Osada,2006;Yang et al.,2009;Poorter et al.,2012).It is reasonable therefore to suppose that the N and P requirements of twigs are similar to those of leaves, particularly since twigs are often photosynthetic organs in addition to their other functions (Sterner and Elser,2002; Ghimire et al., 2017). Previous studies have shown a strong correlation between the chemical composition of twigs and leaves (Ishida et al., 2008; Yang et al., 2014; Yan et al., 2016). Given the functional linkages between twigs and leaves, it is reasonable to expect that the N and P concentrations between these two organs will be tightly correlated and that it will conform generally to the 2/3 scaling relationship.
Twig specific density(TSD)and twig dry matter content(TDMC)are two important functional traits(Cornelissen et al.,2003).Several studies have shown that twig specific density(TSD)is negatively correlated with photosynthetic capacity (Santiago et al., 2004) and radial growth rates(Fujimoto et al., 2006), whereas TSD is reported to be positively correlated with twig dry matter content(TDMC)(Yao et al.,2015).Thus,TSD and TDMC are reasonable surrogates for plant growth rates(Chave et al.,2009). Therefore, based on the growth rate hypothesis (GRH), stating that plants with greater metabolic and growth rates require more P than N content to ensure rapid protein(N-rich)synthesis supported by P-rich rRNA. We hypothesized that TSD and TDMC will correlate negatively with increasing rRNA(P)investments relative to protein(N)investments.
We compiled a large comprehensive dataset of paired twig N and P data for woody species to test whether this hypothesis is supported by the data. Specifically, we focused on three questions. (1) Do twig N and P concentrations and N:P ratios vary within and across functional groups and biomes? (2) Is the numerical value of the twig N versus P scaling exponent uniform across different functional groups and biomes? And(3),if the scaling exponent is uniform,how is it related to the predictions of the GRH? We also examined whether local climate and soil nutrient conditions influenced the twig N and P scaling relationship.
2. Materials and methods
2.1. Data compilation
A large dataset of pairwise twig N and P concentrations for woody species and their associated twig specific density (TSD) and twig dry matter content (TDMC) compiled for the goals of this study. The data were compiled from a broad range of peer-reviewed publications from 1980 to 2021 using the keywords“twig”,“nitrogen”and“phosphorus”in the Web of Science(http://apps.webofknowledge.com), Google Scholar(http://scholar.google.com), and National Knowledge Infrastructure Database (http://cnki.net). All of the data were taken directly from the tables, figures, appendices, and the main text of the original papers.When data were only presented graphically, the data were extracted using GetData Graph Digitizer 2.26(http://getdata-graph-digitizer.com).We gathered data only from publications reporting the paired N and P concentrations of twigs with species name and detailed site information,and excluded data from fertilized or polluted sites, or plants grown in greenhouses.The final dataset includes 2,038 paired observations of twig N and P from a total of 536 woody species spanning 75 sites worldwide(Fig. 1; A list of the literature sources is provided in the supplementary material).
2.2. Statistical analysis
Fig. 1. The distribution of the 75 sampling sites used in this study.
After a preliminary analysis of distributions, the N and P concentration data were log10-transformed to assure normality, and subsequently subjected to reduced major axis (RMA) regression analyses using the‘lmodel2’ function of the R package LMODEL2 (Legendre, 2018) to determine the N versus P scaling exponents for different functional groups and biomes. The species were first sorted into two phylogenetic groups (i.e., angiosperms and gymnosperms) and four life-form groups(i.e.,tree,shrub,evergreen and deciduous species).We then divided the data into three biomes (i.e., tropical 0–25°, temperate 25°–50°, and boreal >50°). The likelihood-ratio test was used to evaluate the heterogeneity of the numerical values of the RMA scaling exponents within the functional groups and biomes.To evaluate whether the GRH predictions regarding P and N requirements for plant growth are consistent with the scaling exponents for twig P than twig N, we evaluated the scaling relationships of twig N and P concentrations in relation to TSD and TDMC using RMA regression.All statistical analyses were conducted in R 3.4.2(R Core Team,2017).
To determine the numerical values of the N versus P scaling exponents at each site, we analyzed the scaling exponent for each of the 24 local sites with 10 or more samples using RMA regression protocols(Wang et al.,2021b).We also used‘Funnel plot’analyses to evaluate the scaling exponents in relation to the sample size for the same 24 local sites(Palmer, 1999; Wright et al., 2005; Reich et al., 2010; Wang et al.,2020c). We recorded the mean annual temperature (MAT) and mean annual precipitation(MAP),and soil N(STN)and P(STP)concentrations from each site, and examined their influence on variation in scaling exponents. When this information was missing in a paper, we used WorldClim global climatic database (http://www.worldclim.org/) to determine MAT and MAP data with a grid precision of 0.5°×0.5°based on each site's geographical coordinates.Missing STN and STP data were derived from a global harmonized database (http://www.openlan dmap.org/) with a grid precision of 0.5°× 0.5°(WISE30sec; Batjes,2016). A generalized linear model (GLM) was applied to evaluate the relative effects of climate factors(MAT and MAP)and soil nutrients(STN and STP) on the scaling exponents using the ‘glm’ function in the R package STATS(R Core Team,2017).
3. Results
3.1. Twig N and P concentrations and their ratios in functional groups and biomes
Across all observations, the mean values of twig N and P concentrations and N:P ratios were 9.33 mg·g-1, 1.12 mg·g-1and 10.16, respectively.However,N and P concentrations and N:P ratios varied within and across the different functional groups and biomes(Table 1).For example,N concentrations ranged from 7.98 mg·g-1for gymnosperms to 9.68 mg·g-1for shrubs,whereas P concentration ranged from 1.04 mg·g-1for gymnosperms to 1.19 mg·g-1for deciduous species.In a similar manner,N:P ratios ranged from 8.06 for gymnosperms to 11.53 for evergreen species. Among the different biomes, tropical biomes had the highest N and P concentrations and N:P ratios(i.e.,9.94 mg·g-1,1.19 mg·g-1and 13.02, respectively), whereas boreal biomes had the lowest N and P concentrations and N:P ratios (i.e. 8.13 mg·g-1, 1.09 mg·g-1and 7.48,respectively).
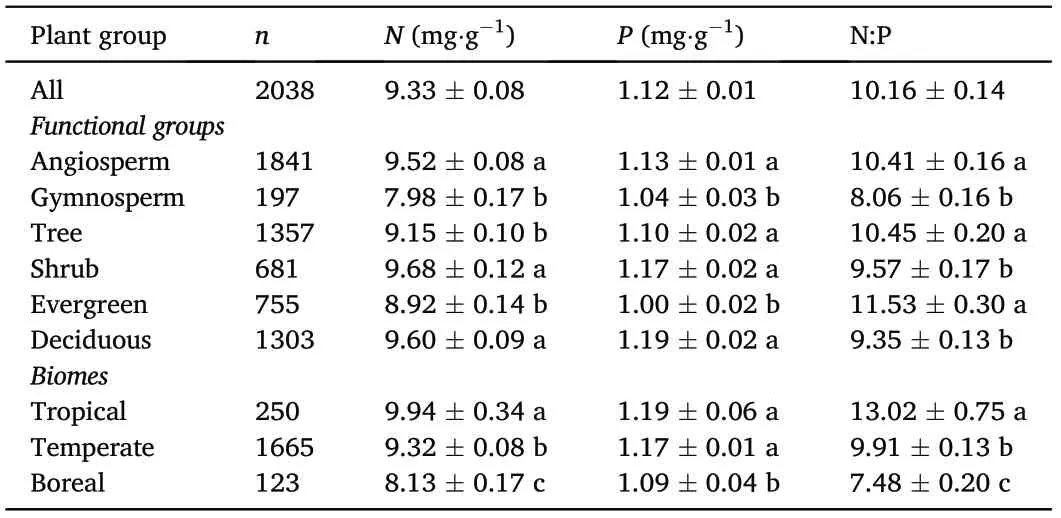
Table 1 The means of twig N and P concentrations and N:P ratios for different functional groups and biomes. n represents the number of samples. Different letters represent significant differences at 0.05 level.
3.2. Twig N versus P scaling exponent across different functional groups and biomes
Across all observations (n = 2,038), twig N increased with twig P,with a scaling exponent numerically equal to 0.67(95%CIs,(0.64,0.69),R2= 0.15, p <0.001) (Fig. 2a; Table 2). Likewise, among the major functional groups, all of the numerical values of the scaling exponents were close to 0.67(Fig.2b–d;Table 2), i.e.,the numerical values of the scaling exponent showed no significant differences among the contrasting functional groups. There were also similar twig N versus P scaling relationships among the biomes,with the scaling exponent very near to 0.67,i.e.,α=0.65 for boreal,α= 0.67 for temperate,and α =0.67 for tropical biomes(Fig.3;Table 2).Thus,the scaling exponents for all of the data,within the different plant functional groups,and within each of the biomes were approximately numerically equal to 2/3.
3.3. Scaling relationships of twig N and P concentrations in relation to TSD and TDMC
The twig N and P concentrations decreased with TSD,with α=-1.1(n = 671, 95% CIs, (-1.18, -1.03), R2= 0.08, p <0.001) for N versus TSD (Fig. 4a) and α = -1.58 (n = 671, 95% CIs, (-1.70, -1.47), R2=0.08, p <0.001) for P versus TSD (Fig. 4b). Likewise, the twig N and P concentrations also decreased with TDMC, with α = -1.27 (n = 1,524,95% CIs, (-1.33, -1.20), R2= 0.07, p <0.001) for N versus TDMC(Fig.4c)and α=-1.97(n=1,524,95%CIs,(-2.07,-1.48),R2=0.06,p <0.001)for P versus TDMC(Fig.4d).
3.4. Twig N versus P scaling exponents across local sites
The numerical values of the twig N versus P scaling exponents statistically differed among 24 local sites, ranging from 0.35 to 1.12(Fig. 5a). A ‘Funnel plot’ analysis of the scaling exponent in relation to the sample size for the 24 local sites showed that these scaling exponents numerically converge to a mean value of 0.66 with increasing sample size(Fig. 5b). The over effect of climate factors (MAT and MAP) and soil nutrients (STN and STP) explained 59.26% of the variation in the numerical values of the N versus P scaling exponents for 24 local sites.When taken separately,climate and soil nutrients accounted for 18.18%and 40.48% of the variation in twig N versus P scaling exponent,respectively.Of the climate and soil factors,MAP and STP accounted for the greater proportions of variation in the N versus P scaling exponent(6.11%and 22.47%,respectively) (Fig.5c).
4. Discussion
This study employs a comprehensive world-wide dataset for pairwise twig N and P concentrations of woody species to explore whether a general twig N versus P scaling relationship exists. The analyses presented here reveal that a uniform 0.67 scaling exponent for the twig N versus P scaling relationship exists across different functional groups and biomes. Thus, a general 2/3-power law appears to hold true for twigs.This numerical value is observed to vary among different local sites,but converges onto 0.67 as the sample size of sites increases.
4.1. Variations in twig N and P concentrations and N:P ratios across functional groups and biomes
Our results show that the global mean values of twig N and P concentrations and N:P ratios are 9.33 mg·g-1, 1.12 mg·g-1and 10.16,respectively. These values are obviously lower than the global mean values for leaves reported by Tian et al. (2018). This difference can be attributed to the different physiological functions of twigs and leaves(Westoby and Wright, 2003). Twigs primarily function mechanically to support leaves and to provide nutrients and water, whereas leaves are responsible for carbon gain.However,in addition to their other functions twigs are often photosynthetic.
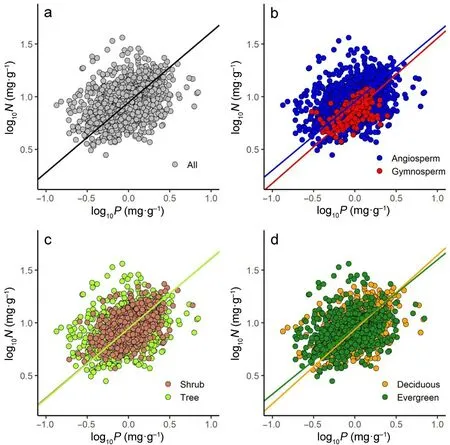
Fig. 2. Scaling relationships of twig nitrogen (N) and twig phosphorus (P) for all species pooled (a), angiosperm and gymnosperm (b), tree and shrub (c), and evergreen and deciduous (d).
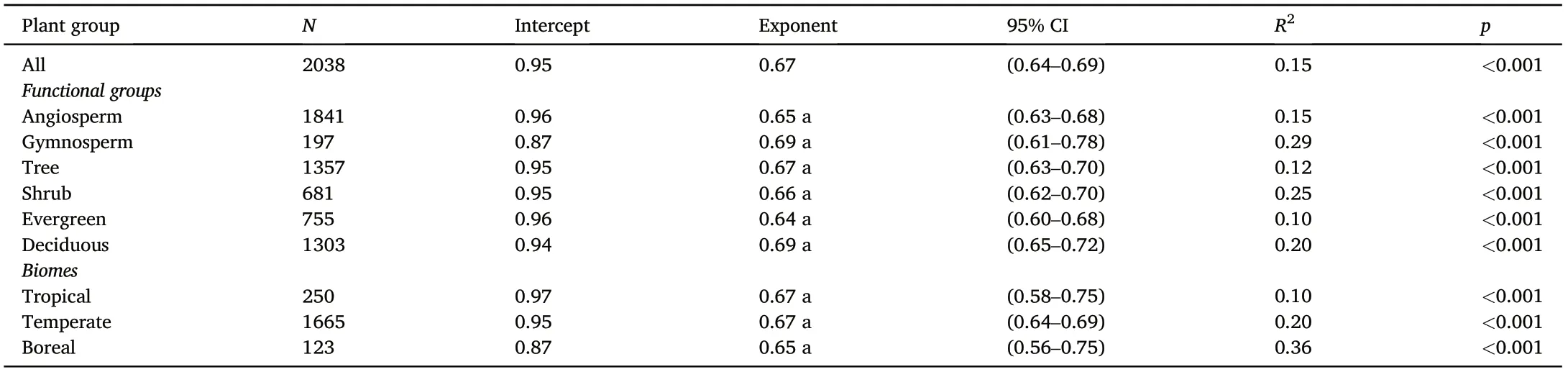
Table 2 Summary of reduced major axis (RMA) regression results of log-transformed twig N versus P for woody species across different functional groups and biomes (all relations were statistically significant with p <0.001).n,the number of observations, and letters represent significant differences in exponents based on a likelihood ratio test.
The varied twig N and P concentrations and N:P ratios across functional groups are not surprising given their functional diversity and the diversity of plant nutrient use strategies. For instance, N and P concentrations and N:P ratios are lower in evergreen species than in deciduous species, supporting the notion that slow-growing species have lower N and P concentrations than those of fast-growing species(Güsewell,2004;Wang et al., 2015). Variations in twig N and P concentrations and N:P ratios among biomes are also statistically discernible. Tropical biomes tend to have the highest N and P concentrations and N:P ratios,whereas boreal biomes tend to have the lowest N and P concentrations and N:P ratios. Our results support the notion that plants growing in warm conditions may have higher N and P concentrations perhaps to decrease nutrient limitation and to increase water use efficiency(Palmroth et al.,2013;Wang et al.,2020b).
4.2. A constant twig N versus P scaling exponent across different functional groups and biomes
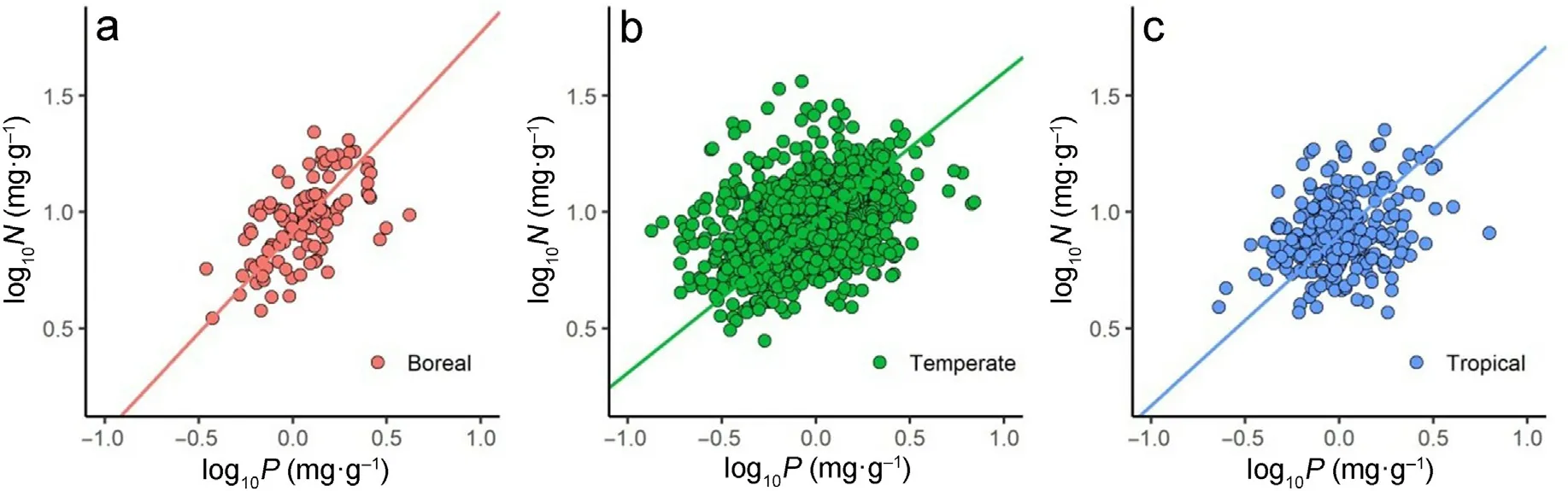
Fig. 3. Scaling relationships of twig nitrogen (N) and twig phosphorus (P) in different biomes. (a) boreal; (b) temperate; and (c) tropical.
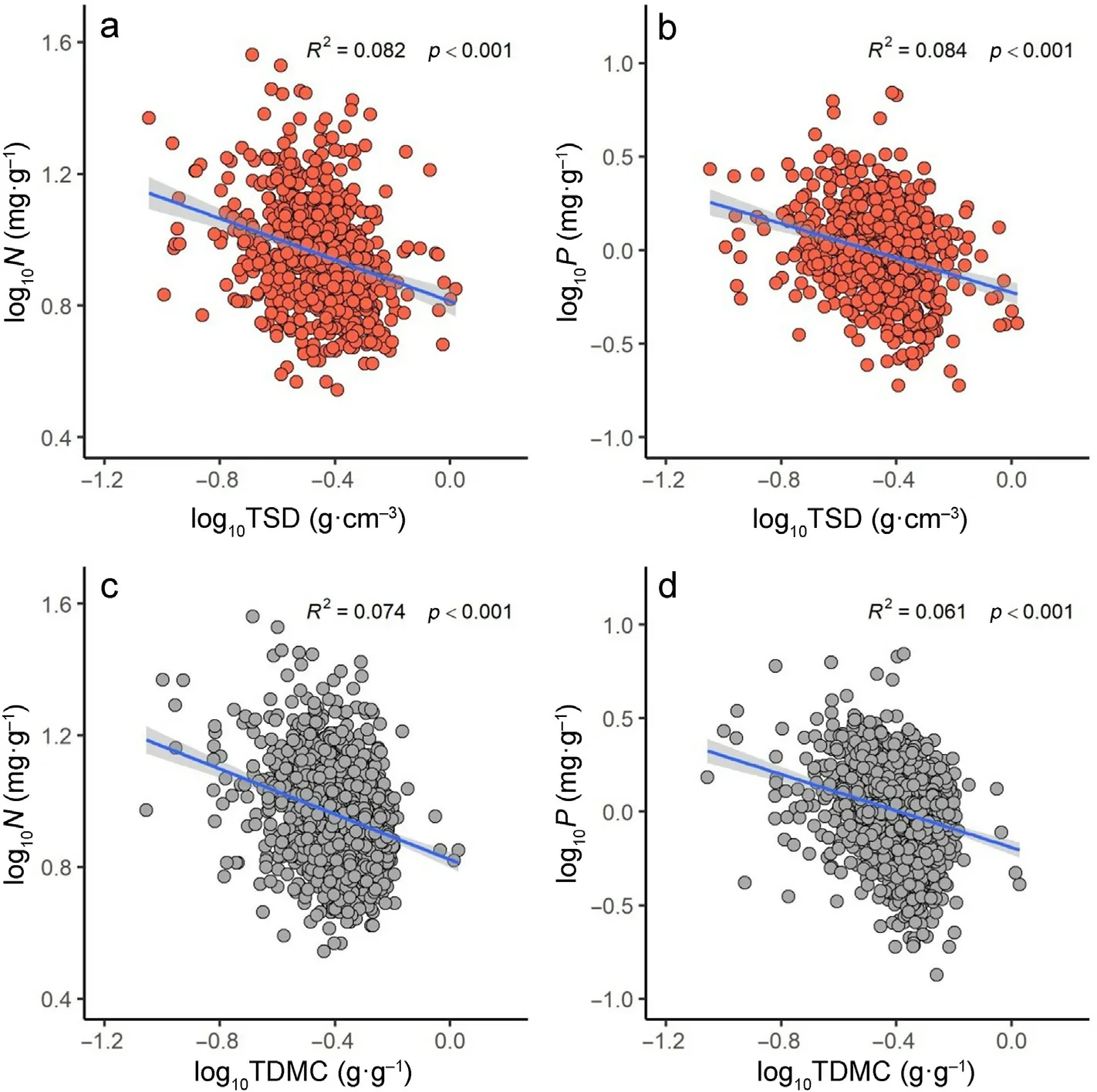
Fig.4. Scaling relationships of twig specific density(TSD)and twig nitrogen(N)(a),and twig phosphorus(P)(b);twig dry matter content(TDMC)and twig nitrogen(N) (c), and twig phosphorus (P) (d).
As noted,the numerical value of the scaling exponent governing the twig N versus P scaling relationship is 0.67 across all the woody species examined in this study, and for the different functional groups and the biomes included in our dataset (Figs. 2 and 3; Table 2). These results support the hypothesis that the twig N versus P scaling exponent is conserved across the different functional groups and biomes as a result of structural and biochemical constraints, and evolutionary history(McGroddy et al., 2004; Wright et al., 2004). Structural constraints are relevant to the hierarchical branching networks of vascular plants,where fractal-like networks are posited to maximize resource uptake and minimize energy consumption at the organ level of organization (West et al.,1997).N and P are transported through the vascular plant network in such a manner that the scaling of N to P is constrained or at least profoundly influenced by vascular architecture (Enquist, 2002).Biochemical constraints are also associated with the stoichiometry of nutrient balance within plant organs (Sterner and Elser, 2002; Ågren,2008),i.e.,plants need to maintain a constant internal N and P balance in their organs to maintain growth (Sterner and Elser, 2002). Therefore,both structural and metabolic functional traits have direct effects on twig N versus P scaling exponents regardless of the functional group a plant belongs to or where it grows.Importantly,this result supports the GRH,which posits that plant organs with high growth rates (e.g., twigs and leaves)require relatively more P than N to ensure rapid protein synthesis(Elser et al., 2000, 2003; Ågren, 2004), thereby leading to N versus P scaling exponents with numerical values less than 1.0 (i.e., α <1)(Sterner and Elser,2002).
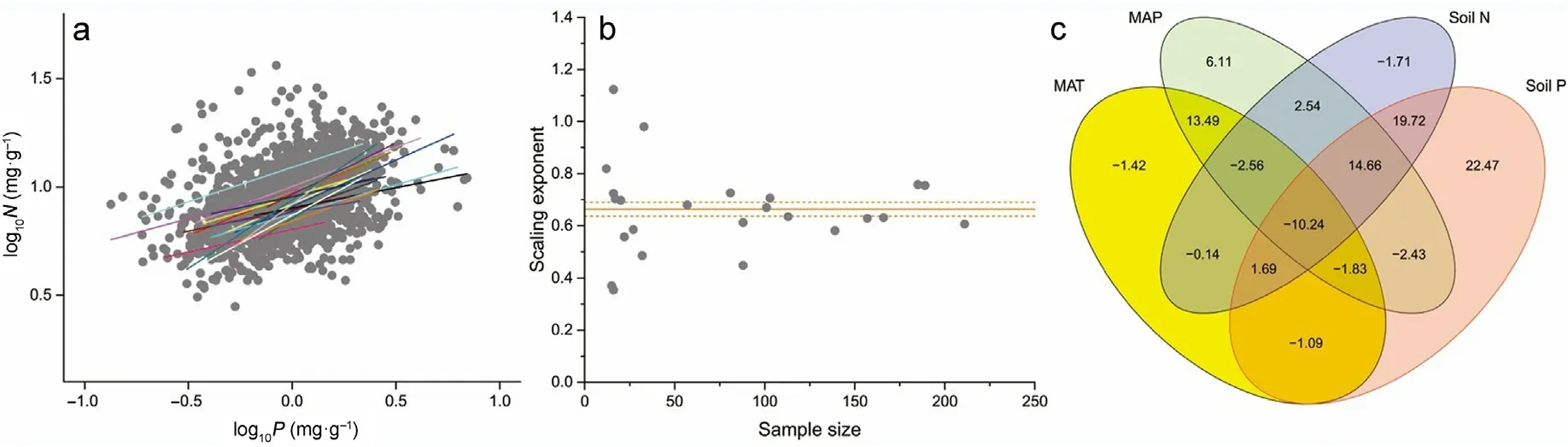
Fig. 5. Scaling relationships of twig nitrogen(N)and phosphorus(P)for 24 local sites with more than ten records(a).Relationship between twig N versus P scaling exponents and sample size for 24 local sites(b).The solid and dashed lines denote the mean(0.66)and 95%CI for the scaling exponent.The effects(R2,%)of MAT,MAP, STN, and STP and their interaction on variation in scaling exponent for 24 local sites (c). The red ellipse presents the dominant group of variables. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The numerical value of the twig N versus P scaling exponent is statistically indistinguishable from 2/3, which has been also reported for leaves(e.g.,Niklas,2006;Reich et al.,2010).N and P play critical roles in the core metabolic pathways of plants and link the three different primary plant organs into integrated phenotype (Li et al., 2021). For example, N and P are absorbed by roots and transported through the trunk,branches,twigs,leaves,and reproductive organs to maintain all of the multi-functional requirements of the whole plant (Westoby et al.,2002;Craine et al.,2005;Li et al.,2010;Minden and Kleyer,2014;Reich,2014). Furthermore, leaves are important nutrient storage and assimilation organs wherein N and P are required for photosynthesis and respiration (Zhao et al., 2016b). In addition, the twigs of many woody species are photosynthetic (owing to the absence of phellem and the presence of photosynthetic hypodermal cells that intercept sunlight)(Wang et al.,2018;Zhang et al., 2018;Chen et al.,2020). For example,especially in arid ecosystems,many woody species have become leafless and evolved photosynthetic twigs to adapt to water stress(e.g.,Haloxylon ammodendron and Calligonum mongolicum) (Su and Yan, 2006; Wang et al., 2021, 2022). Thus, it is not surprising that the twig and leaf N versus P scaling relationships are similar if not identical.
In addition,our results indicate that the scaling of twig P versus TSD is governed by a greater scaling exponent than the scaling of twig N versus TSD. Similarly, the scaling exponent of twig P versus TDMC is numerically higher than twig N versus TDMC exponent (Fig. 4). Both of the scaling differences translate into the 2/3 scaling exponent observed for the twig N versus P relationships seen within and among different functional groups and biomes (Table 2). These relationships are consistent with the GHR prediction that plants with high growth rates require more P than N to support protein synthesis.
4.3. Variant twig N versus P scaling exponent within local sites
Large variations in the numerical values of the twig N versus P scaling exponent are observed across the 24 local sites examined in this study(Fig.5a).These values are close to 0.67 with sufficient sample sizes,and converge onto the scaling exponent observed for the pooled data,and for the different functional groups or biomes examined in this study.Due to the effects of climate and soil nutrients on the numerical values of leaf and fine root N versus P scaling exponent(Tian et al.,2018;Wang et al.,2019), we speculated that climate (MAT and MAP) and soil nutrients(STN and STP) would play important roles in determining these numerical values across different local sites.As reported for leaves and fine roots(Tian et al.,2018;Wang et al.,2019),STP is observed to be the most important factor explaining the variation in the numerical value of the twig N versus P scaling exponent across different local sites. This result supports the notion that P tissue contents are directly related to soil nutrient concentrations (Sterner and Elser, 2002). Moreover, this result also supports the idea that the positive correlation between twig P and soil P is primarily a consequence of root absorption (Yao et al., 2015).Soil P availability clearly differs across the 24 local sites in our data base,and tends to increase with increasing latitude or from the humid to arid regions.In this context it is useful to note that species growing in tropical forests tend to be more P limited and uptake excess N,thereby leading to higher numerical values of the N vs.P scaling exponent,whereas species growing in boreal habitats tend to be N limited and tend to absorb excess P when soil N availability is deficient,resulting in lower numerical values of the N vs.P scaling exponent.These features may explain the variation of twig N vs.P scaling exponents across the local sites.However,due to the limited local sites,this inference requires more paired twig N and P data from different local sites along with associated soil nutrients to be evaluated.
5. Conclusion
Our results show that the twig N versus P scaling relationship is governed by an exponent approximately equal to 0.67 across different functional groups and biomes, supporting the GRH. However, this numerical value differs among different local sites as a function of climatic and soil nutrient.STP is the most important factor explaining differences in the numerical values of twig N versus P scaling exponents. These findings advance our understanding of the whole plant nutrient allocation strategy, and have important implications for predicting plant growth and nutrient cycling in terrestrial ecosystems in response to global environmental changes.
Funding
This study was financially supported by the National Science Fund for Excellent Young Scholars (No. 31822010), the National Key Research and Development Program of China (No. 2020YFA0608102), the Biodiversity Survey and Assessment Project of the Ministry of Ecology and Environment, China (No. 2019HJ2096001006), National Scientific and Technological Program on Basic Resources Investigation (No.2019FY102002)and the Innovation Base Project of Gansu Province(No.20190323), the Sichuan Science and Technology Program (No.2020YFH0005).
Availability of data and materials
Any data that support the findings of this study are included within the article.The list of references for dataset is publicly available and can be accessed in the appendix.
Authors’contributions
ZQW conceived the idea and designed the research. ZQW, DCJ and ZQM collected the data, ZQW,JMD,ZQM,and KJN performed the data analysis and contributed to the writing of the paper. All authors gave final approval for publication.
Declaration of competing interest
We declare that we have no competing financial and personal relationships with other people or organizations that could have appeared to influence our work reported in this paper.
Acknowledgements
We thank the scientists who contributed the valuable data for this study.We also thank the two anonymous referees for their comments to improve this study.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://do i.org/10.1016/j.fecs.2022.100049.
ReferencesÅgren, G.I., 2004. The C:N:P stoichiometry of autotrophs-theory and observations. Ecol.Lett. 7, 185–191.
Ågren, G.I., 2008. Stoichiometry and nutrition of plant growth in natural communities.Annu. Rev. Ecol. Systemat. 39, 153–170.
Batjes, N.H., 2016. Harmonized soil property values for broad-scale modelling(WISE30sec) with estimates of global soil carbon stocks. Geoderma 269, 61–68.
Chave,J.,Coomes,D.,Jansen,S.,Lewis,S.L.,Swenson,N.G.,Zanne,A.E.,2009.Towards a worldwide wood economics spectrum. Ecol. Lett. 12, 351–366.
Chen, X.P., Wang, M.T., Li, M., Sun, J., Lyu, M., Zhong, Q.L., Cheng, D.L., 2020.Convergent nitroge-phosphorus scaling relationships in different plant organs along an elevational gradient. AOB Plants 12 (3), plaa021.
Core Team,R.,2017.v.3.3.3.R:a Language and Environment for Statistical Computing.R Foundation for Statistical Computing, Vienna, Austria. https://www.r-project.org/.(Accessed 8 August 2019).
Cornelissen, J.H.C., Lavorel, S., Garnier, E., Diaz, S., Buchmann, N., Gurvich, D.E.,Reich, P.B., Ter Steege, H., Morgan, H.D., van der Heijden, M.G.A., Pausas, J.G.,Poorter,H.,2003.A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 51, 335–380.
Craine, J.M., Lee, W.G., Bond, W.J., Williams, R.J., Johnson, L.C., 2005. Environmental constraints on a global relationship among leaf and root traits of grasses.Ecology 86,12–19.
Elser, J.J., Sterner, R.W., Gorokhova, E., Fagan, W.F., Markow, T.A., Cotner, J.B.,Harrison,J.F.,Hobbie,S.E.,Odell,G.M.,Weider,L.J.,2000.Biological stoichiometry from genes to ecosystems. Ecol. Lett. 3, 540–550.
Elser, J.J., Acharya, K., Kyle, M., Cotner, J.B., Makino, W., Markow, T., Watts, T.,Hobbie, S.E., Fagan, W.F., Schade, J., Hood, J., Sterner, R.W., 2003. Growth ratestoichiometry couplings in diverse biota. Ecol. Lett. 6, 936–943.
Elser, J.J., Fagan, W.F., Kerkhoff, A.J., Swenson, N.G., Enquist, B.J., 2010. Biological stoichiometry of plant production: metabolism, scaling and ecological response to global change. New Phytol. 186, 593–608.
Enquist, B.J., 2002. Universal scaling in tree and vascular plant allometry: toward a general quantitative theory linking plant form and function from cells to ecosystems.Tree Physiol. 22, 1045–1064.
Fujimoto,T.,Kita,K.,Uchiyama,K.,Kuromaru,M.,Akutsu,H.,Oda,K.,2006.Age trends in the genetic parameters of wood density and the relationship with growth rates in hybrid larch (Larix gmelinii var. japonica × L. kaempferi) F1. J. For. Res. 11, 157.
Ghimire,B.,Riley,W.J.,Koven,C.D.,Kattge,J.,Rogers,A.,Reich,P.B.,Wright,I.J.,2017.A global trait-based approach to estimate leaf nitrogen functional allocation from observations. Ecol. Appl. 27, 1421–1434.
Guo,Y.P.,Yan,Z.B.,Gheyret,G.,Zhou,G.Y.,Xie,Z.Q.,Tang,Z.Y.,2020.The communitylevel scaling relationship between leaf nitrogen and phosphorus changes with plant growth, climate and nutrient limitation. J. Ecol. 108, 1276–1286.
Güsewell, S., 2004. N:P ratios in terrestrial plants: variation and functional significance.New Phytol. 164, 243–266.
Han, W.X., Fang, J.Y., Guo, D.L., Zhang, Y., 2005. Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. New Phytol. 168,377–385.
Ishida, A., Nakano, T., Yazaki, K., Matsuki, S., Koike, N., Lauenstein, D.L., Shimizu, M.,Yamashita,N.,2008.Coordination between leaf and stem traits related to leaf carbon gain and hydraulics across 32 drought-tolerant angiosperms. Oecologia 156,193–202.
Kerkhoff,A.J.,Fagan,W.F.,Elser,J.J.,Enquist,B.J.,2006.Phylogenetic and growth form variation in the scaling of nitrogen and phosphorus in the seed plants.Am.Nat.168,E103–E122.
Legendre, P., 2018. lmodel2: Model II Regression. R Package v. 1.7-3. https://cran.r-project.org/web/packages/lmodel2/index.html. (Accessed 15 March 2022).
Li, A., Guo, D.L., Wang, Z.Q., Liu, H.Y., 2010. Nitrogen and phosphorus allocation in leaves,twigs and fine roots across 49 temperate,subtropical and tropical tree species:a hierarchical pattern. Funct. Ecol. 24, 224–232.
Li, J.L., Chen, X.P., Niklas, K.J., Sun, J., Wang, Z.Y., Zhong, Q.L., Hu, D.D., Cheng, D.L.,2021. A whole-plant economics spectrum including bark functional traits for 59 subtropical woody plant species. J. Ecol. 110, 248–261. https://doi.org/10.1111/1365-2745.13800.
McGroddy, M.E., Daufresne, T., Hedin, L.O., 2004. Scaling of C:N:P stoichiometry in forests worldwide: implications of terrestrial Redfield-type ratios. Ecology 85,2390–2401.
Minden, V., Kleyer, M., 2014. Internal and external regulation of plant organ stoichiometry. Plant Biol. 16, 897–907.
Niklas, K.J., 1994. Plant Allometry: the Scaling of Form and Process. University of Chicago Press, Chicago.
Niklas, K.J., 2006. Plant allometry, leaf nitrogen and phosphorus stoichiometry, and interspecific trends in annual growth rates. Ann. Bot. 97, 155–163.
Niklas, K.J., Cobb, E.D., 2005. N, P, and C stoichiometry of Eranthis hyemalis (L). Salib.(Ranunculaceae) and the allometry of plant growth. Am. J. Bot. 92, 1263–1268.
Niklas, K.J., Owens, T., Reich, P.B., Cobb, E.D., 2005. Nitrogen/phosphorus leaf stoichiometry and the scaling of plant growth. Ecol. Lett. 8, 636–642.
Osada,N.,2006.Crown development in a pioneer tree,Rhus trichocarpa,in relation to the structure and growth of individual branches. New Phytol. 172, 667–678.
Palmer,A.R.,1999.Detecting publication bias in metaanalyses:a case study of fluctuating asymmetry and sexual selection. Am. Nat. 154, 220–233.
Palmroth, S., Katul, G.G., Maier, C.A., Ward, E., Manzon, S., Vico, G., 2013. On the complementary relationship between marginal nitrogen and water-use efficiency among Pinus taeda leaves grown under ambient and CO2-riched environments. Ann.Bot. 111, 467–477.
Poorter, H., Niklas, K.J., Reich, P.B., Oleksyn, J., Poot, P., Mommer, L., 2012. Biomass allocation to leaves, stems and roots: meta-analyses of interspecific variation and environmental control. New Phytol. 193, 30–50.
Reich, P.B., 2014. The world-wide ‘fast–slow’ plant economics spectrum: a traits manifesto. J. Ecol. 102, 275–301.
Reich, P.B., Oleksyn, J., Wright, I.J., Niklas, K.J., Hedin, L.O., Elser, J.J., 2010. Evidence of a general 2/3-power law of scaling leaf nitrogen to phosphorus among major groups and biomes. P. Roy. Soc. B.-Biol. Sci. 277, 877–883.
Santiago, L.S., Goldstein, G., Meinzer, F.C., Fisher, J.B., Machado, K., Woodruff, D.,Jones, T., 2004. Leaf photosynthetic traits scale with hydraulic conductivity and wood density in Panamanian forest canopy trees. Oecologia 140, 543–550.
Sardans, J., Pe~nuelas, J., 2015. Trees increase their P:N ratio with size. Global Ecol.Biogeogr. 24, 147–156.
Sterner, R.W., Elser, J.J., 2002. Ecological Stoichiometry: the Biology of Elements from Molecules to the Biosphere. Princeton University Press, Princeton.
Su, P.X., Yan, Q.D., 2006. Photosynthetic characteristics of C4desert species Haloxylon ammodendron and Calligonum mongolicum under different moisture conditions. Acta Ecol. Sin. 26, 75–82.
Tian, D., Yan, Z.B., Niklas, K.J., Han, W.X., Kattge, J., Reich, P.B., Luo, Y.K., Chen, Y.H.,Tang, Z.Y., Hu, H.F., Wright, I.J., Schmid, B., Fang, J.Y., 2018. Global leaf nitrogen and phosphorus stoichiometry and their scaling exponent.Natl.Sci.Rev.5,728–739.
Wang,Z.Q.,Ji,M.F.,Deng,J.M.,Milne,R.I.,Ran,J.Z.,Zhang,Q.,Fan,Z.X.,Zhang,X.W.,Li, J.T., Huang, H., Cheng, D.L., Niklas, K.J., 2015. A theoretical framework for whole-plant carbon assimilation efficiency based on metabolic scaling theory: a test case using Picea seedlings. Tree Physiol. 35, 599–607.
Wang, Z.Q., Huang, H., Li, X.W., Mao, K.S., Ran, J.Z., Deng, J.M., 2018. Allocation of nitrogen and phosphorus within and between needles, stems and roots of Picea seedlings. Nord. J. Bot. 36, e01952.
Wang, Z.Q., Yu, K.L., Lv, S.Q., Niklas, K.J., Mipam, T.D., Crowther, T.W., Uma~na, M.N.,Zhao, Q., Huang, H., Reich, P.B., 2019. The scaling of fine root nitrogen versus phosphorus in terrestrial plants: a global synthesis. Funct. Ecol. 33, 2081–2094.
Wang,Z.Q.,Bu,H.Y.,Wang,M.C.,Huang,H.,Niklas,K.J.,2020a.Allocation strategies for seed nitrogen and phosphorus in an alpine meadow along an altitudinal gradient on the Tibetan Plateau. Front. Plant Sci. 11, 614644.
Wang, M.C., Zhao, Q., Jiang, D.C., Wang, Z.Q., 2020b. Complete chloroplast genome sequence of Stachys japonica(Labiatae).Mitochondrial DNA B Resour 5,2675–2676.
Wang,Z.Q.,Lv,S.Q.,Song,H.,Wang,M.C.,Zhao,Q.,Huang,H.,Niklas,K.J.,2020c.Plant type dominates fine-root C:N:P stoichiometry across China: a meta-analysis.J. Biogeogr. 47, 1019–1029.
Wang, M.C., Gu, Z.J., Fu, Z.X., Jiang, D.C., 2021. High-quality genome assembly of an important biodiesel plant, Euphorbia lathyris L. DNA Res. 28, dsab022.
Wang, Z.Q., Huang, H., Yao, B.Q., Deng, J.M., Ma, Z.Q., Niklas, K.J., 2021a. Divergent scaling of fine-root nitrogen and phosphorus in different root diameters, orders and functional categories: a meta-analysis. For. Ecol. Manag. 495, 119384.
Wang, Z.Q., Wang, M.C., Yu, K.L., Hu, H.F., Yang, Y.H., Ciais, P., Ballantyne, A.P.,Niklas, K.J., Huang, H., Yao, B.Q., Wright, S.J., 2021b. Global synthesis for the scaling of soil microbial nitrogen to phosphorus in terrestrial ecosystems. Environ.Res. Lett. 16, 044034.
Wang,M.C.,Zhang,L.,Tong,S.F.,Jiang,D.C.,Fu,Z.X.,2022.Chromosome-level genome assembly of a xerophytic plant, Haloxylon ammodendron. DNA Res. 29, dsac006.
West, G.B.,Brown, J.H., Enquist, B.J., 1997. A general model for the origin of allometric scaling laws in biology. Science 276, 122–126.
Westoby, M., Wright, I.J., 2003. The leaf size-twig size spectrum and its relationship to other important spectra of variation among species. Oecologia 135, 621–628.
Westoby, M., Falster, D.S., Moles, A.T., Vesk, P.A., Wright, I.J., 2002. Plant ecological strategies: some leading dimensions of variation between species. Annu. Rev. Ecol.Systemat. 33, 125–159.
Wilson, B.F., 1989. Tree branches as populations of twigs. Can. J. Bot. 67, 434–442.
Wright, I.J., Reich, P.B., Westoby, M., Ackerly, D.D., Baruch, Z., Bongers, F., Cavender-Bares, J., Chapin, T., Cornelissen, J.H.C., Diemer, M., Flexas, J., Garnier, E.,Groom, P.K., Gulias, J., Hikosaka, K., Lamont, B.B., Lee, T., Lee, W., Lusk, C.,Midgley, J.J., Navas, M., Niinemets, U., Oleksyn, J., Osada, N., Poorter, H., Poot, P.,Prior, L., Pyankov, V.I., Roumet, C., Thomas, S.C., Tjoelker, M.G., Veneklaas, E.J.,Villar, R., 2004. The worldwide leaf economics spectrum. Nature 428, 821–827.
Wright, I.J., Reich, P.B., Cornelissen, J.H.C., Falster, D.S., Garnier, E., Hikosaka, K.,Lamont, B.B., Lee, W., Oleksyn, J., Osada, N., Poorter, H., Villar, R., Warton, D.I.,Westoby, M., 2005. Assessing the generality of global leaf trait relationships. New Phytol. 166, 485–496.
Xiang, S., Wu, N., Sun, S.C., 2009. Within-twig biomass allocation in subtropical evergreen broad-leaved species along an altitudinal gradient: allometric scaling analysis. Trees (Berl.) 23, 637–647.
Yan,Z.B.,Li,P.,Chen,Y.H.,Han,W.X.,Fang,J.Y.,2016.Nutrient allocation strategies of woody plants: an approach from the scaling of nitrogen and phosphorus between twig stems and leaves. Sci. Rep. 6, 20099.
Yang,D.M.,Niklas,K.J.,Xiang,S.,Sun,S.C.,2009.Size-dependent leaf area ratio in plant twigs: implication for leaf size optimization. Ann. Bot. 105, 71–77.
Yang, X., Tang, Z.Y., Ji, C.J., Liu, H.Y., Ma, W.H., Mohhamot, A., Shi, Z.Y., Sun, W.,Wang,T.,Wang,X.P.,Wu,X.,Yu,S.L.,Yue,M.,Zheng,C.Y.,2014.Scaling of nitrogen and phosphorus across plant organs in shrubland biomes across Northern China.Sci.Rep. 4, 5448.
Yao, F.Y., Chen, Y.H., Yan, Z.B., Li, P., Han, W.X., Fang, J.Y., 2015. Biogeographic patterns of structural traits and C:N:P stoichiometry of tree twigs in China's forests.PLoS One 10, e0116391.
Yuan,Z.Y.,Chen,H.Y.H.,Reich,P.B.,2011.Global-scale latitudinal patterns of plant fineroot nitrogen and phosphorus. Nat. Commun. 2, 344.
Zhang, J.H., He, N.P., Liu, C.C., Xu, L., Yu, Q., Yu, G.R., 2018. Allocation strategies for nitrogen and phosphorus in forest plants. Oikos 127, 1506–1514.
Zhao,N.,Yu,G.Y.,He,N.P.,Xia,F.C.,Wang,Q.F.,Wang,R.L.,Xu,Z.W.,Jia,Y.L.,2016a.Invariant allometric scaling of nitrogen and phosphorus in leaves, stem, and fine roots of woody plants along an altitudinal gradient. J. Plant Res. 129, 647–657.
Zhao, Y.T., Ali, A., Yan, E.R., 2016b. The plant economics spectrum is structured by leaf habits and growth forms across subtropical species. Tree Physiol. 37, 173–185.
Zhao,M.Y.,Luo,Y.K.,Chen,Y.H.,Shen,H.H.,Zhao,X.,Fang,J.Y.,Hu,H.H.,2021.Varied nitrogen versus phosphorus scaling exponents among shrub organs across China.Ecol. Indicat. 121, 107024.
- Forest Ecosystems的其它文章
- Prediction of the global potential geographical distribution of Hylurgus ligniperda using a maximum entropy model
- Structure complexity is the primary driver of functional diversity in the temperate forests of northeastern China
- Monitoring the abundance of saproxylic red-listed species in a managed beech forest by landsat temporal metrics
- Trade-offs among fine-root phosphorus-acquisition strategies of 15 tropical woody species
- Evaluating and quantifying the effect of various spruce budworm intervention strategies on forest carbon dynamics in Atlantic Canada
- Consistent response of nematode communities to management of coniferous plantations

