Soil chemical fertility change over four decades in the Morvan Mountainsand influence of tree species (Burgundy, France)
Margaux Clesse, Arnaud Legout, Jacques Ranger, Bernd Zeller, Gregory van der Heijden
INRAE, BEF, F-54000, Nancy, France
Keywords:Nutrient pools Tree species Acidification Podzolisation Recovery Ecosystem services
ABSTRACT
1. Background
In Europe and North America,forest land cover reached its minimum around the beginning of the 18th century due to the overexploitation of forest resources (Kaplan et al., 2009). Forest land cover has since then greatly increased helped by the afforestation policies and the use of new energy sources during the second half of the 20th century. Vast areas were planted with productive coniferous tree species that were translocated within Europe(e.g.Norway spruce(Picea abies Karst.)and Scots pine (Pinus sylvestris L.)) or imported from North America (e.g. Sitka spruce (Picea sitchensis Bong.) and Douglas fir (Pseudotsuga menziesii Mirb.)).In many cases,native deciduous forest stands such as beech and oak were converted to coniferous plantations to increase forest productivity and these plantations still remain a common sylvicultural practice.However,tree species may impact many different processes and fluxes at different ecosystem levels as reported by the numerous reviews and studies (Augusto et al., 2002, 2015; De Schrijver et al., 2007). It may influence i) the geochemical cycling (atmospheric deposition, mineral weathering, leaching) and ii) the biological cycling (uptake, immobilization in biomass, litterfall, mineralization) (Legout et al., 2020), as detailed below:· Tree species scavenge dry and occult atmospheric deposition depending on their canopy architecture, height, foliage type and stand structure. Atmospheric deposition and concentrations of nitrogen and sulfur in throughfall are generally higher under coniferous stands compared to hardwood stands (Augusto et al., 2002; De Schrijver et al., 2007). According to Augusto et al. (2002), Norway spruce stands collect more atmospheric deposition than beech or oak(respectively +187%, +59% and +99% sulfur deposition under canopy compared to bulk deposition) and nitrogen and sulfur deposition is higher under conifers than deciduous trees (Rothe et al.,2002;Verstraeten et al.,2012).Augusto et al.(2002)also showed that mineral weathering was enhanced under Norway spruce stands(two to three times higher) compared to hardwood stands. Lastly, tree species may also influence nutrient leaching (Augusto et al., 2002;Legout et al., 2016) and may exert a control over N mineralization and nitrification processes (Andrianarisoa et al., 2010; Zeller et al.,2019),leading to an increase in soil acidity when nitrate production exceeds nitrate consumption(Legout et al.,2016).
· Tree species can also influence biological cycling and uptake of nutrients.Nutrient immobilization in aerial biomass is usually higher in hardwood species than for coniferous species(Augusto et al.,2002).However, the coniferous species produce more biomass and their rotation lengths are shorter than hardwood species (Binkley, 1995;Augusto et al., 2002). Depending on litterfall mass and chemical composition, tree species may also influence carbon and nutrient pools in the organic layer (Moukoumi et al., 2006; Vesterdal et al.,2008;Carnol and Bazgir,2013).Slower litter decomposition rates in coniferous stands have been reported (Binkley, 1995; Rovira and Vallejo, 2002) which may also contribute to the release of organic acids further contributing to soil acidification.
As a result of these different processes, tree species influence soil acidification and chemical fertility. It has been shown that soil acidification processes are enhanced under Norway spruce stands compared to other species (oak, beech, birch, Douglas fir, pine) (Bergkvist and Folkeson, 1995; Augusto et al., 1998, 2000, 2002). Several studies also demonstrated that exchangeable Ca,Mg and K pools are generally lower under coniferous stands compared to broadleaved stands(Ranger,1995;Cremer and Prietzel, 2017), while solution concentrations in Al and H+are generally higher (Brown and Iles, 1991; Legout et al., 2016). Gruba and Mulder (2015) also showed that tree species influence the cationic exchange capacity in the soil.
Despite numerous studies, our understanding of the impact of different tree species on soil chemical fertility is still incomplete. Very few studies have been able to report the effects of different tree species on the soil chemical fertility over several decades. Long-term monitoring and common garden experimental sites are for this aim necessary because tree species induced changes may be small due to the slow soil processes involved. Yet,understanding the effects of tree species on the long-term fertility of forest soils is of utmost importance to ensure its sustainability because i) forests generally grow on acidic and nutrient poor soils, ii) which in many cases have been severely impacted by the elevated acidic atmospheric inputs during the 20th century,iii)although atmospheric deposition of sulfur has decreased since the 1980s(Prechtel et al., 2001; Boxman et al., 2008; Engardt et al., 2017), nitrogen inputs remain in many cases elevated (Boxman et al., 2008; Vuorenmaa et al.,2017), iv) forest endure today increased pressure due to the intensification of silvicultural practices and harvesting (De Turckheim, 1990;Achat et al.,2015;Par′e and Thiffault,2016),and v)the current context of global and climate change may encourage foresters to convert tree species in order to adapt forest stands to the changing climate and/or increase forest productivity (Bolte et al.,2009;Thurm et al.,2018).
The common garden experiment of the Breuil-Chenue site(Burgundy,France), installed in 1976 and monitored since 2001 (see details in material and methods section),is a fairly unique experiment that compares 6 monospecific plots:oak(Quercus sessiliflora Smith.),beech(Fagus sylvatica L.), Norway spruce (P. abies.), Douglas fir (P. menziesii.), Nordmann fir(Abies nordmanniana Spach.)and Laricio pine(Pinus nigra Arn.ssp.laricio Poiret var. corsicana). Based upon soil sampled in 2001, Mareschal et al.(2010)showed that tree species had an impact on the chemical properties of the topsoil(specifically the cationic exchange capacity and soil pH)25 years after plantation. Since 2001, several studies have shown that tree species influence numerous soil biogeochemical processes such as organic matter decomposition,nitrification or soil solution speciation(Moukoumi et al., 2006; Zeller et al., 2007; Andrianarisoa et al., 2010; Trum et al.,2011;Legout et al.,2016).However,the impact of such processes on the chemical properties of the soil has not be assessed since 2001. Furthermore,because changes in forest soils may be very slow in temperate forest ecosystems, studies require long-term monitoring datasets to precisely conclude on tree species effects.
The main objective of this study is to quantify the effect of tree species on the soil chemical fertility over time during the first 45 years after plantation. The detailed objectives are i) quantify the change of soil chemical fertility and the effect of tree species over the 45 years after plantation and,ii)compare the measured trends between the 1976–2001 and 2001–2019 periods.An attempt to integrate these results in a more global approach to evaluate tree species effect based on several criterions(soil fertility, water quality, wood production, etc.) was made. To meet these objectives,we studied the humus layer,mineral soil layers down to 70-cm depth from samples collected in 1974, 2001 and 2019 in the 6 monospecific stands of the Breuil-Chenue experimental site. Based on previous studies (Moukoumi et al., 2006; Zeller et al., 2007; Mareschal et al., 2010; Legout et al., 2016), we hypothesize that chemical fertility degradation between 2001 and 2019 occurred as follows:elevated in the Douglas fir and Laricio pine, due mainly to acidolysis related to high nitrification rate and nutrient cation leaching, intermediate in the Nordman fir,Norway spruce and oak due to acidolysis and chelation(in relation to the release of organic acids from organic matter transformation)and less intense in the beech plot.
2. Methods
2.1. Experimental site
The experimental site of Breuil-Chenue is located in the Breuil-Chenue state forest in the Morvan Regional Natural Park, Burgundy,France (latitude 4°18′10′′, longitude 4°4′44′′) at 640-m elevation. The annual rainfall is on average 1,180 mm and the annual temperature 9°C(over the period 2006–2012). In 1975, the native forest (coppice with standards(CwS)composed of beech and oak)located on a homogeneous soil type was clear-cut. The organic layer and harvest residues were removed with a bulldozer. Monospecific plots (1,000 m2each) of oak(Q.sessiliflora),beech(F.sylvatica),Norway spruce(P.abies),Douglas fir(P.menziesii),Nordmann fir(A.nordmanniana)and Laricio pine(P.nigra ssp.laricio var.corsicana)were planted and a plot of native forest was left unharvested (Fig. 1). This study focuses on the unfertilized plots of the block 1.The chemical properties of the soil are described in Table S1.
The soil is developed on the “La Pierre qui Vire” leucogranite the composition of which is poor in Mg,K and particularly Ca.The deep soil(70–90 cm)contains 67.32%of SiO2,21.82%of Al2O3,2.86%of Fe2O3,0.1%of MnO,0.42%of MgO,<0.02%of CaO,1.54%of Na2O,4.90%of K2O,0.31%of TiO2and 0.10%of P2O5(van der Heijden et al.,2013).The soil is an Alumic Cambisol(WRB FAO)close to cryptopodzolization in the upper horizons(Ranger et al.,2004).The soil is acidic and nutrient poor with a saturation rate below 10%in the A horizon in the native forest plot(Mareschal et al.,2013).The humus type in all plotsis a mesomull(Br^ethes et al.,1995)with a layer L and F for all species except in the native forest plot where the humus type is a moder(Moukoumi et al.,2006).
The silvicultural management of the different plots followed the regional guidelines, forest planning and management practices defined by the French National Forest Office(ONF).No thinning occurred during the first 24 years.From 2000 onwards,thinnings occurred approximately every seven years for the coniferous plots and every 9 or 10 years for hardwood plots.All above ground biomass of thinned trees was exported.The thinnings were carried out manually (i.e. no mechanization of felling, logging or skidding)as follows(Legout et al.,2016):

Fig. 1. Diagram of the Breuil-Chenue experimental site adapted from van der Heijden et al.(2013).Location of the various plots and sampling points in 1974, 2001 and 2019.
· November 2000: 1st thinning of the Norway spruce (30% removed)and Douglas fir(43%removed)stands.
· November 2001:1st thinning of the beech(48%removed),oak(14%removed), Nordmann fir (15% removed) and Laricio pine (10%removed)stands.
· March 2007:2nd thinning of the Norway spruce(24%removed)and Douglas fir(36%removed)stands.
· November 2008: 2nd thinning of the oak (7% removed), Nordmann fir(35%removed)and Laricio pine(27%removed)stands.
· January 2014:2nd thinning of the beech(36%removed)stand.
· An additional thinning for the plots of Norway spruce (15%removed), Nordmann fir (10% removed) and Douglas fir (15%removed)was carried out in the fall of 2016.
2.2. Soil and organic layer sampling and analysis
In 1974, soil samples were collected at 7 systematic depths: 0–10,10–20, 20–30, 30–40, 40–50, 50–60 and 70–80 cm. Eight replicate soil profiles were sampled. Exchangeable Ca, Mg and K were extracted on a percolation column with buffered ammonium acetate at pH=7(25 g fine earth and 200 mL ammonium acetate solution at 1.5 g·L-1)(Duchaufour,1970) and dosed by ICP-AAS. The density of fine earth was measured with a cylinder of 503 cm3.
In 2001 and 2019, soil samples were collected with a cylindrical auger at seven systematic depths: 0–5, 5–10, 10–15, 15–25, 25–40,40–55 and 55–70 cm. For each plot, 16 soil profiles were collected in 2001 and 5 in 2019. The samples were air-dried, grinded and sieved (2 mm).The organic layer(Ol,Of and Oh)was collected directly above the mineral soil sample profile using a 0.1-m2quadrat and dried at 65°C to constant weight.In 2001,the organic layer dry mass was measured for all replicates but its chemical composition was only measured for 8 out of the 16 replicates.In 2019,all samples were weighed and analyzed.N and C concentrations in the organic layer were determined in 2001 and 2019 by an elemental analyzer, while K, Ca, Mg, P were determined by ICPAES after a nitric acid digestion.
In 2001,the density of fine earth was measured for each stand and horizon. The texture was measured for each stand and each depth of four profiles.The pH water and pH KCl of soil samples were measured using a Mettler pH meter TSDL25 after 12 h of contact with respectively distilled water and a 1-mol·L-1solution of KCl (soil/solution ratio: 1/5). The exchangeable pools of Mg2+, Ca2+, Na+, Fe3+, Al3+, and Mn2+were determined from two sequential soil extractions using a 1 mol·L-1KCl reagent.The exchangeable K pools were determined from two sequential soil extractions using a 1 mol·L-1NH4Cl reagent(Espiau and Peyronel,1976).Extractedsolutions were dosed byICP-AES(JY180 ULTRACE,Horiba Jobin Yvon,Longjumeau,France).H+ions were extracted with a solution of KCl 1 mol·L-1and titrated with an automatic titrimeter (Mettler TS2DL25)(Rouiller et al., 1980). Concentrations of C and nitrogen (N) were determined by elemental analysis from a test sample of 0.5 and 1 g soil,respectively (Anne (1945) and Kjeldahl method). Poorly crystalized and amorphous aluminium(hydr)oxides were extracted by a Tamura extraction(Tamura, 1958) (0.5 g of soil in 40 mL of sodium tricitrate reagent at 1 mol·L-1, pH = 7.3) and analyzed by ICP-AES (Jobin-Yvon Instruments,Longjumeau,France,model JY 180)(Mareschal,2008).
In 2019, the chemical properties of soil samples were obtained by analyses at the INRAE-Arras laboratory:pH water(soil water pH–1:5 soil to water volume ratio)and pH KCl(soil KCl pH–1:5 soil to 0.1 mol·L-1KCl reagent volume ratio) (NF ISO 10390), C and N content, cationic exchange capacity and exchangeable pools(Mg,Ca,K,Na,Al,H,and Mn)extracted with a cobaltihexamine reagent (5 g of soil in a 100 mL of Co(NH3)63+solution at 1 molc·L-1) (NF X 31–130). Tamura extraction(same protocol applied as in 2001) were carried out at the INRAE BEF laboratory and dosed by ICP-AES (Agilent 7500series). Though cobaltihexamine, KCl and NH4Cl extractions showed similar results (Ciesielski et al., 1997a, 1997b), we carried out a comparison between these methods:a subset of 48 samples(2019 campaign)was randomly selected and no significant differences were observed between extraction methods for Ca,Mg and K(p-value <0.001).
The exchange acidity(EA)is defined as the sum of H+and Al3+,the CEC as the sum of the exchangeable cations(Mg2+,Ca2+,Na+,K+,Fe3+,Mn2+,H+,Al3+)and the sum of basic exchangeable cations(S)as the sum of Mg2+,Ca2+and K+.
2.3. Correction of the organic layer thickness sampling bias
Correctly separating the organic layer from the mineral topsoil layer is very complex and may lead to an important overestimation of the organic layer mass. The loss on ignition (LOI) was measured for organic layer samples collected in 2001 and 2019(for 2001,5 samples were randomly selected)to check for possible bias between campaigns.The organic layer was sieved(<0.2 mm),oven-dried at 105°C for a minimum of 24 h and then heated in the mitten oven(Herrmann Moritz West 2050)following a temperature increase ramp: the temperature was gradually increased by 100°C over 20 min and maintained stable for 30 min.This sequence was repeated until the temperature reached 500°C. Then, the samples were maintained at 500°C for 4 h.LOI data showed that the 2001 organic layer samples contained a substantial amount of mineral material.
The organic layer dry weight in 2001 was thus corrected(DWcorrected)for the sampling bias by assuming that the LOI in the 2001 samples should be equal to the LOI measured in 2019 as follows(1)(Table S2):

We estimated that the excess dry mass measured in the organic layer in 2001 was caused by a 0–5 cm organo-mineral soil layer inclusion of less than 1 mm of thickness. No correction on soil stocks was applied because i) this soil thickness was very small compared to the sampled 0–5 cm layer and ii) the estimated Mg, Ca and K pools in a millimetre thick layer were much smaller than the Ca,Mg and K pool correction in the organic layer.
2.4. Quantification limits and date outliers
We chose to replace values in the dataset that were below the analytical quantification limit by the quantification limit value. Changes in quantification limits can result in a considerable bias(Hansson et al.,2020).In order to avoid such biases, i) quantification limit values were set to the highest value between the 2001 and 2019 campaign and ii) we replaced the values below the analytical quantification limit by the quantification limit values.These changes affected 9.4%,0.2%,15.4%,55.6%and 1.4%of the exchangeable Fe,Mg,Ca,Na and H values in the initial dataset.
Potential outliers were identified based on the distributions of each measured variable. Outliers were identified if they were outside the boundaries A and B defined in equations(2)and(3)as:
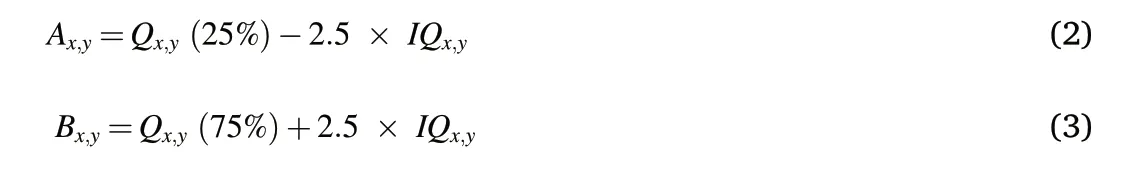
where Ax,yand Bx,yare the criteria below and above which concentrations of element x are considered abnormally low or high in soil y;Qx,y(25%)is the 25%quantile of element x in soil y;Qx,y(75%)is the 75%quantile of element x in soil y;IQx,yis the interquantile of element x in soil y.
No organic layer data and no 2019 mineral soil data were removed.
2.5. Pool size calculation methodology
As described previously, fnie earth bulk density was measured in 1974 and 2001 but not in 2019. AAn average of fine earth density measured in 1974 was calculated for each sampled soil layer.
A statistical test (ANOVA) on bulk density was carried out and no significant difference between the plots was observed in 2001 (Tukey test;p-value >0.05).We therefore assumed that fine earth bulk density was constant between 2001 and 2019. The average fine earth bulk density was calculated for each stand and each sampled soil layer from the 2001 data.The pools of nutrients in the soil in 2001 and 2019 were calculated from the averaged stand fine earth bulk density.
For comparison, stocks in equivalent soil layers were calculated for the 1974 soil profile to match those of 2001 and 2019.
2.6. Multi-criteria approach
Be interested in only chemical parameters is not enough to judge the tree species effect. A multi-criteria approach was established to better compare the tree species at the ecosystem scale by including soil chemical fertility criteria as well as other ecosystem compartments and functions.For this approach,the following criteria were considered:
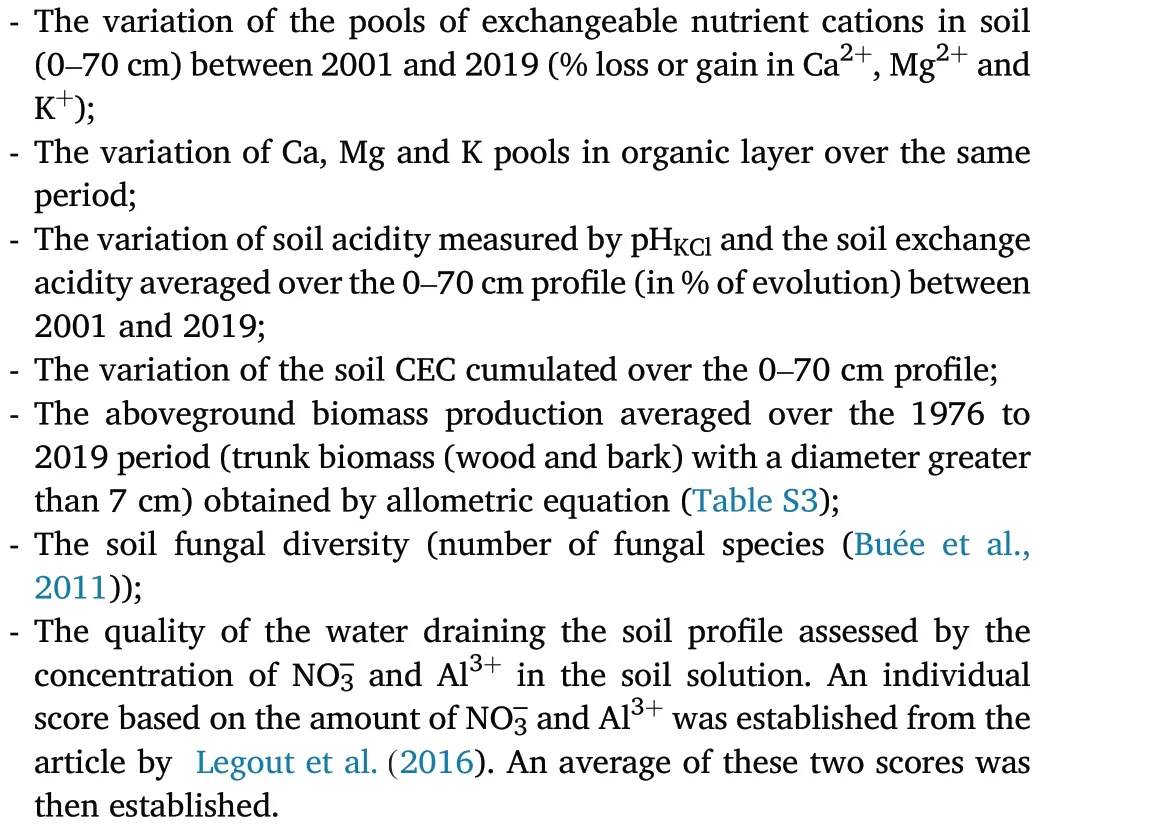
For comparison,each criteria was translated into a score ranging from 1 to 7,7 corresponding to the“best observed condition”for each criteria(Table S4).
2.7. Statistical tests
In order to test differences between tree species stands for each depth and for both dates (2001 and 2019), an ANOVA (where normality and equality of variances have been checked beforehand) and a Tukey test(test post hoc by peers)were performed with a 5%level of significance to test differences between tree species. The differences between both sampling dates for each stand were tested with a Student test when the data followed a normal distribution(Shapiro test)with a significance of p-value <0.05 or 0.1 or with a Wilcoxon test when the data failed the normal distribution test. Though the design of this experiment did not include replicated blocks (simple pseudoreplication according to Hurlbert(2004)), the comparison of the different tree species plots is nevertheless valuable to understand tree species effects on soil chemical fertility(Davies and Gray,2015).Moreover,a detailed study of the Breuil site showed that the soil under these different tree species is comparable(Ranger et al., 2004). What is more, each individual tree species plot of this common garden experiment covers a large surface(1,000 m2)given them a strong spatial representativeness in contrast to a “classic block experiment”where the plots may be much smaller.
Correlation between different data series were tested with Pearson(data followed a normal distribution) or Kendall (data not followed a normal distribution)tests with a significance of p-value <0.05.
Monte-Carlo simulations were carried out to estimate uncertainties associated with the differences in soil chemical properties or nutrient pools between 2019 and 2001(noted Δ2019-2001).For each variable,an average value was calculated for both 2001 and 2019 by randomly selecting values within a normal distribution defined from the measured average and stand deviation of the variable in 2001 and 2019.The Δ2019-2001value was then calculated. This calculation procedure was repeated to generate a population(n=1,000)of Δ2019-2001values and the standard deviation of this population was calculated. Two Δ2019-2001values were considered significantly different when the difference was greater than the sum of both standard deviations(i.e.no standard deviation overlap).
3. Results
3.1. Pool size change in the mineral soil
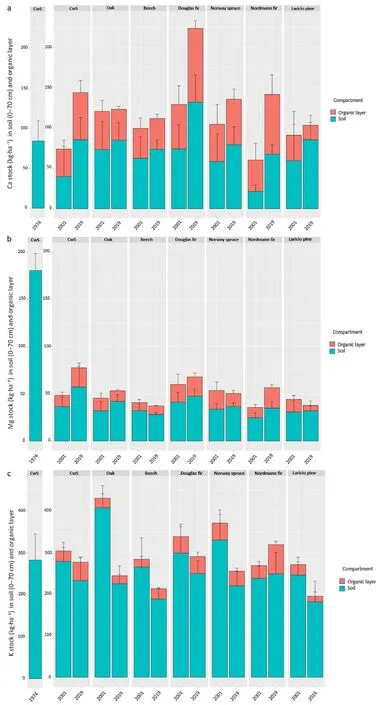
Fig. 2. Pools of calcium (a), magnesium (b) and potassium (c) in the organic layer and the 0–70 cm mineral soil (exchangeable pools) in 1974, 2001 and 2019 in the different monospecific plots of the Breuil-Chenue site.The 1974 soil samples were collected in the native coppice with standards forest prior to the site installation.
Over the 1974–2001 period,the stocks of exchangeable Mg in the soil decreased strongly in all the stands: an average net loss of 148 kg·ha-1and a maximal loss of 156 kg·ha-1in the Nordmann fir stand(Fig.2).The strongest decrease in exchangeable Ca and K pools(respectively-63 and-45 kg·ha-1)was also observed for the Nordmann fir stand.For the other stands, soil exchangeable Ca pools only slightly decreased whereas exchangeable K pools increased in the Norway spruce, Douglas fir and oak stands by respectively+47,+15 and++124 kg·ha-1.
Between 2001 and 2019, soil exchangeable Ca and Mg pools increased in all stands except for Mg in the beech stand(-4 kg·ha-1)but these trends were not all statistically significant (Table 1). Differences between tree species were small in 2001 for both Ca and Mg and only the CwS and Douglas fir plots differed from the other species. The largest losses in the soil were observed for the exchangeable K pool and the exchangeable pools of K decreased significantly for all stands except the CwS and Nordmann fir stands. For this nutrient, the largest decrease between 2001 and 2019 was recorded in the oak stand(-182 kg·ha-1).In the Nordmann fir stand,the exchangeable K pool remained stable(+4 kg·ha-1).Large differences between tree species were observed in 2001.The exchangeable K pool in 2001 was largest in the Nordmann fir and Norway spruce stands mainly due to the elevated exchangeable K pools in the topsoil layers (0–5 cm). No significant differences between tree species were observed in 2019.
The nitrogen pool in the 0–40 cm of the soil profile increased between 2001 and 2019 in all stands(Table S1)mainly in the 0–15 cm soil layer.The C:N ratio in the 0–40 cm layer decreased over the same period for all stands except for the Nordmann fir(in surface)and the beech for which it increased(Table S1).
3.2. Pool size change in the organic layer between 2001 and 2019
In the organic layer, the variations of nutrient pool sizes were very variable depending on the nutrient and the tree species.The pools of Ca,Mg and K increased in the CwS, Douglas and Nordmann fir stands but decreased in the Laricio pine and oak stands.The largest loss of nutrients was observed in the Laricio pine stand with -13.6 kg·ha-1of Ca, -7.3 kg·ha-1of Mg and -11.5 kg·ha-1of K. The Ca pool remained stable in the beech stand. In the Norway spruce stand, the Ca pool increased but the Mg and K pools decreased. Differences between tree species were more significant in 2019 compared to 2001.
The change in nutrient pool sizes in the organic layer is partially explained by the decrease in organic layer dry weight observed in all stands except for the Nordmann fir and CwS stands in which it increased.The decrease in organic layer dry weight in the Douglas fir stand was smaller than in the other stands and was not statistically significant(Table S5). Differences between tree species were larger in 2019 compared to 2001 and more pronounced in the organic layer compared to the mineral soil.
3.3. Soil chemical property change over time
Even though few differences (between tree species and between sampling dates)were significant at the soil layer scale(Figs.3–6),similar behaviors were observed. The oak and the beech showed similar behaviors:the pHKClin these two stands increased over time and over the entire soil profile while the exchangeable acidity (EA), the sum (S) of nutrient cations (Ca + Mg + K) and the CEC decreased (Figs. 3–6). The Nordmann fir, Norway spruce and CwS stands also showed similar behaviors:the CEC,EA and S increased in the topsoil layers and decreased or remained stable in the deeper soil layers.The behavior of the Douglas fir and Laricio pine stands was intermediary compared to the Nordmann fir/Norway spruce/CwS and oak/beech.The EA and CEC in the Douglas fir and Laricio pine stands increased in the topsoil layers and soil pHKCldecreased but less remarkably than in the Nordmann fir,Norway spruce and CwS stands (Fig. 3). The general behavior of the Douglas fir was closer to that of the Nordmann fir, Norway spruce and CwS stands whereas the behavior of the Laricio pine was closer to that of the oak and beech stands (the Laricio pine stand differed from the oak and beech stands only in the 0–5 cm soil layer).
Soil carbon content increased significantly in the 0–10 cm for all tree species except for the oak and beech stands for which the increase was not significant. The increase in carbon occurs mainly in the 0–5 cm horizons and weaker over 5–10 cm(Fig.7).
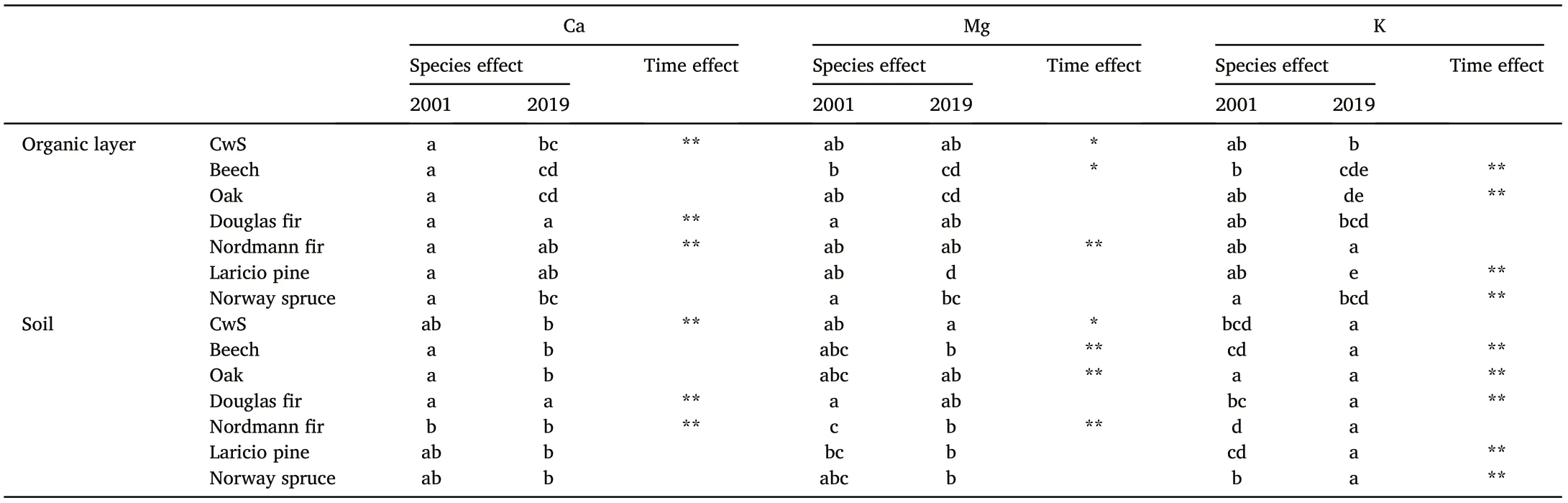
Table 1 Statistical significance(95%confidence)of differences between tree species in 2001 and 2019(letters)and between the two sampling dates(asterisks;*:p-value <0.1,**: p-value <0.05) for Ca, Mg and K stocks in the soil (exchangeable pools)and organic layer.

Fig. 3. Soil pHKCl for the different tree species and sampled soil layers between 2001 and 2019. Different letters indicate statistical significant (95% confidence)differences between tree species in 2001 and 2019 and asterisk indicate differences between 2001 and 2019 (*: p-value <0.1; **: p-value <0.05).
3.4. Correlations between different soil chemical properties
In the soil layers down to 15 cm,the variations of S and CEC between 2001 and 2019 were positively related to the variations of soil carbon content(Fig.8e).
The relation between the variations of S(ΔS)and the variations of soil pHKCl(ΔpHKCl) (p-value <0.05) (Fig. 8d) shows that, in the deeper soil layers, when pHKClincreases S slightly increases but that S is stable or decreased in the 0–15 cm soil layers for the beech and oak stands despite an increase in pHKCl. The variations in exchange acidity (EA) was positively related to the variations of S with a correlation coefficient of 0.71(p-value <0.05)(Fig.8a)but this relation was mainly supported by the variations in the 0–15 cm soil layers of the Norway spruce, Douglas fir,Nordmann fir and CwS stands.EA decreased for all soil layers below 15 cm depth in all the stands and in the 0–15 cm layers in the beech and oak stands.As EA decreased,S remained stable in the deeper soil layers of the coniferous stands and decreased in the topsoil layers in the beech and oak stands. The variations of CEC were negatively correlated to the variations of soil pHKCl(p-value <0.001), positively correlated to the variations of exchangeable Al (p-value <0.001) (Figs. 8c and 9d), and negatively correlated to the variations in Tamura-extractible Al content for the topsoil layers but not for the deeper soil layers(Fig.8b).
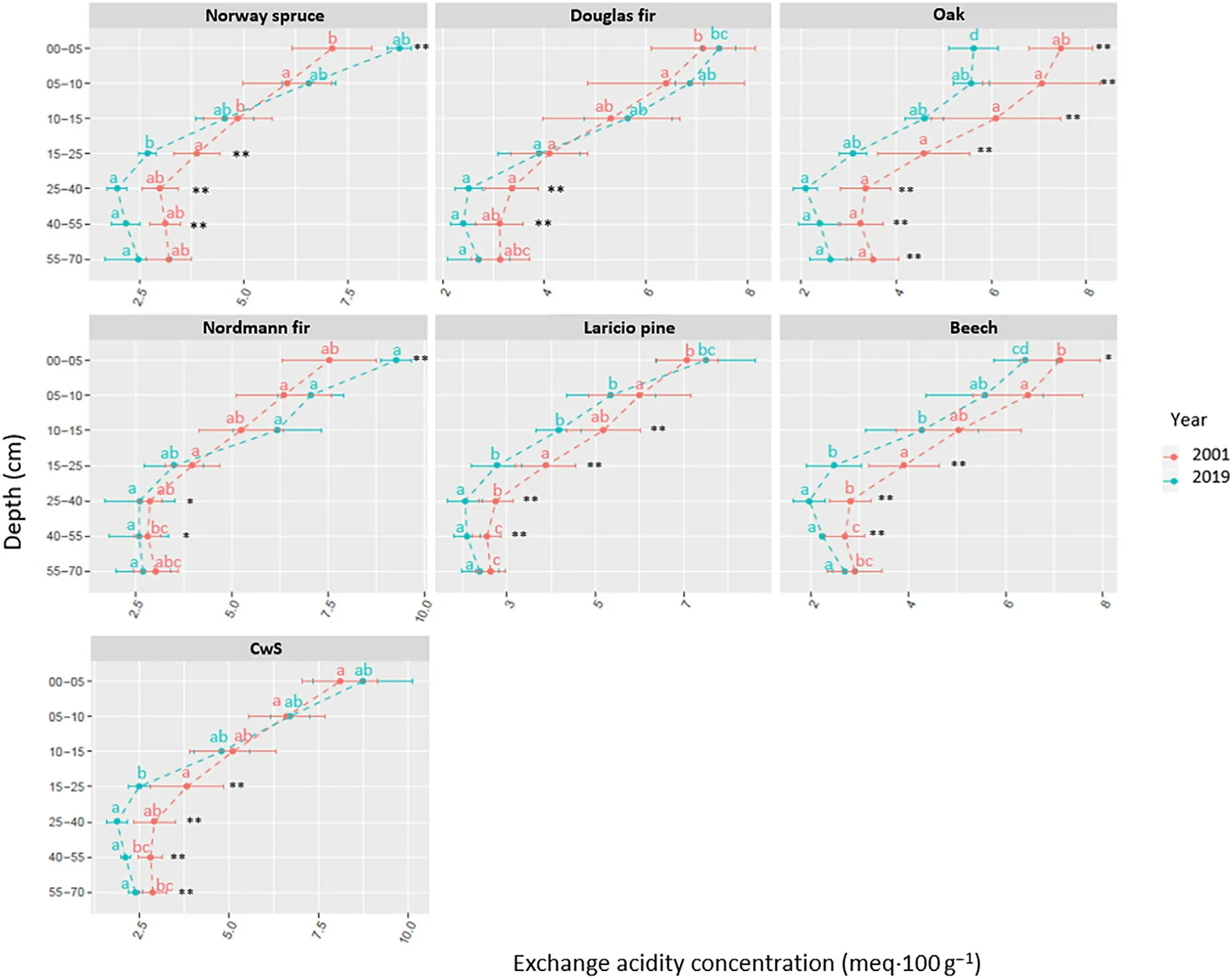
Fig.4. Exchange acidity of soil(EA)for the different tree species and sampled soil layers between 2001 and 2019.Different letters indicate statistical significant(95%confidence) differences between tree species in 2001 and 2019 and asterisk indicate differences between 2001 and 2019 (*: p-value <0.1; **: p-value <0.05).
Observing the exchangeable cations individually(Fig.9)showed that as the variations in exchangeable K were strongly correlated to the variations of the CEC (correlation coefficient of 0.75, p-value <0.05)whereas exchangeable Mg and Ca remained relatively stable despite the CEC decreased (except for the exchangeable Ca in the beech and oak stands).The oak and beech stands differed from the general trend in the topsoil layers (0–15 cm)for both exchangeable Ca and Al.
3.5. Multi-criteria approach
For most criteria, the CwS plot was among the best graded plots:fungal biodiversity, chemical quality of draining water and temporal change in plant-available nutrient cations (Ca, Mg and K). This suggests that a certain balance between the different ecosystem functions has taken place in this stand. However, the CwS plot had poor grades for the criteria assessing the change over time of soil CEC, pHKCland exchangeable acidity (Fig. 10). The oak and beech plots had the best grades for the criteria assessing the change over time of soil pH and exchangeable acidity in the 0–70 cm soil profile. The coniferous stands had the highest grades for the criteria assessing the change over time of soil CEC. The Douglas fir and to a lesser extent the Norway spruce and Laricio pine stands displayed a much higher biomass production compared to the beech and oak stands. However, the Douglas fir and Laricio pine stands had the lowest grades for the criteria assessing the chemical quality of draining waters and the fungal biodiversity(Fig. 10).
4. Discussion
4.1. Contrasting signs of soil recovery from past acidification
4.1.1. Chemical fertility change in the mineral soil between 1974 and 2001
It is difficult to decisively conclude on the exchangeable Ca,Mg and K pool size change between 1974 and 2001 because the soil sampling and analytical methods are never strictly comparable. However, the very small differences observed between both dates for the exchangeable Ca and K pools strongly suggest that the reservoirs of these nutrients remained stable. In the same time, exchangeable Mg pools decreased very strongly(Fig.2).As discussed in a previous study(van der Heijden et al.,2013),this net loss of Mg is most likely explained by elevated Mg leaching in the first years following the clear cut in 1975 when the site was setup caused by a rapid increase in the organic matter decomposition after the opening of the canopy (Vitousek et al., 1979; Dahlgren and Driscoll, 1994; Legout et al., 2009). Ca was probably better retained in the soil profile due to its greater affinity for organic cation exchange and adsorption sites compared to Mg and K (van der Heijden et al., 2014).The different behaviors could also be explained by the different relative abundances of Mg,Ca and K on the CEC in 1974.Mg occupied a greater proportion of the CEC in 1974 compared to Ca and K and may thus have been more easily available. The exchangeable Mg/Ca and Mg/K ratios averaged over the 0–70 cm soil profile were respectively 2.1 and 1.6 in 1974(Table S6).
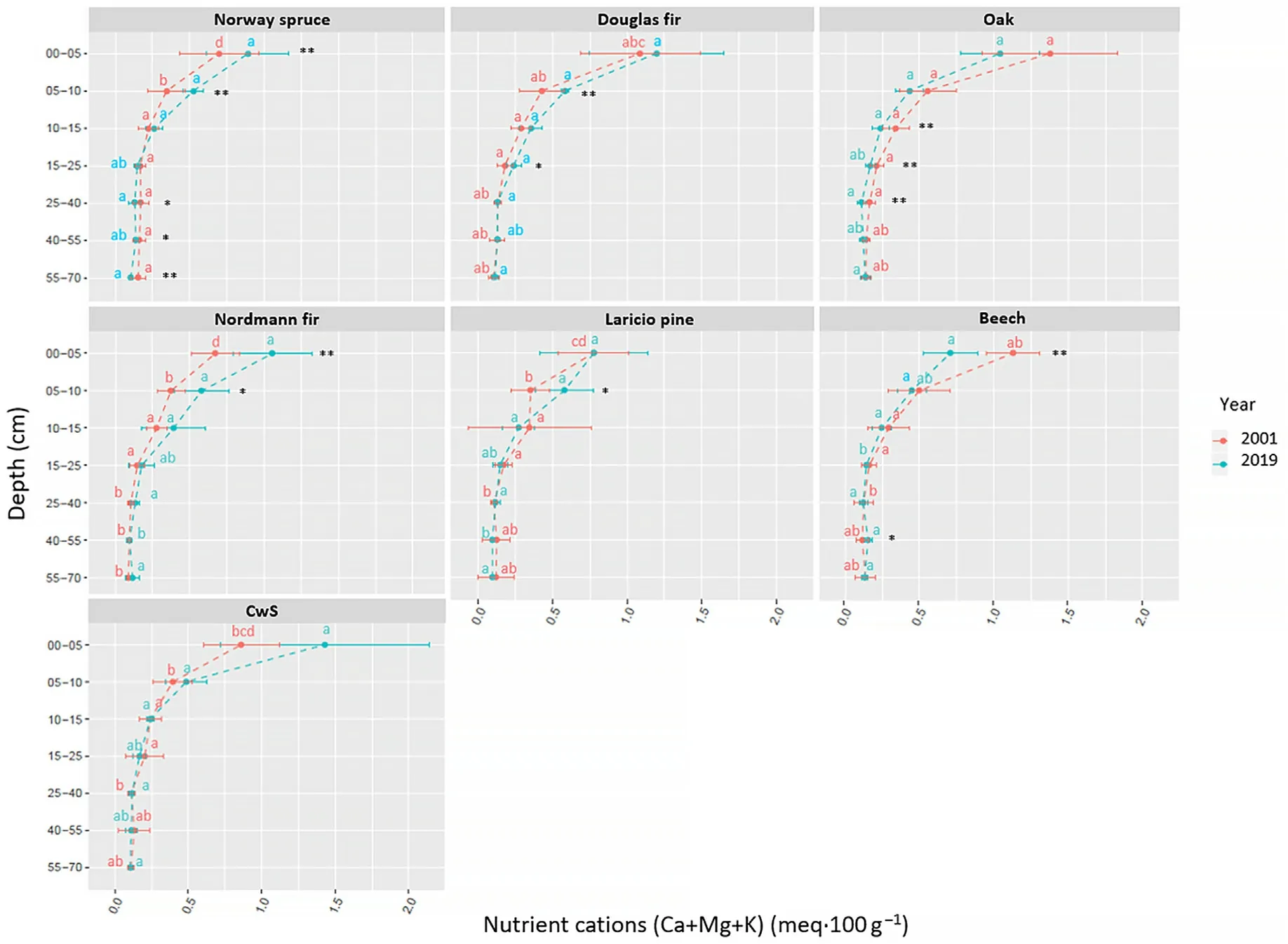
Fig.5. Sum of nutrient cations in soil(S)for the different tree species and sampled soil layers between 2001 and 2019.Different letters indicate statistical significant(95%confidence)differences between tree species in 2001 and 2019 and asterisk indicate differences between 2001 and 2019(*:p-value <0.1;**:p-value <0.05).
One might have also expected the exchangeable K pools to decrease between 1974 and 2001 but they remained stable and in certain stands slightly increased. It is possible that the K weathering flux over the 1974–2001 period may have compensated for net losses in the water drainage flux. Indeed, in the beech plot, the K-mineral weathering flux estimated for the 2002–2008 period was 6 fold greater than the Mg weathering flux (respectively 2.9 and 0.5 kg·ha-1) (van der Heijden et al., 2013). The amount of acid atmospheric deposition during the 1974–2001 period was also probably greater than over 2002–2008 which would probably have enhanced the mineral weathering in the soil.Finally,specific K exchange sites in the interfoliar space of phyllosilicates may also have contributed to better retaining K in the soil compared to Mg(Poonia and Niederbudde,1990).
4.1.2. Chemical fertility change in the mineral soil between 2001 and 2019
The change in a certain number of soil properties between 2001 and 2019 suggests that the soil chemical fertility is slowly recovering from past acidification. Indeed for most stands, the exchangeable Ca and Mg pools in the 0–70 cm soil profile increased (even if not always significantly) (Fig. 2) and the soil pH (pHH2Oand pHKCl) increased in the soil layers deeper than 15 cm for all stands(Fig. 3 and Table S1). However,many other soil properties indicated an on-going acidification and a degradation of soil chemical fertility. Indeed, except for the oak and beech stands, the soil pH decreased in the topsoil layers (Fig. 3 and Table S1).Moreover,the exchangeable K pools in the 0–70 cm soil profile decrease in all stands except for the Nordmann fir stand(Fig.2).Finally,the cationic exchange capacity in the 0–70 cm soil profile decreased for all stands(Table S1)despite the observed increase in CEC in the 0–15 cm soil layers in the Douglas fir,Norway spruce and Nordmann fir stands.Despite the fact that these changes in nutrient pools occurred over 45 years,the tree species effect and the induced changes may be qualified as rapid because 45 years represents at the most (for the fastest growing species) only a little over half the life span of a forest stand. In many cases, the impact of forest disturbance on exchangeable nutrient pools and soil chemistry may not be quantifiable over periods less than a century(Richardson et al.,2017).
These results illustrated the necessity to study and compare different soil indicators to correctly assess the evolution of soil chemical fertility and also showed that tree species effect must be taken into account.Even if compensation may occur at the scale of the soil profile(0–70 cm),tree species effect is more pronounced in the topsoil layers,as pointed out by other studies(Augusto et al.,2002).
Soil cationic exchange capacity change: The change in CEC likely played a major role in the temporal changes observed in the different tree species plots. Several causes may have led to these CEC changes in the different stand and soil layers. These changes cannot be explained by a change in the contribution of CEC variable charges (activation or deactivation of exchange sites) because the variations in CEC were negatively correlated to the variations in soil pH(Fig.8c).The dynamics of the CEC may be,at least partially,explained by the dynamics of the soil carbon pool(Fig. 8e). Indeed, a large proportion of the total CEC originates from organic cationic exchange sites (Mareschal et al., 2010; van der Heijden et al., 2014), and for the coniferous (Norway spruce, Nordmann fir,Douglas fir) and CwS stands, the increase in soil CEC was related to the increase in carbon content in the 0–15 cm soil layers (Fig. 8e). The dynamics of carbon cannot, however, account alone for the observed CEC changes because i)the soil carbon content increased in the topsoil but not or very little in depth(no significant differences)(Figs.7 and 8e),ii)for the beech and oak stands,the carbon content increased in the topsoil but the CEC decreased (Figs. 6, 7 and 8e), and iii) although the CEC and carbon content were positively correlated in 2001 (Mareschal et al., 2010) and 2019,the slope of the relation was different in 2019(data not shown).
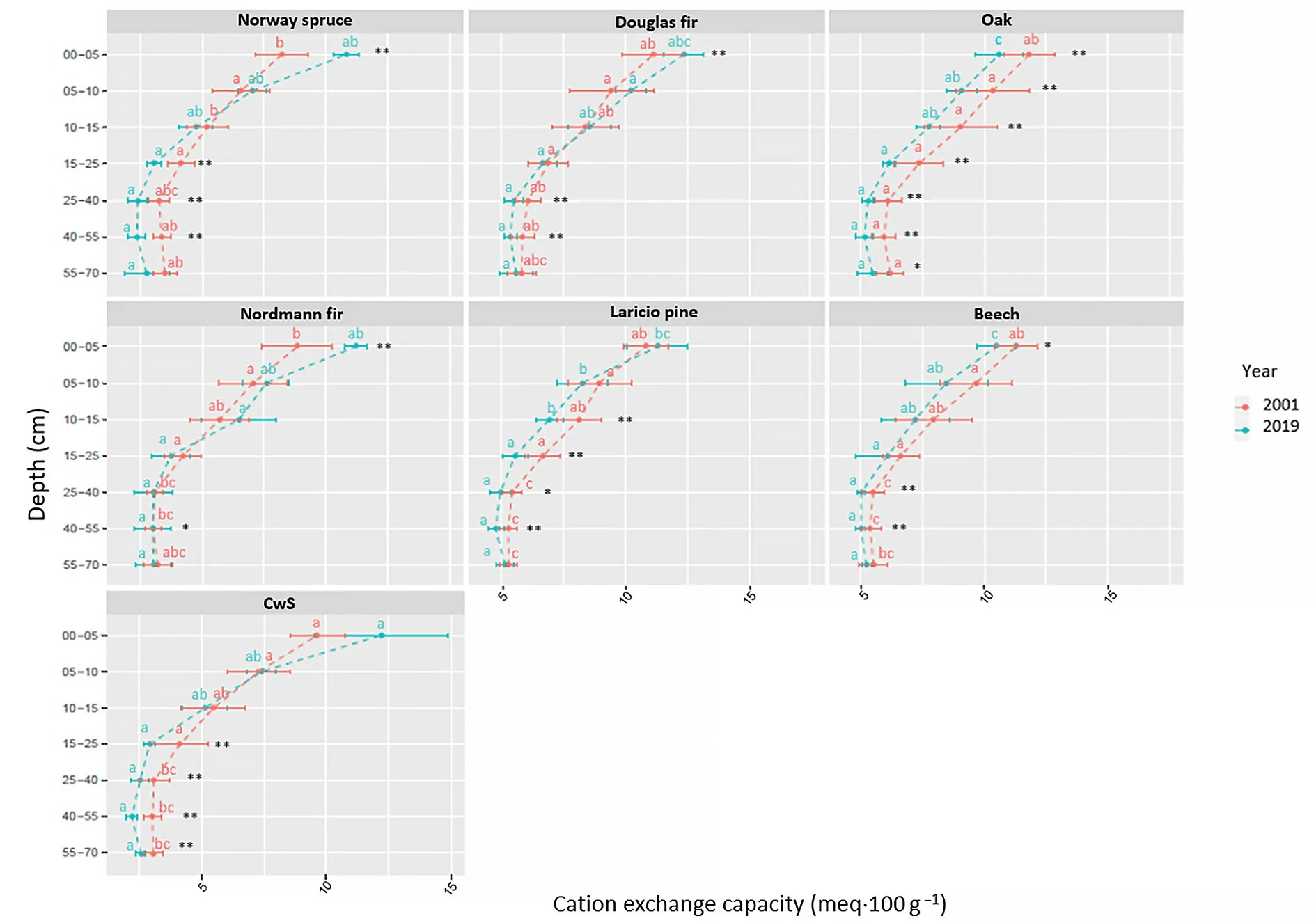
Fig. 6. Soil CEC for the different tree species and sampled soil layers between 2001 and 2019. Different letters indicate statistical significant (95% confidence)differences between tree species in 2001 and 2019 and asterisk indicate differences between 2001 and 2019 (*: p-value <0.1; **: p-value <0.05).
Mareschal et al. (2013) demonstrated a strong relation between the CEC and the precipitation/dissolution of aluminium hydroxides in the interfoliar space of phyllosilicates at the Breuil site: the precipitation of Al hydroxides blocks the accessibility of cationic exchange sites thus reducing the CEC. These mechanisms probably greatly contribute to explaining the observed dynamics of the CEC between 2001 and 2019 in all the stands.The negative correlation between the CEC variations and the variations of Tamura extracted Al content(Fig.8b)and the negative relationships between Tamura extracted Al and the CEC(Fig.11)in 2001 and 2019 support this hypothesis.
This hypothesis enables to explain the surprising negative correlation found between the variation of soil pH and the variation of CEC(Fig.8c):as the pH increases,Al solubility decreases causing the precipitation of Al hydroxides in the phyllosilicate interfoliar space as suggested by Mareschal et al. (2013); conversely, as the pH decreases, Al solubility increases and favoring the dissolution of interfoliar Al hydroxides.
Consequences for the nutrient cations: The dissolution and precipitation of interfoliar Al hydroxides may impact more severely the exchangeable K pools because the freed or blocked sites by interfoliar Al hydroxides are likely to be more frequently specific K exchange site(interfoliar sites)(Mareschal et al.,2009).Our results showed a positive correlation between the variations of the CEC and exchangeable K pools(Fig. 9c and Table S1) but no relation between on the one hand the variations of the CEC and on the other hand the variations of the exchangeable Mg and Ca pools. This suggests that the precipitation of interfoliar Al hydroxides during the 2001–2019 period caused a net desorption flux of K from the interfoliar space of phyllosilicates and would explain the decrease in exchangeable K pools over this period for almost all studied tree species. The dynamics of Al are most likely the main driver of the variations of exchangeable nutrient cations in the soil of the different tree species over the 2001–2019 period.
Exchangeable Mg and Ca pools were less affected by CEC variations than exchangeable K pools except in the topsoil of the oak and beech stands(Figs.9a and b).This could be explained by the fact that potassium is mainly adsorbed on the mineral fraction of the CEC (Poonia and Niederbudde, 1990; van der Heijden et al., 2014), notably on exchange sites specific to K as previously discussed. Mg and Ca are adsorbed on both the mineral and organic fraction of the CEC. What is more, the exchangeable Ca pool variations in the 0–70 cm soil profile were correlated to the variations of the Ca pool in the organic layer(data not shown)but no such relation was found for Mg and K.This relation suggests that Ca accumulated in the soil through the biological cycle and was retained more strongly in the mineral soil compared to Mg and K due to Ca higher affinity for organic cationic exchange sites(DeSutter et al.,2006;van der Heijden et al.,2014).The different behavior of K compared to Mg and Ca could also be due to the smaller exchangeable Ca and Mg pools in 2001 compared to K (the average pools for all tree species were respectively 54,33 and 293 kg·ha-1).The lower availability of Mg and Ca compared to K may have led to a relative higher leaching flux of K.
The behavior of exchangeable Ca and Al in the beech and oak stands differed slightly form the other stands: the decrease in soil CEC in the topsoil layers seemed to impact more strongly exchangeable Ca and less strongly exchangeable Al pools(Fig.9a).Despite the soil pH increase,Al remained very competitive with other basic cations under beech and oak in the topsoil layers. Since aluminum has a strong affinity for organic matter and remained abundant in these two stands, Al may have substituted Ca adsorbed on organic exchange sites,but the origin of this pattern which seems specific to the hardwood stands remains unclear.
4.1.3. Pool size change in the organic layer between 2001 and 2019

Fig.7. Soil carbon content(g·kg-1)for the different tree species and sampled soil layers between 2001 and 2019.Different letters indicate statistical significant(95%confidence) differences between tree species in 2001 and 2019 and asterisk indicate differences between 2001 and 2019 (*: p-value <0.1; **: p-value <0.05).
The organic layer dry weight decreased between 2001 and 2019 for all stands except for the CwS and Nordmann fir stands (Table S5) most probably because of the successive thinnings.Indeed,between 1976 and 2001,the densely planted stands were not thinned.At the plantation,the density was 1,600 tree·ha-1for coniferous and 15,000 tree·ha-1for oak and beech. The opening of the canopy due to the thinnings carried out between 2001 and 2019 most likely increased the decomposition of the organic layer that had accumulated over the 1976–2001 period,as suggested by previous studies(Vitousek et al.,1979;Dahlgren and Driscoll,1994;Legout et al.,2009).In the CwS stand,the increase in organic layer dry weight is in agreement with the accumulation of organic matter as the stand matures and may also be explained by the fact that no thinning or harvesting operations were carried between 1976 and 2019. In the Nordmann fir stand,the increase in organic layer dry weight may partly be due to an important production of fruit recorded in 2017. However,for both the CwS and Nordmann fir stands,an increase in carbon content in the topsoil layers was also recorded (Fig. 7) which suggests that the increase in organic layer dry weight is due to a slower organic matter decomposition. The strong increase in Ca stored in the organic layer of the Douglas fir stand can be explained by the biological cycle in this stand: the Ca concentration in the litterfall was higher in this stand compared to all others (data not shown). The mineral soil often represents the largest proportion of available nutrients in forest ecosystems and is often the only compartment taken into account in fertility budget studies. However, the organic layer cannot be neglected because it directly contributes to the replenishment of the plant-available pools in the mineral soil and represents in many cases an important pool of nutrients(Jonard et al.,2009;Prescott et al.,2000).
4.2. Tree species effect on soil chemical fertility
While the tree species effect on soil chemical fertility and its change over time varied between the different tree species, similar behaviours were identified which led us to define three groups:i)Norway spruce and Nordmann fir, ii) Douglas fir and Laricio pine, and iii) oak and beech(Fig. 12). Several studies have focused on the effect of tree species on pedogenetic processes at the Breuil site (Mareschal,2008;Legout et al.,2016)and we discuss hereafter the consistency between these processes and our observations.
4.2.1. Norway spruce, Nordmann fir
Organic acids are released during the decomposition of organic matter contributes to the acidification of soils (Dijkstra et al., 2001). In the Nordmann fir stands, crypto podzolisation (i.e. acidolysis and chelation) seem predominant among the pedogenetic processes (Legout et al.,2016).Indeed,nitrate leaching is very limited in these stands and the vertical transfer of Al in the soil profile is mainly ensured by dissolved organic compounds, as suggested by the predominance of organically chelated Al species in the soil solutions (Mareschal, 2008; Legout et al.,2016).This is also the case under Norway spruce,but nitrate leaching is far from negligible(Legout et al.,2016),which reinforces the acidolysis process. Our results agree with this view and the important role of dissolved organic carbon and chelation processes in these stands. Indeed,the increase in soil carbon content between 2001 and 2019 suggests a slower and less complete decomposition of organic matter which is likely to release organic acids. The pHKClaverage over the entire soil profile decreased for all two stands and this decrease is linked in the topsoil to the increase in carbon content(data not shown).The increase in carbon content also increased the number of negative charge balanced by H+and more protons could be exchanged with the solution,thus decreasing the soil pH value. Lower soil pH may have promote the dissolution of interfoliar Al hydroxides thus increasing the CEC in the topsoil; this increase may have partly compensated for the loss of nutrient cations.Acidolysis and chelation occurring in the topsoil layers in these stands did not result in the depletion of nutrient cations from the CEC over time and our hypothesis of moderate nutrient cation depletion for these stands was not verified.
4.2.2. Laricio pine and Douglas fir

Fig. 8. Relationships between the variations, noted Δ, (between 2001 and 2019) of different soil chemical properties for the different tree species and sampled soil layers. a) ΔS = f(ΔEA), b) ΔAl Tamura = f(ΔCEC), c) ΔCEC = f(ΔpHKCl), d) ΔS = f(ΔpHKCl), e) ΔCEC = f(ΔC).
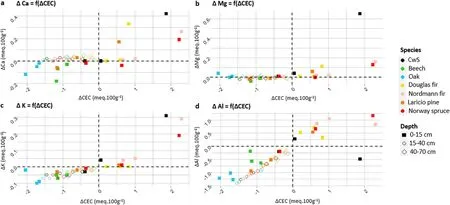
Fig. 9. Relationship between the variations of the CEC and exchangeable cations for the different tree species and sampled soil layers.a)ΔCa=f(ΔCEC),b)ΔMg=f(ΔCEC), c) ΔK = f(ΔCEC), d) ΔAl = f(ΔCEC).

Fig. 10. Comparison of multiple criteria each assessing different functions of the ecosystem for the different tree species.Each criteria corresponds to a grade between 1 (center of the diagram) and 7 (outer ring) and corresponds to a relative classification of the seven tree species, 7 being the best grade.
In these stands, the excess of nitrate production (nitrification) over consumption is the cause of the higher acidity and the higher Al concentrations with a predominant Al3+species in the soil solution as well as the higher leaching fluxes of Mg,Ca and K compared to the other stands(Mareschal, 2008; Legout et al., 2016). In these stands,acidolysis is the main pedogenetic process(Legout et al.,2016).The C:N ratio decreased in the entire soil profile of the Douglas fir stand between 2001 and 2019 which concurs with elevated nitrification and elevated nitrate leaching.However, our initial hypothesis that nutrient depletion is more intense under these two stands is only partly validated. The depletion of exchangeable K pools was not greater than in the other stands. Soil pH decreased in the topsoil but not as strongly as in the Norway spruce and Nordmann fir stands(Fig.12).What is more,the exchangeable pools of Mg and Ca increased in the Laricio pine and Douglas fir stands between 2001 and 2019 and the increase in exchangeable Ca pools in the Douglas fir stand was the greatest of all stands, probably in relation with biological cycling and the high Ca concentration in the litterfall under Douglas fir (data not shown). The strong on-going acidification evidenced by the monitoring of soil solutions(Legout et al.,2016)was not obvious for the solid phase of the soil when focusing on indicators such as soil pH, exchangeable acidity and exchangeable nutrient cation pools.This discrepancy between the solid and liquid phase may be caused by i)higher mineral weathering in these stands (in relation to the acidity produced by nitrification), ii) higher inputs of nutrient cations by atmospheric deposition and/or iii) the CEC increase. In the Douglas fir stand,Legout et al.(2016)showed that atmospheric deposition of Ca,Mg and K were higher compared to the other stands. In the Laricio pine stand,atmospheric deposition levels were similar to the other stands but it is likely that the Ca, Mg and K losses in the leaching flux were compensated by the vertical transfer of Ca, Mg and K from the organic layer to the mineral soil.
4.2.3. Oak and beech
The two broadleaved stands show signs of recovery from past acidification over the 2001–2019 period. The soil pH increased strongly (pvalue <0.05)in the 0–10 cm and over the entire soil profile but the CEC decreased over the entire soil profile as well as the nutrient cation pools.This significant behavior stands out from other coniferous species(Fig. 12). The decrease in soil CEC is most likely due to both the precipitation of Al hydroxides in the phyllosilicate interlayer space (pH increase) and the better mineralization of the organic matter in these stands (the carbon content increase in the topsoil was the weakest for these two stands). For the same site, Trum et al. (2011) showed that organic matter decomposition was the fastest in the oak stand. The results support our initial hypothesis for the beech stand but the high demineralization in the oak stand hypothesis was not validated. According to Legout et al.(2016),the oak stand was the third stand with the highest excess of nitrate production but,contrarily to the Laricio pine and Douglas fir,the excess of nitrate production in the topsoil is compensated over the entire soil profile by nitrate consumption.This may contribute to explain why soil acidification in this stand was not intense despite the nitrification dynamics.
According to Mareschal (2008), the coniferous species at the Breuil-Chenue experimental site cause a higher acidification and a higher depletion of the exchangeable pools of nutrient cations in the following order: Norway spruce ≥Laricio pine = Douglas fir >oak = beech. In a general way, our study agrees with this classification but the effect of acidolysis and chelation processes in Nordmann fir stand seems to be more damaging for the solid phase than previously, with a substantial decrease of pH and an increase of exchange acidity in the topsoil layers.The observed change over time of the exchangeable pools of nutrient cations in the soil may be partly explained by differences in net immobilization in aboveground biomass (Sicard et al., 2006). However, the data from this study shows that tree species directly continuously influenced the pedogenetic processes over 43 years at this site.
4.3. Ecosystem scale multi-criteria approach
Used individually, static indicators such as soil pH, CEC, pools of exchangeable cations may be misleading and their change over time does not necessarily reflect the processes occurring in the soil or the ecosystem. For instance, an increase in soil CEC over time is generally considered to be a sign of recovery from past soil acidification: an increase in soil pH often increases the number of active cationic exchange reaction sites (variable charge component of the CEC) (Helling et al.,1964;Camberato,2001).In our study,we showed that the CEC increase under certain tree species (Douglas fir, Norway spruce, Nordmann fir,etc.) is associated with a decrease in soil pH and thus indicates a degradation of the soil for these species rather than a recovery.Yet,this CEC increase also resulted in an increase in exchangeable nutrient cations available for tree uptake and growth.This illustrates both i)the necessity to study and compare different static soil indicators in order to assess the intensity of on-going soil processes and their consequences in terms of chemical fertility and ii)the importance of assessing the consequences of these processes and changes over time on the other components and functions of the ecosystem.
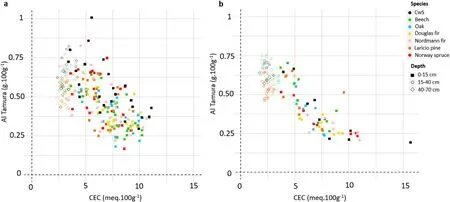
Fig. 11. Relationship between CEC and Al Tamura in 2001 (a) and 2019 (b) for the different tree species and sampled soil layers.
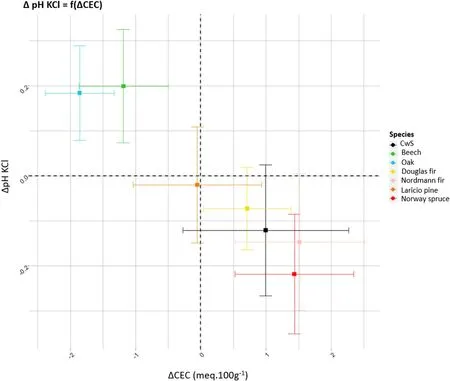
Fig.12. Relationship between the variations of the CEC and pHKCl,noted Δ(difference between the 2019 and 2001 values)in the 0–10 cm soil layer for the different tree species. The standard deviations were estimated with a Monte-Carlo simulation.
The multi-criteria approach that is proposed here aims at better assessing the functioning of the ecosystem and how tree species influence its functioning as a whole. Such multi-criteria approaches (which may encompass broader criteria than those selected in this study) coupled with the knowledge of the processes and functioning of ecosystems enable to help rational forest management (Schwaiger et al.,2019).
For this purpose, tree species effects on soil chemical fertility were compared to other ecosystem functions reported in previous studies carried out at the Breuil-Chenue experimental site such as wood/biomass production, chemical quality of the draining water and soil fungal biodiversity(Fig.10).
Depending on the criteria used the interpretation of the tree species effect on the functioning of the forest ecosystem can be very different.Our results underline the importance of taking into account the capacity of the soil to sustain over the long term the different ecosystem functions.This is best illustrated by the Douglas fir case (Fig. 10) although the biomass production for this species is remarkable, other ecosystem functions are severely degraded(chemical quality of draining water and fungal diversity)and the‘cost’of this elevated production in terms of soil chemical fertility is very high.The very strong on-going acidification may currently enable to maintain sufficient amounts of plant-available nutrients in the soil for tree growth but it is highly uncertain whether this tree growth rate may be sustained over the long term(more than a forest revolution). What is more, the strong modifications induced by this species in less than 50 years questions the capacity of the soil to supply available nutrients over the long term thus ensuring all ecosystem function necessary for the growth of future stands.Our results also raise concerns for the other coniferous stands although their tree species effect is often not as strong as the Douglas fir.
5. Conclusion
The increase in soil pH and in exchangeable Mg and Ca in most stands and soil layers between 2001 and 2019 may suggest that the soil at the Breuil-Chenue experimental site is recovering from past acidification.However, the decrease of pH and the increase of exchange acidity and cationic exchange capacity (CEC) in the topsoil layers under coniferous stands and the overall decrease of exchangeable K pools in most stands highlighted that soil acidification and/or crypto podzolisation are still on-going at this site. Tree species influence the pedogenetic processes occurring at the Breuil Chenue site,but our initial hypothesis that these processes would be accompanied by the depletion of exchangeable nutrient cation pools was not validated since Mg and Ca pools increased between 2001 and 2019. The changes in soil chemical properties over time were mainly explained by the changes in soil CEC which was probably due to the increase in carbon content in the topsoil and/or to the dynamics of precipitation/dissolution of Al (hydr)oxides in the interfoliar space of phyllosilicates. The increase in soil CEC over time may have partly compensated for the loss of nutrient cations,especially in the coniferous stands.Our results illustrate how important the choice of the adequate indicators is to characterize and quantify the changes in soil fertility over time because different indicators may lead to opposed conclusions.
Our study demonstrate that tree species strongly influence how nutrient pools in the soil change over time.Our hypotheses which were that the chemical degradation of fertility would be i)high in Douglas fir and Laricio pine, due mainly to acidolysis linked to the high rate of nitrification and leaching of nutrient cations, ii) intermediate in the Nordman fir,Norway spruce and oak due to acidolysis and chelation(in connection with the release of organic acids from the transformation of organic matter)and iii)less intense in the plot beech are not completely verified.
Based on their behavior,we were able to distinguish three groups of species:i)Nordmann fir and Norway spruce with acidolysis and chelation processes (related to the release of organic acids from organic matter transformation), resulting in the most intense changes in topsoil properties (e.g. decrease in pH, increase in exchange acidity), ii) Douglas fir and Laricio pine,where the strong acidification occurring(related to high nitrification rate and nutrient cation leaching)is probably compensated by a greater weathering and/or atmospheric depositions fluxes, and iii)Oak and beech with a lower soil acidification compared to all the other stands. However, even if signs of soil acidification recovery occurred in these deciduous stands between 2001 and 2019,the decrease in soil CEC did not allow to improve significantly the exchangeable nutrient pools over the soil profile.
Finally, our study highlights the difficulties of comparing multiple indicators and shows just how necessary multi-criteria approaches are to properly understand how the ecosystem functions.Tree species selection by the forest manager should be based on such multi-criteria approaches to properly account for the different ecosystem functions and services,opportunities and threats.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Authors’contributions
MC conceptualized, investigated, analyzed, wrote the original draft,reviewed and edited the study. AL conceptualized, investigated,analyzed,reviewed,edited,supervised the study and acquired funds.BZ reviewed, edited and supervised the study. JR provided the data resources, reviewed and edited the study. GvdH conceptualized, investigated, analyzed, reviewed, edited, supervised the study and acquired funds.
Funding
The study was funded successively by GIP ECOFOR,AllEnvi,ANAEE France, the LTSER Zone Atelier Bassin Moselle and INRAE (DISC,ECODIV).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing of interest
The authors declare that they have no competing interests.
Acknowledgements
This site belongs to French national research infrastructure ANAEEFrance (ANR-11-INBS-0001). We gratefully acknowledge the financial support received successively from GIP ECOFOR,AllEnvi,ANAEE France,the LTSER Zone Atelier Bassin Moselle and INRAE(DISC, ECODIV).We are also grateful the Parc Naturel R′egional du Morvan.We thank INRAE and Office National des For^ets for financing Margaux Clesse-s doctoral thesis. We would also like to thank l’Office National des For^ets, Dominique Gelhaye, Pascal Bonnaud, Serge Didier, Benoît Pollier and Gilles Nourrisson for their contribution to the Breuil-Chenue site management,sampling and analysis over the years. The UR-1138 INRAE – Biogeochimie des Ecosystemes Forestiers is supported by a grant overseen by the French National Research Agency (ANR) as part of the ‘‘Investissement d’Avenir’’ program (ANR-11-LABX-0002-01, Lab of Excellence ARBRE).
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://do i.org/10.1016/j.fecs.2022.100043.
- Forest Ecosystems的其它文章
- Prediction of the global potential geographical distribution of Hylurgus ligniperda using a maximum entropy model
- The 2/3 scaling of twig nitrogen to phosphorus in woody plants
- Monitoring the abundance of saproxylic red-listed species in a managed beech forest by landsat temporal metrics
- Trade-offs among fine-root phosphorus-acquisition strategies of 15 tropical woody species
- Structure complexity is the primary driver of functional diversity in the temperate forests of northeastern China
- Consistent response of nematode communities to management of coniferous plantations

