Consistent response of nematode communities to management of coniferous plantations
Hifng Yin, Yu Su, Siz Liu, Xingjun Li, Xinwi Li,d,*, Chun Fn,d, Pingting Gun,Zhijing Xi, Simin Wng, Stfn Shu,f, Vlntyn Krshvsk
a College of Forestry, Sichuan Agricultural University, Chengdu, 611130, China
b J.F. Blumenbach Institute of Zoology and Anthropology, University of G¨ottingen, G¨ottingen, 37073, Germany
c Sichuan Academy of Forestry, Chengdu, 610036, China
d State Forestry and Grassland Administration Key Laboratory of Forest Resources Conservation and Ecological Safety on the Upper Reaches of the Yangtze River,Sichuan Agricultural University, Chengdu, 611130, China
e State Environmental Protection Key Laboratory of Wetland Ecology and Vegetation Restoration, School of Environment, Northeast Normal University, Changchun,130117, China
f Centre of Biodiversity and Sustainable Land Use, University of G¨ottingen, G¨ottingen, 37073, Germany
Keywords:Soil nematodes Crop-tree thinning Understory vegetation Forest management Soil fauna
ABSTRACT
1. Introduction
Natural forests in China have been replaced in large by plantations,and this also applies to the Sichuan Basin,China(Xianghao et al.,2010;Qin et al.,2018).In the Sichuan Basin,the main plantations include pine(Pinus massoniana Lamb.), Chinese fir (Cunninghamia lanceolata (Lamb.)Hook.) and cypress (Cupressus funebris Endl.). These plantations are an essential ecological barrier in the upper reaches of the Yangtze River,conserving water and soil and protecting biodiversity (Wang et al.,2017). However, these plantations were established with high tree density,and due to inefficient management,they are of low productivity and yield, associated with scarcity of understory vegetation, difficulties in natural regeneration and poor soil fertility(Masiero et al.,2015;Cheng et al., 2017; Lei et al., 2018). Understory vegetation is essential for maintaining forest diversity and providing multiple ecosystem services,such as shelter and food for animals and soil and water conservation(Taki et al.,2010;Fu et al.,2015;Yang et al.,2018).Thus,to improve the services these plantations provide, including their conservation value,more effective forest management needs to be implemented.
As common forest management practice, thinning, has been widely used in the Sichuan Basin(Wang et al.,2018;Liu et al.,2021).Crop-tree thinning (CTT) is a forest management technique used to promote the growth of individual trees by concentrating resources on these target trees by harvesting competitor trees(Heitzman and Nyland,1991;Ward,2002; Miller et al., 2007). Canopy gaps formed by CTT affect the abundance and richness of understory plants due to changes in the light regime and the availability of nutrients(Trentini et al.,2017;Lyu et al.,2018). Therefore, CTT contributes to the formation of heterogeneous stands in even-aged plantations (Arseneault and Saunders, 2012; Junjie et al.,2021).However,the impact of CTT on the belowground system of plantations remains unclear.
As a species-rich and abundant component of the belowground system, soil nematodes occupy a wide range of trophic positions and significantly contribute to soil food web interactions (Bernard, 1992;Yeates et al.,1993;Ferris et al.,2012). In soils, nematodes feed on bacteria, fungi, plants and animals, as well as litter debris and carrion(Yeates et al., 1999; Ferris and Matute, 2003). In particular,microbial-feeding nematodes significantly contribute to carbon mineralization, nutrient cycling and plant growth (Griffiths, 1994; Sarathchandra et al., 2001; Williamson et al., 2005). During the growing season, carbon emissions from soil nematode respiration have been calculated to be equivalent to 15% of the emissions due to global fossil fuel combustion (Van Den Hoogen et al., 2019). Soil nematodes have been shown to be sensitive to ecological disturbances and therefore used as indicators for soil processes(Neher,2001;Neher et al.,2005).For soil nematodes, various indices have been developed to determine the state and extent of ecosystem disturbance (Ferris and Bongers, 2009; Ferris,2010). Trophic groups, colonizer-persister (c-p) value, maturity index,enrichment index and structure index of soil nematodes have been widely used to characterize the structure and functioning of soil food webs(Bongers,1990;Bongers and Bongers,1998;Yeates,2003)as well as changes in environmental conditions and ecosystem functions (Ma et al.,2018; Siebert et al.,2019).
Previous work on the impact of CTT on soil nematodes is limited.Yin et al. (2021) studied the effects of soil nematode communities at early stages(one year)of CTT in pine plantations,showing that CTT increases the density of soil nematodes, in particular the relative abundance of herbivores as well as the structure and enrichment indices,whereas the density of bacterivore and fungivore nematodes remained unaffected.Although forest management changes the habitat and food resources of soil nematodes, their impact on the structure and functioning of soil nematode communities remains controversial (Yeates, 2007). For example, harvesting trees has been reported to have little effect on soil nematode abundance, but to increase nematode diversity and the proportion of herbivores (Neher et al., 2005). Similarly, forest disturbance has been reported to have little effect on soil nematode community structure, with species richness and the maturity index being only affected by strong disturbances such as slash-and-burn agriculture and forest clearance(Bloemers et al.,1997).Additionally,the introduction of silver fir (Abies alba Mill.)into beech-dominated (Fagus sylvatica L.) forests have little effect on nematode density and metabolic activity (Kondratow et al., 2019). Vegetation changes affect soil nematodes directly,but also via changes in microbial assemblages (Wang et al., 2019).However,all these studies focused on singular or two tree species stands,whereas studies across multiple forest ecosystems using the same management measures are lacking.Furthermore,there is a lack of research on the mechanism responsible for changes in the structure and functioning of soil nematode communities due to CTT.
The major objective of this study was to explore whether and how CTT affects the soil nematode community in various types of plantations.Therefore,we investigated the community composition and structure of soil nematodes after three years of CTT in three different plantations in the Sichuan Basin. Moreover, we measured environmental variables related to CTT, such as soil moisture,soil organic matter concentration,microbial biomass carbon and nitrogen, and understory plant diversity,to obtain insight into the drivers of changes in nematode communities due to CTT. We hypothesized that (i) CTT increases the abundance and richness of soil nematodes in each of the three plantations, particularly that of herbivores, (ii) CTT increases the stability of nematode community structure,and(iii)soil microbes and understory vegetation diversity are critical factors affecting soil nematode community composition.
2. Materials and methods
2.1. Study site
This study was performed in three plantations,pine(Pinus massoniana Lamb.),Chinese fir(Cunninghamia lanceolata(Lamb.)Hook.)and cypress(Cupressus funebris Endl.) (Table 1). The plantations are located in the subtropical monsoon climate zone at similar elevations. The study sites represent typical and widespread plantations of coniferous trees in the Sichuan Basin. Except for cutting sick and dead trees, the plantations received little management.
The pine plantation is located in the Tianchi Forest Farm of Huaying City(106.7883°E,30.3392°N)and stocks on nitisol.The average annual temperature is 17.2°C, and the average annual rainfall is 1,087 mm(Sichuan Provincial Meteorological Service). The plantation was established in 1982,trees were originally established in a square pattern.The understory comprises mainly Lindera glauca, Rubus chroosepalus, Litsea cubeba, Humata repens, Dicranopteris dichotoma and Setaria plicata. The Chinese fir plantation is located in Yuejiang Forest Farm, Gaoxian County,Yibin City(104.7056°E,28.6400°N)and stocks on nitisol.The average annual temperature is 17.9°C,and the average annual rainfall is 1,200 mm (Sichuan Provincial Meteorological Service). The plantation was established in 2005, trees were originally established in a square pattern. The understory comprises mainly Rhus chinensis, Ficus tikoua,Parathelypteris glanduligera and Arthraxon hispidus.The cypress plantation is located in Yufeng Town, Anju District, Suining City (105.5331°E,30.4106°N) and stocks on purpli-udic cambosols. The average annual temperature is 17.4°C, and the average annual rainfall is 990 mm(Sichuan Provincial Meteorological Service). The plantation was established in 1990,trees were originally established in a square pattern.The understory comprises mainly Myrsine Africana, Vitex negundo, Rhus chinensis,Ficus tikoua and Cyperus.
2.2. Study design and sampling
Crop-tree thinning(CTT)was implemented in October 2015.In each type of plantation,six plots of 100 m×100 m were established,resulting in a total of 18 plots.The plots were located more than 10 m away from the edge of the forest and any road in the plantation. Half of the plots were assigned to CTT(with crop-tree thinning),and the other half served as a control (without any forest management). Crop trees were selected based on the following criteria:trunks without live branches up to 6 m,well-developed canopy, and no signs of damage. Trees competing with the crop trees were cut using a tree trimming machine and removed from the plot. The trimming was done damaging the soil and ground vegetation as little as possible.Therefore,150 trees were selected as crop trees in each of the CTT plots.
Samples were taken three years after establishment of the plots(September 2018). Five crop trees were randomly selected in each CTT and control plot. The top 15 cm of soil was collected using a soil corer with a diameter of 5 cm at a distance of 1.5–2.5 m from the trunk of the tree;three soil samples were taken from each tree and pooled,resulting in a total of 90 samples.The diversity and species identity of herbaceous plants were determined in 15 randomly selected 1 m×1 m squares perplot. Furthermore, in five randomly selected 5 m × 5 m squares, the diversity and species identity of shrubs were recorded.Soil samples were stored at 4°C and transferred to the laboratory immediately after sampling.

Table 1 Details of the three plantations.
2.3. Soil nematodes
Nematodes were extracted from 50 g soil using modified Baermann funnels (Ruess,1995;Zhao et al.,2014a; Shao et al.,2019).Nematodes were stored in 4% formalin for counting. Nematode density was calculated on the basis of 100 g dry soil (65°C, 72 h). A minimum of 100 nematode individuals were identified to the genus level (see Supplementary Table S1) at 200× to 1000× magnification (Olympus, BX51)following Goodey (1963), Bongers (1988) and Andr′assy (2005). Nematodes were assigned to five trophic groups (bacterivores, fungivores,herbivores, omnivores, predators) according to Yeates et al. (1993).Furthermore, the genera were assigned to colonizer-persister (c-p)groups representing life cycle strategies related to r-or K-strategies(c-p 1 to c-p 5)(Bongers,1990;Bongers et al.,1995;Ferris,2010).In addition,the maturity index based on Bongers(1990)and the structure index and enrichment index based on Ferris et al. (2001) were calculated. The nematode maturity index, serves as a measure for the degree of disturbance of soil systems(Bongers,1990).The enrichment index(EI)reflects the resource provisioning of soil food webs and the structure index (SI)indicates the exposure of soil communities to stress(Ferris et al.,2001).
2.4. Environmental factors
Soil moisture and soil organic matter were determined using standard methods (Gregorich and Carter, 2007). Soil pH level was determined using a pH meter(Sartorius PP-25),using a ratio of sample to water(H2O without CO2) of 1:2.5. Soil microbial biomass carbon and microbial biomass nitrogen were measured by chloroform fumigation extraction(Brookes et al.,1985;Vance et al.,1987).Total carbon in the extracts was measured using a TOC analyzer (TOC-V Series; Shimadzu Corporation,Kyoto, Japan), and total nitrogen was measured by a total N analyzer(TNM-1; Shimadzu Corporation). The diversity of understory plants(herbs and shrubs) was calculated based on the Shannon-Wiener index(Shannon, 1948). Only environmental factors significantly correlating with nematode community composition are shown in the results section;for mean values and SD of all environmental factors,see Supplementary Table S2.
2.5. Statistical analysis
To test hypothesis 1,we inspected the effect of CTT on the abundance and richness of soil nematodes in the three plantations. First, we fitted linear mixed-effects models (LMMs) to analyze responses of soil nematode abundance and richness to plantation type and CTT.Then,we used the Student's t-test to inspect differences between CTT and control plots in each plantation. All models met the assumptions of normality of residuals and homogeneity of variance.
To test hypothesis 2,we used trophic groups,colonizer-persister(c-p)value, maturity, enrichment and structure indices of soil nematodes as dependent variables. First, we calculated the relative abundance of nematode trophic groups.Then,we classified colonizer-persister groups(c-p 1–5) into enrichment opportunists (c-p 1), stress tolerators (c-p 2)and community stabilizers(c-p 3–5) (De Goede et al., 1993), and Fig. 3 was prepared using Origin 2017 (OriginLab, Northampton, MA, USA).We used the least significant difference(LSD)to inspect differences in the maturity index between the CTT and control plots in each plantation.Furthermore, we calculated the structure and enrichment indices and inspected for differences between the CTT and control in each plantation.Statistical analyses for hypothesis 1 and hypothesis 2 were conducted using SPSS 22.0 statistical software(SPSS Inc., Chicago,IL).
To test hypothesis 3,we first standardized nematode community data and environmental data using “Hellinger” and “decostand” in the“vegan” package (Oksanen et al., 2019). Detrended correspondence analysis(DCA)recovered gradients of 2.40(pine),2.40(Chinese fir)and 2.34 (cypress), indicating redundancy analysis (RDA) as an appropriate approach for further analyses.RDA with a forward selection model,using the “ordistep” function in the “vegan” package (Oksanen et al., 2019),was conducted to identify factors significantly affecting soil nematode communities. The following seven environmental variables were included in the RDA, which were a priori assumed to affect nematodes community composition: soil moisture, soil organic matter, microbial biomass carbon and nitrogen, Shannon-Wiener index of herbs and shrubs. The forward selection procedure was stopped if a variable reached a significance level >0.05. Monte Carlo tests with 999 permutations were performed to evaluate the overall model significance for selected factors in each plantation.Variations explained by the selected environmental variables were assessed via adjusted R2values. The 21 species closely correlated with the first two axes are displayed for each plantation.The analyses were performed in R software version 4.0.4 (R Core Team,2021).
3. Results
3.1. Abundance and richness
A total of 41 nematode families and 81 genera were identified(Supplementary Table S1). Nematode abundance was significantly increased after CTT (P <0.05, Supplementary Table S3), most pronounced in cypress(by a factor of 3.64),less pronounced in pine(2.36)and least pronounced in Chinese fir(1.38)(Fig.1a).However,nematode abundance was not significantly different among the three types of plantations (P = 0.09). Nematode richness was also significantly increased after CTT(P <0.05),but only in pine and cypress plantations and not in Chinese fir(Fig.1b).
3.2. Community structure
In general, herbivorous nematodes dominated in each of the plantations(29.7%of total;mean across plantations),followed by bacterivores(21.5%),omnivores(18.1%),predators(16.2%)and fungivores(14.5%)(Fig. 2). In the pine plantation, the relative abundances of omnivores(+16.3%), herbivores (+8.3%) and fungivores (+0.2%) were higher after CTT but lower for bacterivores(-12.8%)and predators(-11.9%).In the Chinese fir plantation, the relative abundances of fungivores(+11.1%) and herbivores (+9.3%) were higher after CTT, while they were lower for omnivores(-13.9%),predators(-6.3%)and bacterivores(-0.2%). In the cypress plantation, the relative abundance of bacterivorous(+9.6%)and herbivorous(+4.6%)nematodes was higher after CTT but lower for omnivores (-7.7%), predators (-5.1%), and fungivores(-1.4%)nematodes.

Fig. 1. Abundance (a) and richness (b) of soil nematodes in pine, Chinese fir and cypress plantations three years after crop-tree thinning (CTT) compared to the respective control. Bars sharing the same letter do not differ significantly (P >0.05; Student's t-test).
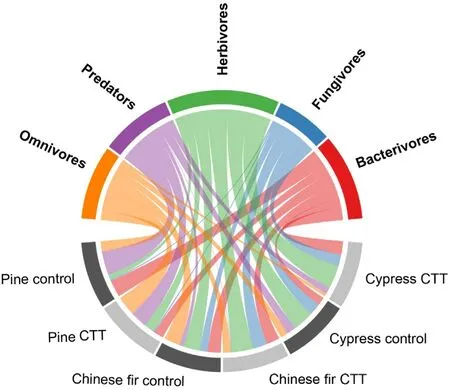
Fig. 2. Relative abundance (percent of total) of soil nematode trophic groups(upper half circle) in pine, Chinese fir and cypress plantations three years after crop-tree thinning(CTT)and in the control(lower half circle).The width of the links represents the relative abundance of trophic groups in the respective treatments in three plantations, which percentage is shown in Table S1.
Among the three colonizer-persister(c-p)classes present,community stabilizers(c-p 3–5)dominated(62.2%of total;mean across plantations),followed by stress tolerators(c-p 2;30.4%)and enrichment opportunists(c-p 1;7.4%;Fig.3).In the pine plantation,the frequency of community stabilizers (c-p 3–5) increased after CTT, which was related to the increase in omnivorous nematodes, whereas the frequency of stress tolerators(c-p 2)decreased.In contrast,in Chinese fir and cypress plantations,the frequency of enrichment opportunists(c-p 1)and stress tolerators(cp 2)increased after CTT,whereas the frequency of community stabilizers(c-p 3–5) decreased, which was related to the decrease in omnivorous and predatory nematodes.
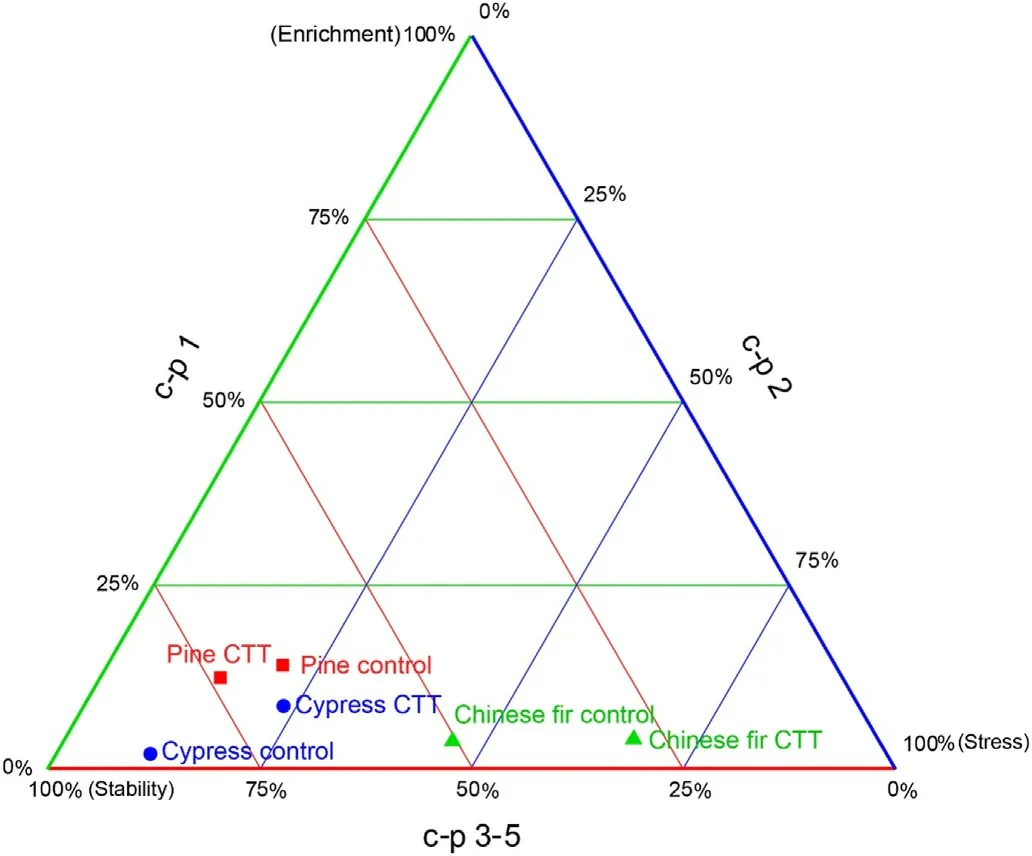
Fig. 3. C-p triangles of unweighted proportions of nematode c-p classes (colonizer-persister; c-p 1–5) in pine, Chinese fir and cypress plantations three years after crop-tree thinning (CTT) and in the respective control. The green line represents enrichment opportunists (c-p 1), the blue line represents stress tolerators(c-p 2),and the red line represents community stabilizers(c-p 3–5).This figure was done using Origin 2017. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The maturity index of nematodes was significantly lower (P <0.05)after CTT in Chinese fir and cypress plantations but not in pine plantations (Fig. 4a). The nematode faunal profile placed nematodes from all plantations in Quadrat B (enriched and structured), except Chinese fir after CTT(in Quadrat C),which was positioned at the transition between Quadrats B and C (resource-limited and structured; Fig. 4b). In cypress plantations,no bacterivores and fungivores c-p 2 nematodes were found in control treatments, resulting in the structure index and enrichment index of 100%. The enrichment index and structure index were lower after CTT in Chinese fir and cypress plantations,while they changed little in the pine plantation. Changes in the position due to CTT were mainly due to the structure index in the Chinese fir plantation, whereas it was mainly due to the enrichment index in cypress plantations.
3.3. Factors affecting soil nematode community composition

Fig.4. Effects of crop-tree thinning on ecological indices of soil nematodes in pine,Chinese fir and cypress plantations three years after crop-tree thinning(CTT)and in the respective control; maturity index (a) and nematode faunal profile (b).Quadrats refer to the faunal profile A,B, C,and D(Ferris and Bongers, 2009) (b). Bars sharing the same letter do not differ significantly (P <0.05; least significant difference test).

Fig. 5. Redundancy analysis (RDA) of nematode genera in control and crop-tree thinning (CTT) plantations as related to microbial biomass nitrogen and Shannon-Wiener index of herbs or shrubs(blue arrows)in pine(a),Chinese fir(b)and cypress plantations(c).The 21 most abundant genera are shown.Only significant(P <0.05)environmental factors were retained.(For interpretation of the references to colour in this figure legend,the reader is referred to the Web version of this article.)
In each of the plantations, two of the seven environmental variables significantly correlated with species composition (RDA, forward selection, overall Monte Carlo test P = 0.001), together explaining 68.8%,64.0% and 72.4% of the variation in nematode species composition across the control and CTT treatments in the pine,Chinese fir and cypress plantations, respectively (Fig. 5a-c). Irrespective of plantation type,nematode communities in the control and CTT treatments separated mainly along Axis 1. Strikingly, in each of the plantations, microbial biomass nitrogen and the Shannon-Wiener index of herbs (pine and Chinese fir plantations) or shrubs (cypress plantation) correlated positively with nematode communities in the CTT treatment.In the pine plantation, this separation was mainly due to high densities of Prionchulus, Hemicaloosia, Rhabditis, Helicotylenchus, Eudorylaimus and Pristionchus in the CTT treatment, whereas the control treatment was associated with high densities of Mononchus and Diplogasteriana(Fig.5a).In the Chinese fir plantation,the CTT treatment was associated with high densities of Meloidogyne, Criconema, Pungentus, Ditylenchus and Laimydorus,whereas the control treatment was associated with high densities of Prodorylaimus,Sakia and Thorneella(Fig.5b).In the cypress plantation,the CTT treatment was associated with high densities of Campydora,Alaimus, Tylencholaimus, Hemicycliophora, Diphtherophora and Aphelenchus,whereas the control treatment was associated with high densities of Iotonchus and Tylencholaimellus(Fig.5c).
4. Discussion
4.1. Abundance and richness of soil nematodes
Supporting our first hypothesis, CTT significantly increased the abundance and richness of soil nematodes in each plantation,indicating that CTT generally beneficially affects nematode communities. As indicated by RDA, this was mainly due to Eudorylaimus in pine, Ditylenchus,Laimydorus and Psilenchus in Chinese fir and Helicotylenchus and Paratylenchus in cypress plantations, all known to easily adapt to new environmental conditions (ˇCerevkov′a et al., 2021; Yin et al., 2021). On the other hand, perturbations such as CTT may also eliminate or reduce nematode species unable to adapt to the new environment(Yeates,2007;Liu et al., 2019). Bloemers et al. (1997) found that soil nematode abundance decreased significantly due to strong disturbances in the forests. However, Yang et al. (2018) found that moderate forest management increased the abundance of nematodes, potentially due to fostering understory vegetation (Zhao et al., 2013). In fact, CTT at our study site likely functioned as a moderate disturbance(Yin et al.,2021)and was associated with increased understory vegetation diversity(Supplementary Table S2),which likely explains the beneficial effects on nematode abundance.
Compared to nematode abundance, the response of nematode diversity to CTT was less uniform among plantations. CTT significantly increased the richness of soil nematode genera in pine and cypress plantations but not in Chinese fir plantations.This may have been due to the lack of vegetation and food resources in the Chinese fir plantation,which was younger than the other two plantations(Zhang et al.,2015).Nonetheless, CTT as important forest management practice beneficially affects soil organisms as indicated by the overall response of soil nematode abundance and richness.
4.2. Variations in nematode community structures
In line with our first hypothesis, CTT increased the relative abundance of herbivorous nematodes in each of the three plantations, presumably reflecting that CTT increased understory vegetation cover and diversity. As primary consumers, herbivore nematodes directly respond to changes in understory vegetation (Li et al., 2015). Presumably,increased understory vegetation diversity provided a wider range of food resources for herbivorous nematodes, allowing a wider range of nematode genera to coexist (Ruijven et al., 2005). However, earlier studies also found herbivorous nematodes to be sensitive to disturbances (Bergeson, 1986; Pen-Mouratov et al., 2012), but at our study site the beneficial effects of CTT prevailed.
In addition to herbivores, CTT increased the relative abundance of omnivorous nematodes, while it reduced the relative abundance of predatory nematodes, but only in the pine plantation. The increase in omnivorous nematodes conforms to the results of earlier studies showing that omnivorous nematodes,using a wide spectrum of food sources,are well adapted to variations in environmental conditions (Sohlenius and Wasilewska, 1984; Ritter and Bjørnlund, 2005). In contrast, predatory nematodes are typically slow-growing, thereby benefitting from stable habitat conditions(Wilschut and Geisen,2021; ˇCerevkov′a et al.,2021).Their decline due to CTT likely reflects habitat destruction due to tree harvesting, indicating that recovery of the food web structure was incomplete even three years after CTT.
The increased proportion of K-strategists (c-p 3–5 class) in the CTT treatment of the pine plantation suggests that recovery of the food web was most pronounced in this plantation, indicating more stable environmental conditions in pine than in Chinese fir and cypress plantations.This is supported by the generally higher frequency of predatory nematodes in pine than in Chinese fir and cypress plantations. Furthermore,supporting this conclusion,CTT increased the proportion of r-strategists(c-p 1 and c-p 2 class)in Chinese fir and cypress plantations.Presumably,this again reflects that the pine plantation is older and that the trees are larger than those in the Chinese fir and cypress plantations. It is known that with the consolidation of the plant community and availability of food resources with forest age, the community structure of nematodes changes toward K-strategists(Yeates et al.,2000;Yeates,2007).In fact,the understory vegetation diversity of Chinese fir and cypress plantations was lower and less consolidated than that in the pine plantation (Supplementary Table S2), and this presumably favored taxa with short generation times and high reproduction rates(c-p 1 and c-p 2)(Bongers,1999;Krashevska et al.,2019).
Contrasting our second hypothesis, however, CTT did not significantly affect the maturity index of nematodes in pine plantations,which resembles earlier findings(Yin et al.,2021).In contrast,CTT significantly reduced the maturity,structure and enrichment indices of nematodes in Chinese fir and cypress plantations,again suggesting that the recovery of the nematode community after CTT was slower in Chinese fir and cypress plantations than in the pine plantation.Presumably,this was also due to the younger age and lower coverage and diversity of understory plants in the Chinese fir and cypress plantations than in the pine plantation,resulting in lower food resources in the former (Roberts and Gilliam,1995;Bengtsson et al.,2000).
4.3. Factors affecting soil nematode community composition
Supporting our third hypothesis, microbial biomass nitrogen and understory vegetation diversity were identified as significant factors affecting soil nematode community composition. Notably, this consistently applied to each of the plantation systems. These findings are consistent with the results of the study of Wang et al. (2019) reporting that plants indirectly affect nematode communities via changes in bacterial and fungal community composition.In fact,nematodes are among the major predators of microorganisms and benefit from microorganisms becoming more abundant (Griffiths, 1994; Sarathchandra et al., 2001;Williamson et al.,2005).The fact that microbial biomass nitrogen,rather than microbial biomass carbon, functioned as a major driver of soil nematode community composition in each plantation suggests that it is not the quantity of microorganisms,but their quality as a food resource,that determines nematode community composition (Nieminen and Set¨al¨a, 2001; Zhao et al., 2014a). Overall, the results indicate that CTT indirectly affects the belowground food web through changes in resource availability, with the resources being most important are consistent across plantations.
5. Conclusions
Our study provides comprehensive insight into the influence of CTT on belowground nematode communities in tree plantations. The results suggest that CTT changes the structure of nematode communities,generally favoring an increase in nematode abundance and the frequency of herbivorous nematodes. However, the response of nematode communities varied among plantation types, with the effect of CTT being more pronounced in Chinese fir and cypress plantations than in the pine plantation,likely due to more consolidated environmental conditions in older pine plantations than in the younger Chinese fir and cypress plantations.Notably,nematode community composition in each plantation was primarily determined by microbial biomass nitrogen and understory/shrub diversity,suggesting that the effect of CTT on different plantations was mainly due to changes in food resource availability and quality.
Ethics approval and consent to participate
Not applicable.
Funding
This work was supported by the National Key Research and Development Program of China(Grant No.2017YFD060030205),the German Government loans for Sichuan Forestry Sustainable Management(Grant No.G1403083),and the“Tianfu Ten Thousand Talents Plan”of Sichuan Province (Grant No. 1922999002). H. Y. appreciates the financial support from the China Scholarship Council(Project No.202006910045).
Authors’contributions
Haifeng Yin, Xianwei Li, Stefan Scheu, Valentyna Krashevska designed this research, collected and analysed the data and wrote the manuscript draft; Yu Su, Size Liu, Xiangjun Li conducted the fieldwork and analysed the data; Chuan Fan, Pingting Guan, Zhijing Xie, Simin Wang analysed the data. The author(s) read and approved the final manuscript.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Declaration of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could influence the work reported in this paper.
Acknowledgements
Persons who helped with field work, lab analysis and manuscript preparation were highly acknowledged, namely Maojin Guo, Zheng Zhou,Ting-wen Chen,Yi Shen,Junjie Liu.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://do i.org/10.1016/j.fecs.2022.100045.
- Forest Ecosystems的其它文章
- Trade-offs among fine-root phosphorus-acquisition strategies of 15 tropical woody species
- Structure complexity is the primary driver of functional diversity in the temperate forests of northeastern China
- The 2/3 scaling of twig nitrogen to phosphorus in woody plants
- Monitoring the abundance of saproxylic red-listed species in a managed beech forest by landsat temporal metrics
- Evaluating and quantifying the effect of various spruce budworm intervention strategies on forest carbon dynamics in Atlantic Canada
- Prediction of the global potential geographical distribution of Hylurgus ligniperda using a maximum entropy model

