单原子掺杂二硫化钼析氢催化的进展和展望
林高鑫,王家成
(1.中国科学院上海硅酸盐研究所高性能陶瓷与超微结构国家重点实验室,上海 201899;2.中国科学院大学材料科学与光电工程中心,北京 100049)
1 Introduction
The consumption of fossil fuel involves the imbalance of carbon cycle in the environment,resulting in the global warming and sea level rise[1,2].Recently,hydrogen is considered as an alternative to fossil fuels due to its high energy density and zero carbon emission.Electrochemical water splitting is an efficient and green method for hydrogen generation[3—9].To increase cathodic hydrogen evolution reaction(HER)kinetics,there is the urgent demand of high activity electrocatalysts[10—12].Platinum(Pt)is the most efficient catalyst for HER with low onset potential and fast catalytic kinetics owing to the moderate hydrogen adsorption and desorption properties[13].However,the high cost and low durability of Pt limit its large-scale application in industrial hydrogen production[14—16].Therefore,designing earth abundant,active and stable electrocatalysts for HER is of great importance for hydrogen production.
Recently,two-dimensional molybdenum disulfide(MoS2)has been developed as an advanced HER catalyst due to its low cost and high stability[17—21].However,the HER activity of MoS2is inferior to that of noble metal Pt.Previous reports suggested the edge sites of MoS2are the main catalytic centers of HER,and the basal plane is inert to HER[22—25].In the layered MoS2,basal plane occupies the majority of surface.There⁃fore,the performance of MoS2can be dramatically improved through activating the intrinsic activity of basal plane.Numerous strategies including heterostructure constructing,defect engineering,single atom(SA)doping and phase transition have been developed to increase the active sites and intrinsic activity[26—34].Among these methods,SA doping attracts lots of attention due to the facile synthesis process and efficient activation effect.SA doping not only optimizes the hydrogen adsorption property of S atoms at basal plane and edge to increase active sites,but also can induce defect and phase transition into the basal plane to boost the HER performance[24,35].Noble metal SAs(Pt,Ru,Pd,Rh and Ag),transition metal SAs(Fe,Co,Ni,Cu,Mo,W,etc.)and non-metal SAs(O,Se,N and P)have been reported to enhance the HER catalytic activity of MoS2and some of them even show better performance than Pt catalyst.Considering the rapid progress in this field,more attention should be paid to the advanced SAs-MoS2electrocatalysts for efficient HER.
Lots of previous reports demonstrated the positive effect of SAs in MoS2for enhanced HER performanceviacombining the structural and electrochemical characterizations.However,the details of the doping methods in optimizing the HER activity are less discussed.And the summary of the role of SAs in MoS2with adjusted catalytic performance is urgent.In this perspective,we will firstly introduce the relationship between MoS2structure and HER activity,and the basic mechanism of SA doping for enhanced HER performance is discussed(Fig.1).Secondly,the leading synthesis methods and characterization techniques of isolated SAs on MoS2are summarized.Furthermore,the advanced SA-MoS2catalysts for HER are investigated.Finally,we put forward future research challenges and development of high-activity SA-MoS2for industrial hydrogen production.
Fig.1 Schematic illustration of SAs doped MoS2 with different structural configurations
2 Structure and Active Sites for MoS2
The single layer MoS2includes trigonal prismatic phase(1H)and octahedral phase(1T,1T′and 1T‴)[36,37].1H MoS2is the thermodynamically stable phase in which the Mo-S coordinates in a trigonal prismatic model with hexagonal symmetry.1H MoS2is a semiconductor with a band gap of 1.7 eV[38].The semiconductor character of 1H MoS2results in low conductivity,which is not conducive to the electrochemical reactions.Ordered stacking of 1H phase can form two bulk crystals,including hexagonal 2H phase and rhom⁃bohedral 3R phase.The 2H phase is obtained by AbA BaB stacking of 1H layer and the 3R phase is in the stacking model of AbA CaC BcB[39].1T MoS2is the metastable phase with octahedral coordination(D3dgroup).The AbC AbC stacking sequence of 1T monolayers constitute metallic 1T crystals[40].The enhanced electronic conductivity in 1T phase leads to the better catalytic ability in HER compared to the stable phase.
The classic volcanic plot of hydrogen adsorption energy and exchange current density provides an effec⁃tive method to evaluate the HER activity of catalysts by calculation[41—43].In layered MoS2,the pristine basal plane is hydrogen inert,which has no adsorption of hydrogen during HER.The Mo or S atoms at edges show a stronger hydrogen adsorption ability due to the low coordination states.Increasing the density of active sites and enhancing the intrinsic activity are two main methods to improve electrocatalytic ability[2].Therefore,the morphology engineering of decreasing the thickness and exposing more edges of MoS2is beneficial to the HER process.Besides,constructing defects(phase,S vacancy and strain)into the basal plane also leads to the optimization of intrinsic activity,and the catalytic performance can be dramatically enhanced[Fig.2(A)][32,44].SAs in MoS2can not only be served as the active sites for HER but also trigger the nearby sites by inducing phase,S vacancy and strain[Fig.2(A)and(B)].Kang[45]conducted density functional theory(DFT)to prove that transition metal SAs promote the formation of S vacancy.The binding energy between SAs(Rh,Pt,Pd and Ir)and S vacancies are more than 2 eV,indicating the highly stable state of defects.And the SAs and S vacancy are likely to make defect complexes to smooth the HER.Ma and co-workers[46]demonstrated that In or Ge SA doped MoS2(6% doping concentration)with S defect possesses high HER activity.The hydrogen adsorption energies of In-SA-MoS2and Ge-SA-MoS2are 0.020 and ‒0.004 eV,respectively,which are ever better than that of Pt.In 1T MoS2,Ni SAs can tailor the electronic structure of nearby S atoms,and thereby optimize the hydrogen bonding capacity to achieve a near zero hydrogen adsorption energy[47].Crystal orbital Hamilton populations results show the bond strength between S and H atoms in Ni-1T-MoS2is stronger than that of 2H MoS2but weaker than that of 1T MoS2.

Fig.2 Active sites(edges,1T phase,S vacancies and strain)of MoS2 for HER(A)and schematic illustration of Mo⁃SA⁃MoS2 in which Mo SAs are active sites for HER(B)[32]
3 Materials Synthesis
3.1 Hydrothermal Synthesis
Hydrothermal synthesis has been widely used to prepare SA-MoS2because of its controllable and facile features[23,48].In general,molybdenum source and sulfide source are mixed in an aqueous solution and heated in a confined space to generate MoS2.SA-MoS2can be formed in the bottom-up hydrothermal process or produced by bonding with sulfide atoms through a hydrothermal process with metal ions and MoS2.The morphology,layer thickness,electronic structure and content of SAs can be well controlled by adjusting the reaction conditions.
Xu and co-workers[49]designed a facile hydrothermal method to obtain porous Cu@MoS2nanosheets using MoO3as the molybdenum source.The hydrothermal process started with the sulfuration of MoO3into MoS3,and MoS3was subsequently reduced to the MoS2nanosheets[Fig.3(A)].Meanwhile,Cu source played an important role in balancing the thioacetamide content.A large amount of Cu source led to the excessive consumption of thioacetamide,which inhibited the following reactions.Songet al.[50]reported a one-step hydrothermal synthesis of ultrathin Pt-doped MoS2nanosheet.Ammonium molybdate tetrahydrate,thiourea and chloroplatinic acid were mixed inN,N-dimethylformamide under a certain molar ratio and heated at 180 ℃for 24 h.X-Ray diffraction(XRD)pattern showed the obtained Pt-SA-MoS2is 1T′phase with metallic nature.And it achieved high HER activity and stability in an acid electrolyte.
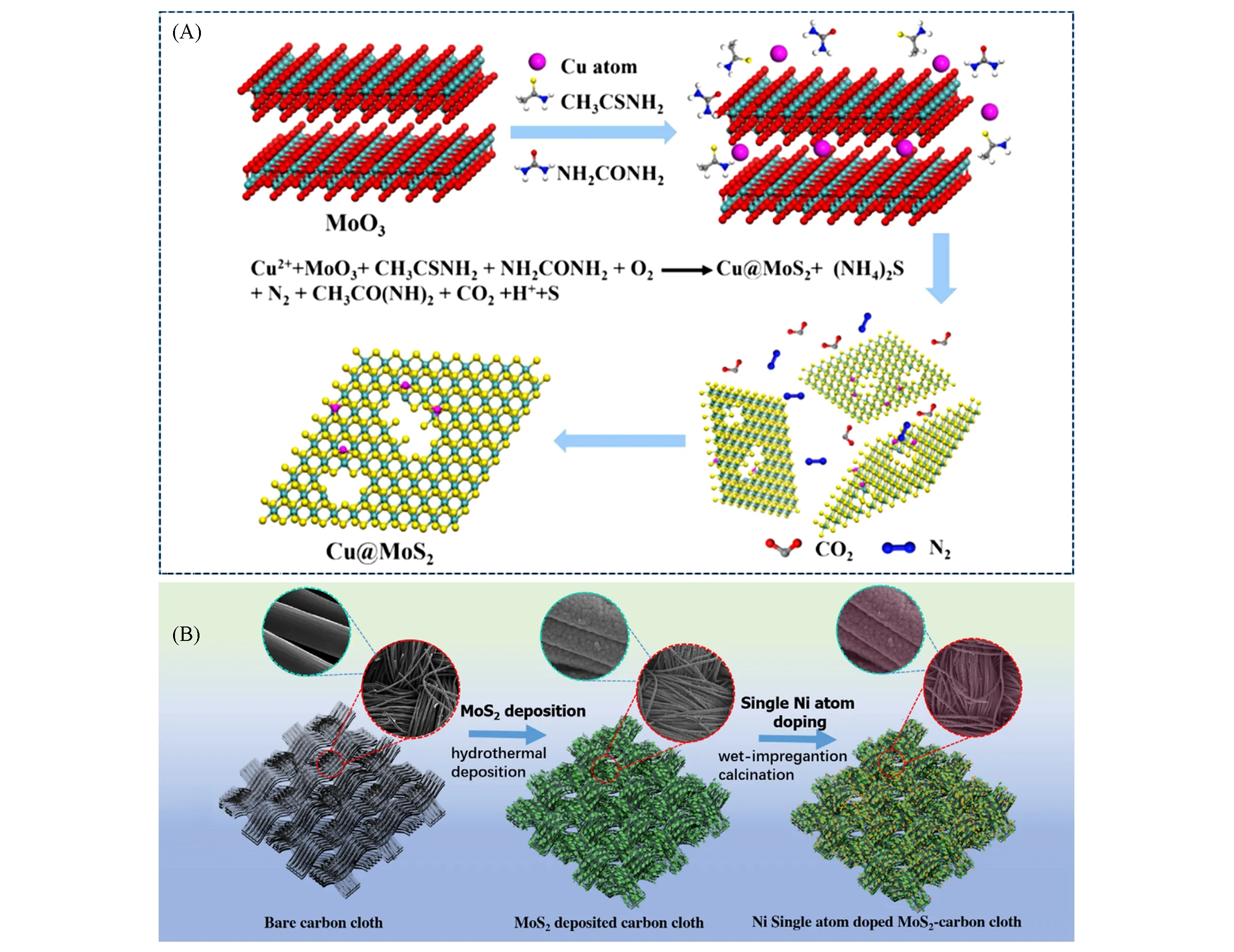
Fig.3 Schematic procedure for syntheszing Cu@MoS2(A) [49] and schematic illustration of the preparation of Ni⁃SA⁃MoS2(B)[52]
3.2 Wet-chemistry Method
Driven by the high surface energy of sulfur atoms,metal ions can spontaneously bond on the surface of MoS2and form SAs.To obtain high-quality SA MoS2,it is essential to increase the specific surface area of MoS2and prevent the agglomeration of metal ions into cluster or nanoparticles.For example,MoS2nanosheet was dispersed in the mixture of deionized water and ethanol containing Ru3+ions at first,and Ru-SA-MoS2was obtained after stirring for 1 h[51].By adjusting the concentration of Ru3+in above mixture,the Ru content in Ru-SA-MoS2ranged from 0.2%to 7.5%.Combined with high-angle annular dark field scanning transmission electron microscopy(HAADF-STEM),Raman spectra and electron spin resonance(EPR)spectra,the authors found that Ru SAs induce the S vacancies and phase transition of MoS2,which is beneficial to the HER activity.
The facile and easy operation route of wet-chemistry method makes it promising in the industrial applica⁃tion.And the immersion time can also be shortened within 1 min.Guet al.[52]prepared MoS2nanosheet array on carbon cloth by hydrothermal method at first.The precursor was immersed into NiCl2solution for only 10 s and further calcinated at 300 ˚C to obtain the Ni-doped MoS2[Fig.3(B)].And the atomic content of Ni was determined by inductively coupled plasma atomic emission spectrometer(ICP-AES)to be(1.8±0.1)%.The catalyst achieved low overpotentials of 110 and 98 mV at the current density of 10 mA/cm2in 0.5 mol/L H2SO4and 1 mol/L KOH,respectively.
3.3 Other Methods
Other methods have also been used to develop SA-MoS2with high quality and catalytic activity(Table 1).For example,Co-SA-MoS2on graphite paper was prepared by the pyrolysis of Co-Mo-S precursor[53].The in-plane S atoms were activated by neighboring Co atoms,exhibiting a high HER performance similar to Pt.Zhang and co-workers[54]developed a solar-irradiation method to synthesize Pt-SA-MoS2with a low overpoten⁃tial in HER.The heat treatment favored the formation of defect and structure distortion,which are served as sites for Pt atoms anchoring.And the final size of Pt(nanoparticle or single atom)was dependent on the concentration of Pt precursor.Besides,uranium(U)doped MoS2was fabricated by a pulse voltammetry method using radioactive wastewater as U source[55].And the mass loading of U atoms can be precisely controlled during the electrochemical synchesis process.DFT calculation suggested the synergistic effect of U atoms and adjacent S atoms in U-SA-MoS2,which boosts the HER.
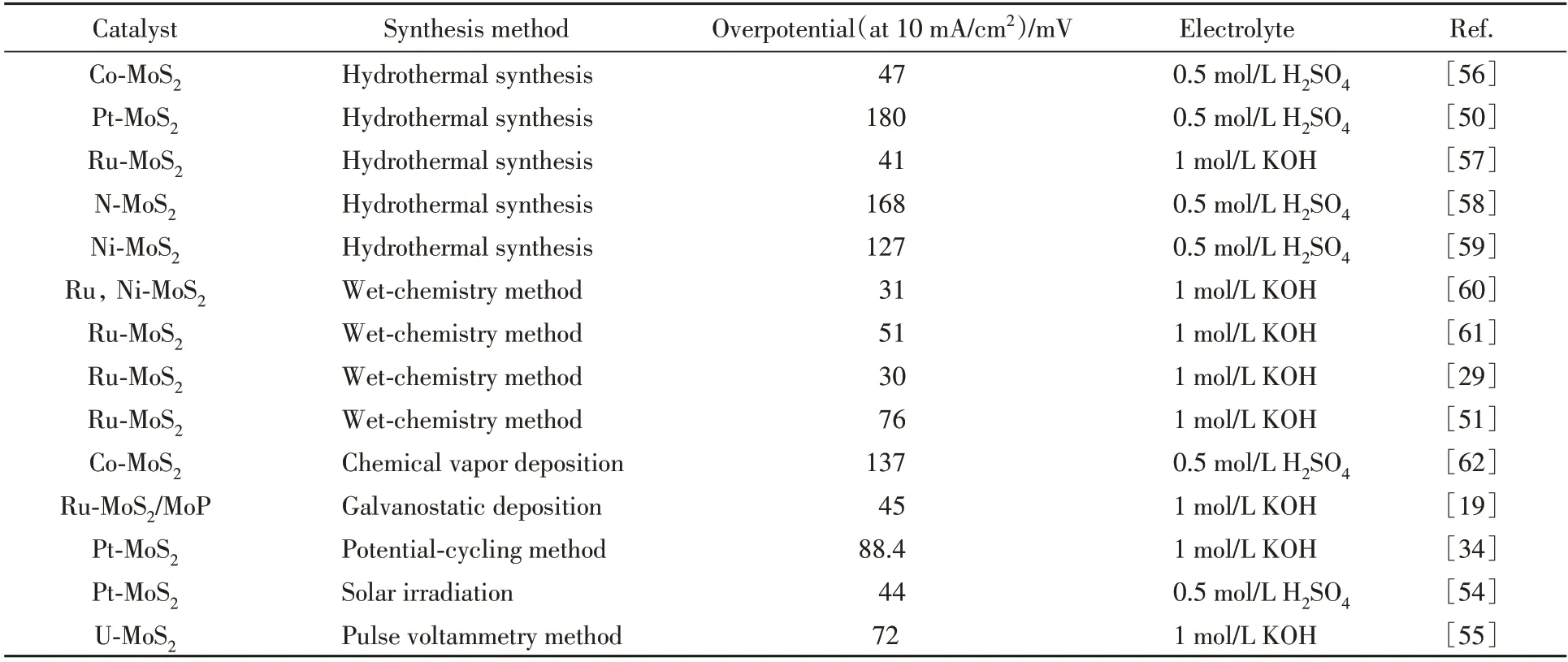
Table 1 Electrocatalytic HER performance of different SAs doped MoS2
4 Structure Characterization
The general characterizations of SA-MoS2are necessary to identifying the atomic sites.The aberrationcorrected high-resolution transmission electron microscopy is able to directly observe the SAs.The surface analysis techniques including atomic force microscopy(AFM)and scanning tunneling microscopy(STM)are capable to identify the local structure of layered SA-MoS2[24,63].X-Ray absorption spectroscopy(XPS)helps to provide coordination and oxidation states of SAs.And the spectroscopy techniques including Fourier transform infrared spectroscopy and Raman spectroscopy are also beneficial to observe the structure of SA-MoS2[64—66].Identifying the active sites in SA-MoS2is an essential precondition for exhibiting the relationship between structure and catalytic activity.
4.1 Transmission Electron Microscopy
The HAADF of transmission electron microscopy(TEM)allows the direct observation of heteroatoms with different atomic number to the substrate[67].Fig.4(A)shows the Ru and Ni atoms in MoS2[60].The atomic num⁃ber of Ni is less than that of Mo and the atomic number of Ru is greater than that of Mo.Therefore,Ru atoms are brighter and Ni atoms are darker compared to Mo atoms in the HAADF image[Fig.4(B)].Combined with intensity line profiles,the detailed atomic structure of Ni and Ru atoms is revealed[Fig.4(C)].Line 2 shows the Ru atom is in the position above the hollow site(site 2);line 4 shows the Ru atom is in the position of Mo atop sites(site 4)and line 1 and line 3 shows the Ru atoms are in the position of Ni atop sites(site 1 and site 3).Therefore,the Ru atoms in different positions were identified by HAADF images.Baoet al.[68]synthe⁃sized flower-like Pt-MoS2nanosheets by chemistry reaction method[Fig.4(D)].XRD patterns confirmed that the Pt-MoS2nanosheets do not has Pt crystal phase,suggesting no Pt nanoparticles.And sub-angstrom resolu⁃tion HAADF image showed the singe Pt atoms on the MoS2substrate[Fig.4(D)—(F)].The atomic number of Pt is greater than that of Mo,and the bright white dots in HAADF images were Pt atoms.It is obvious that Pt atoms replaced the position of Mo atoms,and dispersed on the substrate uniformly[Fig.4(G)].

Fig.4 HAADF⁃STEM image(A)and corresponding enlarged image(B)of Ru/Ni⁃MoS2,HAADF intensity line profiles taken along the appropriately numbered lines indicated in (A) and (B)(C)[60],TEM image of Pt⁃MoS2 with the inset showing a typical MoS2 layer distance of 0.62 nm(D),HAADF⁃STEM images of Pt⁃MoS2 showing that the single Pt atoms marked by red circles uniformly disperse in the 2D MoS2 plane(E),enlarged image showing a honeycomb arrangement of MoS2(F),and the single Pt atoms occupying the exact positions of the Mo atoms(marked by red arrows)(G),the k2⁃weighted EXAFS spectra(H)and the normalized Pt L3⁃edge XANES spectra(I)[68]
4.2 Scanning Tunneling Microscopy
MoS2with layered structure is suitable for the STM.As a surface analysis technique,STM can explore the position of SA at atomic-scale realm and distinguish the elements with close atomic number[63,69].Besen⁃bacher and co-workers[70]used STM to investigate the atomic structure of Co-Mo-S and Ni-Mo-S catalysts.They found that both Co and Ni atoms preferred to substitute the Mo atoms at the(−1010)edge with the S coverage of 50%.Tapasztó and co-workers[71]revealed the slow oxygen-substitution reaction on MoS2basal plane by STM.O-related defects on MoS2showed obvious contrast under STM,and the contrast was dependent on the distance between tip and sample surface.Besides,the authors found that the formed O-SA sites boost the HER activity of the entire MoS2basal plane.
4.3 X-Ray Absorption Spectroscopy
X-ray absorption near-edge structure(XANES)and extended X-ray absorption fine structure(EXAFS)in X-ray absorption spectroscopy(XAS)are able to provide chemical and coordination states of the catalysts[72,73].In particular,the adsorption edge and peak features of XANES are sensitive to the oxidation state and spatial structure of SAs.And the signal of EXAFS relates the coordination number and average bond length between SAs and nearby atoms.For examples,the structure of SA Pt on MoS2was verified by XAS[68].EXAFS spectra showed an obvious Pt-S interaction in Pt-MoS2while no Pt-Pt interaction was detected[Fig.4(H)],confirming the SA nature.And XANES spectra showed the Pt L3-edge of Pt-MoS2is higher than that of Pt foil and 40%Pt/C,suggesting the SA Pt was positively charged through the Pt-S interaction.Tanget al.[29]performed XAS to obtain the coordination state of Ru/np-MoS2.Fourier transform XAFS spectra exhibited characteristic Ru-S and Ru-Mo scattering features,indicating the Ru atoms take the position of Mo atoms.Fitting results suggested isolated Ru coordinates with four S atoms with two S vacancies.
4.4 Other Techniques
The AFM technique is also capable to identify the local structure of SA-MoS2.Zhanget al.[74]took AFM to demonstrate the Fe-SA studding increases the thicknesses of MoS2by 0.15—0.25 nm,indicating the Fe atoms are on the surface,instead of replacing the position of Mo atoms.Combined with HAADF images and EXAFS spectra,the authors revealed the Fe SAs bond with three S atoms on the basal plane.High-resolution X-ray photoelectron spectroscopy(HRXPS)can reflect the valence state information of the measured element.Doping MoS2with SAs leads to the shift of the binding energy for Mo HRXPS due to the electron transfer between SAs and substrate.Positive shift of Mo3dspectra was observed in,and negative shift was detected in
5 Progress on Hydrogen Evolution by SA-MoS2
5.1 Noble Metal Single-atom Doped MoS2
Various noble metals SAs including Ru,Pt,Rh,Ag,etc.have been widely used to improve the catalytic activity of MoS2.Through substituting the position of Mo atoms or bonding with surface S atoms,noble metal SAs can optimize the hydrogen adsorption properties and active basal plane of MoS2to boost HER.Shenet al.[76]revealed the mechanism of enhanced electrocatalytic activity in Ag doped MoS2by DFT calcula⁃tion.ΔGH*was adopted as the descriptor of the HER activity.The ΔGH*of in-plane S atoms for pure MoS2was 1.59 eV,which was a clear departure from 0 eV,suggesting the weak interaction between in-plane S atom and H*.And the calculated band gap for pure MoS2was 1.72 eV.The low electron conductivity limited the electron transfer during HER,which further inhibited the electrochemical performance.When the inter Mo atom was replaced by an Ag atom to form Ag-MoS2,the ΔGH*of in-plane S atom adjacent to the Ag atom sharply decreased to ‒0.17 eV,indicating the enhanced hydrogen bonding ability.And its band gap also decreased to 0.13 eV.Density of states(DOS)showed the localized electron near Femi level of Ag-MoS2,and Ag atom provided extra charge to nearby S atoms.Therefore,the electronic structure and ΔGH*of in-plane S atoms in Ag-MoS2were adjusted.And the authors discovered that the Ag-S distance and ΔGH*showed a volca⁃nic trend.The S atom with Ag-S distance of 0.638 nm showed the optimal activity with the ΔGH*of 0.03 eV.Denget al.[77]synthesized high activity Rh-SA-MoS2by optimizing the inter-Rh distance.Rh-SA-MoS2was pre⁃pared by a solvent-thermal method and the distance between confined-Rh atoms depended on the concentration of Rh ions.The distance synergy in confined Rh atoms played a vital role in adjusting the hydron adsorption energy of nearby S atoms.The optimal catalysts showed a low overpotential of 67 mV at 10 mA/cm2in Arsaturated 0.5 mol/L H2SO4electrolyte.Tsang and co-workers[78]demonstrated that incorporation of SA Pt or Pd into 1T MoS2can significantly increase the performance of catalyst.The optimal mass loading for Pt or Pd-1T-MoS2was 3%.Pd-1T-MoS2and Pt-1T-MoS2showed the overpotentials(at the current density of 10 mA/cm2)of 140 and 230 mV,respectively,which were much lower than the pure 1T-MoS2(300 mV).DFT calculation suggested the doped metal atoms accelerate the rate limiting step of hydrogen recombination.
The doped noble metal SAs can not only play as catalytic sites,but also activate the basal plane to en⁃hance the performance.Tanet al.[29]synthesized Ru-doped nanoporous MoS2(Ru/np-MoS2)by chemical vapor deposition and chemical etching method.The Ru-doping caused the S vacancy generation and phase transition from 2H to 1T in Ru/np-MoS2as confirmed by XANES and HAADF-STEM.And the strain in Ru/np-MoS2was also investigated.EXAFS spectra in Fig.5(A)shows the obvious high-R shift in Ru/np-MoS2,compared to plane MoS2(P-MoS2)without strain and larger ligament nanoporous MoS2(Lnp-MoS2)with a litter strain.The strain in Ru/np-MoS2resulted from its nanotube-shaped ligament,and the strength of strain related to the diameter of ligament[Fig.5(B),(C)].The formed 1T phase,S vacancies and strain in Ru/np-MoS2were beneficial to the HER process by enhancing the hydrogen adsorption ability.In an alkaline electrolyte,Ru/np-MoS2exhibited an overpotential of 30 mV at the current density of 10 mA/cm2with a low Tafel slope of 31 mV/dec[Fig.5(D),(E)].The intrinsic activity was also compared by normalizing the current density to the electrochemical active surface areas[ECSA,Fig.5(F)].Large strain not only provided more active surface atoms,but also optimized the electronic structure of Ru atoms and S vacancy to enhance the intrinsic activity[Fig.5(G)].And the catalyst displayed good durability in an alkaline electrolyte for 40 h[Fig.5(H)].Yanget al.[57]reported the binder-free Ru-SA-MoS2with Ru mass loading of 0.37% for pH-universal HER.The synergistic effect between Ru SAs and MoS2substrate accelerated the catalytic process.Zhanget al.[60]fabricated Ru/Ni co-doped MoS2with a special electronic structure for fast HER.DFT calculations revealed that the S atoms near Ni atoms served as H adsorption sites and Ru atom accelerated hydroxyl adsorption.Therefore,the Ru/Ni co-doped MoS2showed a low overpotential of 32 mV at the current density of 10 mA/cm2with the Tafel slope of 41 mV/dec.
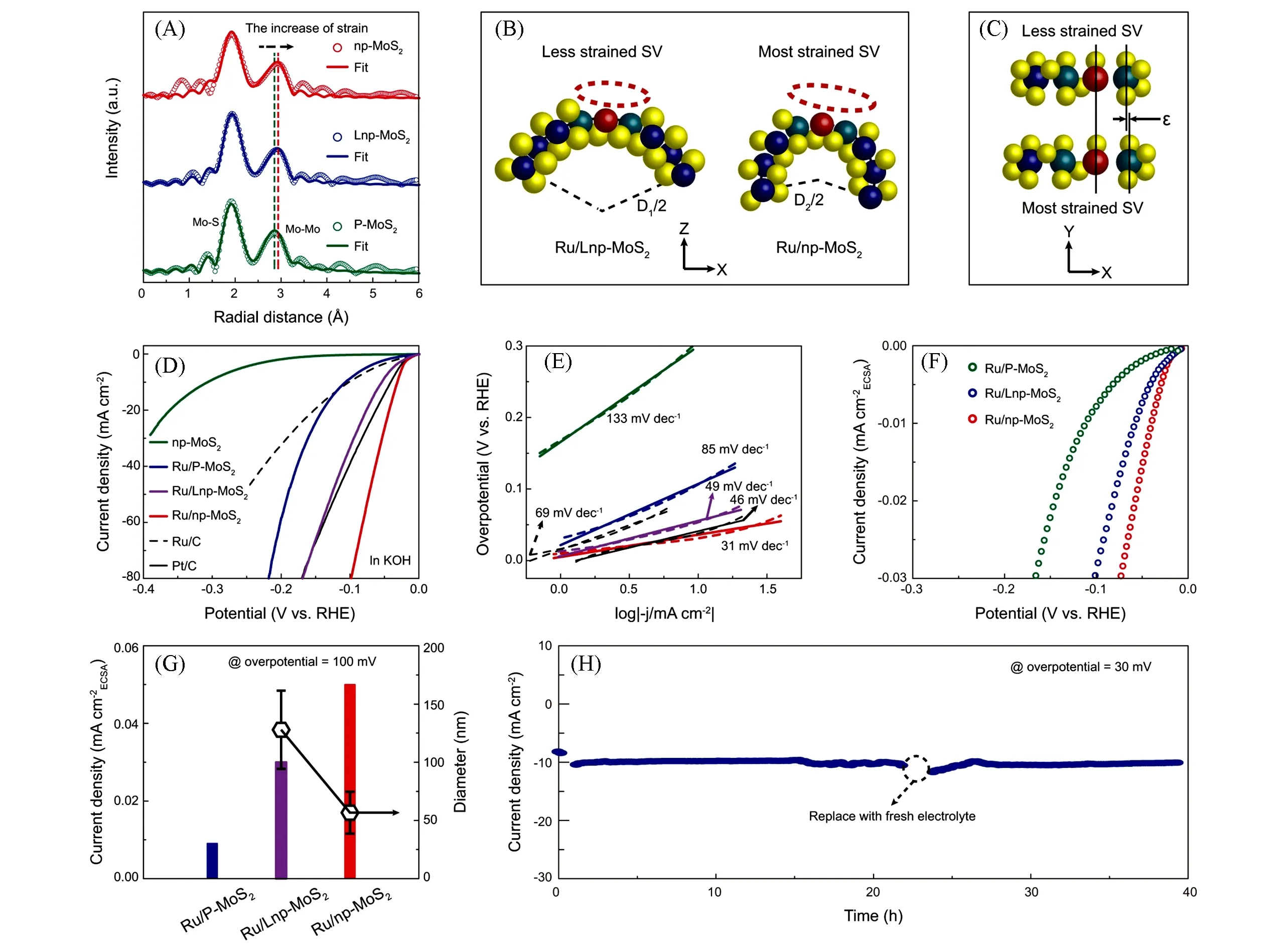
Fig.5 FT⁃EXAFS spectra(A),schematic of the atomic structure of Ru/Lnp⁃MoS2 and Ru/np⁃MoS2 derived from(A)(B,C)[ɛ in (C) represents the amount of deformation],polarization curves(D),corresponding Tafel plots derived from (D)(E),ECSA⁃normalized polarization curves(F),ECSA⁃normalized current density at an overpotential of 100 mV(G)(the average diameters of ligaments for Ru/LnpMoS2 and Ru/np⁃MoS2 were also shown),and stability measurement of Ru/np⁃MoS2 at an overpotential of 30 mV(H)[29]
5.2 Transition Metal Single-atom Doped MoS2
Besides noble metal SA doped MoS2,transition metal SA doped MoS2also shows enhanced HER activity.Guet al.[52]developed Ni-SA-MoS2as the high performance HER catalyst in both acid and alkaline electrolytes.HAADF-STEM images revealed the Ni atoms located at the atop of Mo-S hexagonal sites,and XPS spectrum of Ni-SA-MoS2exhibited a single peak at 855.5 eV,corresponding to Ni-S bonding.DFT calcu⁃lation showed the hydrogen adsorption energies of S atoms at basal plane and edge of pure MoS2were 2.16 and−0.97 eV,respectively,indicating the S at basal plane was hydrogen inert and the S at edge troubles by hydro⁃gen desorption.However,the hydrogen adsorption energies of S atoms at basal plane and edge of Ni-SA-MoS2were 0.8 and 0.1 eV,respectively,confirming the enhanced HER performance.Therefore,introducing Ni SAs significantly increased the intrinsic activity of MoS2.By a one-pot hydrothermal method,Chenet al.[59]substituted Mo sites with Ni atoms for acid and alkaline HER.Compared to pure MoS2,Ni doped MoS2showed additional impurity states in the bandgap,which facilitated the hydrogen adsorption during HER.Louet al.[79]developed the Ni-MoS2on multi-channel carbon matrix(MCM)nanofibers with unique architec⁃ture for electrocatalytic water splitting.Ni SAs could activate the S atoms at basial plane by electronic interac⁃tion to smooth the water dissociation.The catalyst exhibited high electrocatalytic performance and excellent durability for 24 h.
Co SAs can also activate the basal plane of MoS2nanosheet and adjust the electronic structure of coordi⁃nated S atoms.Jia and co-workers[80]compared the HER activity of various transition metals doped 1T′MoS2,and found Co@MoS2was the promising electrocatalyst for HER.Liuet al.[81]synthesized Co-doped MoS2with high HER activity comparable to Pt.The formation of Co-S covalent bond led to the phase transformation from 2H to 1T of MoS2.The best catalyst with Co loading of 3.54% exhibited a high HER performance in an acid electrolyte.Linear sweep voltammetry and constant-current measurements proved the excellent stability of Co-doped MoS2,much better than other MoS2catalysts with Co cluster,nanoparticle or ions.Wanget al.[56]reported Co atoms in Co-SA-MoS2serve as active sites to boost the HER activity.Theorbital perpendicular to the basal plane of Co-SA-MoS2results in the hydrogen Gibbs free energy near 0.Co SAs doped 2H MoS2also exhibited remarkable electrocatalytic performance.CoxMo1‒xS2with conductivity of 0.1 S/m was synthe⁃sized by solid-state reaction method,which was 10 times higher than that of pure MoS2[82].Co-doping induced half-filled intermediate bands in the MoS2,which was responsible for enhanced electrocatalysis.Weiet al.[62]found the long-range ferromagnetic induced by Co doping can activate 50%of S atoms in the basal MoS2plane.After activation,the H adsorption energy of optimal S site was only 0.12 eV,much lower than the 2 eV of pristine MoS2.Tsang and co-workers[83]studied the role of Ni/Co SAs in MoS2.In this work,Ni showed a negative effect on HER due to the Ni-Mo interaction while Co could enhance the HER rateviaactivating the nearby S atoms.
By a cold hydrogen plasma method,Mo-SA-MoS2was synthesized for fast HER in 0.5 mol/L H2SO4[32].Coordinately unsaturated Mo SAs on the surface of MoS2effectively hybridized with hydrogen atom to facilitate the electrocatalysis.Cu SAs on 1T MoS2with large charge transfer(‒0.38 e)was also reported for HER[49].The catalyst showed a low Tafel slope of 51 mV/dec and excellent durability.
5.3 Non-metal Single-atom Doped MoS2
Various non-metal atoms have also been introduced as single atoms into the framework of MoS2to improve the HER performance.For example,N-doped MoS2prepared by hydrothermal reaction shows an overpotential of 168 mV at the current density of 10 mA/cm2for HER,much lower than that of pure MoS2[58].DFT calcula⁃tions indicated the N is not the active sites due to the strong hydrogen bonding property.The S sites influenced by N atoms and the Mo atoms near S defect are responsible for the enhanced activity.Se-SA-MoS2was also re⁃ported for the enhanced HER by increasing active sites and conductivity[84].Xueet al.[85]found the P doping could enhance the conductivity of MoS2,and enlarge the interlayer spacing,which accelerates the hydrogen adsorption and desorption.And the H adsorption free energy of P sites was only 0.04 eV,similar to Pt.The catalyst exhibited a low overpotential of 43 mV at 10 mA/cm2and a small Tafel slope of 34 mV/dec.
Non-metal SAs combining with metal SAs are promising to boost the electrocatalysis.Denget al.[86]reported MoS2nanofoam with confined Se/Co for large-current-density HER.The catalyst possessed a low overpotential of 382 mV at the current density of 1000 mA/cm2and could be stably maintained for 360 h.DFT calculation was performed to understand the role of Se and Co doping.Co atoms led to the enhanced hydrogen adsorption ability of S atoms in basal plane and at edge,and the Se atoms compensated the over-strong adsorp⁃tion sites by electronic interaction[Fig.6(A)].The hydrogen adsorption energy of in-plane,Mo-edge and S-edge for Se/Co co-doped MoS2were −0.21,−0.14 and 0.1 eV,respectively,and all sites seemed to be responsible for the fast HER[Fig.6(A),(B)].Besides,Se-doping promoted the stabilization of the basal plane and edge by Co-Se bonds[Fig.6(C),(D)].The synergistic effect of Co and Se atoms in Se/Co co-doped MoS2significantly boosted the HER process.Moreover,such Se/Co co-doped MoS2electrocatalyst showed both high activity and stability under large current density.Its overpotential at 1000 mA/cm2is only 382 mV with 100%hydrogen production faradic efficiency,which makes it a suitable candidate for industrial catalyst.The synergistic effect of nickel and oxygen atoms were also proved by Gu and co-workers[87].The Ni and O atoms decreased the kinetic energy barrier of water dissociation and accelerated the hydrogen generation during the HER.As a result,the catalyst showed an ultralow onset potential of about 0 V and a high exchange current density of 0.44 mA/cm2.

Fig.6 Adsorption free energies of H*(ΔGH*)at different catalysts(A),adsorption models of H*at different sites of Co/Se⁃MoS2(B),relative formation energies of the Co/Se⁃co⁃doped MoS2 with different doping configurations of Co and Se being separated(Co/Se⁃separated,grid bars) or adjacent(Co/Se⁃adjacent,solid bars)to each other(C),structures of Co/Se⁃co⁃doped basal plane,Mo⁃edge,and S⁃edge with Co/Se⁃separated and Co/Se⁃adjacent configurations(D)[86]
6 Summary and Outlook
This perspective summarizes recent advances of SA-MoS2for HER.The inert basal plane of MoS2results in inferior performance compared to the state-of-the-art Pt catalysts.However,the weakness of MoS2can be optimized by doping SAs on the basal plane.SAs including noble metal,transition metal and non-metal elements not only serve as active sites for HER,but also adjust the hydrogen adsorption property of the whole basal plane by inducing electronic interaction of nearby S atoms,the defect and the phase transition.Typically,the ideal high-performance electrocatalysts require the following properties:(1)near zero hydrogen adsorption energy for high intrinsic activity;(2)dense active sites for fast reaction process;(3)high electronic conductivity for rapid electron transfer;(4)high durability for long-term measurement;(5)low cost in raw materials and the synthesis process for large-scale production.Based on the progress of SA-MoS2,some of them(e.g.Ru-SA-MoS2,Co-SA-MoS2and Ni-SA-MoS2)possess the qualities of high intrinsic activity,dense actives sites,excellent durability and low cost,which meet most requirements of the ideal catalyst.As for conductivity,though the semiconductor character of 2H phase leads to poor electron transfer during HER,the SA-doping or phase transition can make up for the disadvantage.
Based on the rapid development of the hydrogen production,challenges and opportunities still remains for the SA-MoS2.(1)Universal activation mechanism.SAs can adjust the hydrogen adsorption energy of the nearby S atoms and the defects through electronic interactions.However,the relationship between the catalytic activity and the element type or element concentration of SA is still confused in both calculation and experiment.More principles and strategies of designing efficient catalysts are needed.(2)Accurate and controllable method for SA anchoring.Facile synthesis of SA-MoS2with high repeatability is necessary for the mass-production of electrocatalysts.(3)Synergistic effect of double/multi-SAs in MoS2.The coupling of multi-SAs on the basal plane of MoS2can further improve the catalytic performance of SA-MoS2.The catalytic mechanism and simple synthesis method of multi-SA-MoS2are also urgent.(4)Development of theoretical strategy.Though H adsorption energy can efficiently reflect the acid HER activity,an efficient descriptor in non-acid electrolyte is required.And the clarify of relationship between SAs structure and reaction kinetics of electrocatalysts is urgent.(5)Structure design for large current density.The issues of mass transfer and ohmic resistance under high-output show significant influence on the electrocatalysis.The insufficient gas evolution may block the pathway for electrolyte to the active sits,leading to the drop of performance.Ordered layer stacking and hierarchical porous structure of MoS2are promising strategies to enhance energy efficiency of HER at large current density.
To date,SA-MoS2shows great potentials in efficient and long-term hydrogen production.Considering the unique structure and properties of SA-MoS2,the extension of SA-MoS2in nitrogen reduction reaction,oxygen evolution reaction and other energy conversion and storage field is also promising.
——材料科学与工程

