A Method to Predict the Thermochemical Behavior of N-butyl zaideethylnitramine (BuAENA) in DMSO
LU Xu, HU Ling-yan, WANG Tao, LI Bao-jun, LIN Xin-bing, LI Lian-qiang, ZHU Yan-long, DING Li,CHANG Hai
(1.Institute for Hygiene of Ordance Industry, Xi′an 710065, China; 2.Xi′an Modern Chemistry Research Institute, Xi′an 710065, China)
Abstract:The dissolution behavior of N-butyl zaideethylnitramin(BuAENA)dissolved in DMSO (dimethyl sulfoxide) has been studied in the paper, and a method that can predict the dissolution enthalpy was also established. The dissolution properties of BuAENA in DMSO at four different temperatures under atmospheric pressure were measured by using a C80 microcalorimeter. The results show that the dissolution process for the BuAENA in DMSO is endothermic, and the empirical formulate of dissolution enthalpies at 298.15, 303.15, 308.15 and 313.15K are ΔdissH=60.17b-23.02b1/2+5.09, ΔdissH=62.17b-23.58b1/2+5.24, ΔdissH=65.45b-24.01b1/2+5.37 and ΔdissH=68.57b-24.91b1/2+5.60, respectively. The empirical formulate of the dissolution enthalpy of BuAENA dissolved in DMSO can be described as ΔdissH=(-53080.91)/T+237.81b+11329.26/T-60.96b1/2+(-3128.95)/T+15.57. And the accuracy is verified by measuring the dissolution enthalpy of the BuAENA in DMSO at 343.15K. The values of activation energy E and pre-exponential factor A for the dissolution process are 3.35kJ/mol and 10-2.05s-1, respectively.
Keywords:physical chemistry; thermochemical behavior; N-butyl zaideethylnitramin; BuAENA; dimethyl sulfoxide; DMSO
Introduction
In the synthesis process of energy materials, a certain amount of heat is absorbed or released when materials dissolve in a solvent, which leads to a larger error in the calculation of reaction heat[1-10]. In addition, the thermal stability of solution system is imoperant for the safety of reaction[11-17]. So it is necessary to study the dissolution behavior of materials in the solvent and to establish a kind of mathematical model to predict the dissolution enthalpy.
N-butyl zaideethylnitramine (BuAENA) is a kind of energetic plasticizer, which is made by adding n-butyl-n-(2-nitroxyethyl)nitramine (BuNENA) and NaN3into DMSO solvent, has high energy, low sensitivity, and excellent thermal stability[18-19]. So it can be widely used in gun propellant, rocket propellant, and other related fields. Earlier researches have mostly focused on the kinetics of the synthesis of BuAENA[20-21], whereas a few researchers paid attention to the study of thermal behavior in the reaction process.
In this study, C80 microcalorimeter is used to investigate the dissolution behavior of BuAENA in DMSO, the relationships between enthalpies of dissolution(ΔdissH) and molality are studied, the parameters of dissolution behavior, such as ΔdissHpartial(the relative partial molar enthalpy of dissolution) and ΔdissHapparent(the relative apparent molar enthalpy of dissolution), are obtained by calculation, the empirical formula of dissolution enthalpy at different temperatures and different molality are predicted and verified. It also can be obtained the kinetics parameters of the dissolution process, which provide basic guidance for the study of thermal behavior.
1 Experimental
1.1 Materials
BuAENA used in this work was made and purified by Xi′an Modern Chemistry Research Institute. DMSO is a kind of solvent, having a purity of more than 99.99%, made by Chengdu Kelong Chemical Industry Co. Ltd. China. Distilled water was made by distilling apparatus.
1.2 Equipment and conditions
C80 microcalorimeter, made by Setaram instruments from France, was used to study the dissolution behavior. The reliability of this instrument was proved by measuring the enthalpy of the dissolution of KCl in distilled water at 298.15K and the result was (17.23±0.22)kJ/mol, whose relative error was less than 0.05% compared with the literature value 17.24±0.02[22]. It is reliable to measure the enthalpy of the dissolution using this instrument.
The enthalpies of the dissolution of BuAENA in DMSO were measured at 298.15, 303.15, 308.15 and 303.15K, respectively.
2 Results and Discussions
2.1 Theoretical basis
Calorimetric experiment is used to measure the dissolution enthalpy ΔdissHat different temperaturesTand molalityb.
The relationship between the dissolution enthalpy ΔdissHand molalitybis obtained by mathematical fitting as shown Eq.(1).
ΔdissH=Ab+Bb1/2+C
(1)
The relationship between the dissolution enthalpy ΔdissHand temperatureTis constructed by Eq.(2)—(5). And the empirical formula for predicting dissolution enthalpy is finally obtained.
A=X/T+C1
(2)
B=Y/T+C2
(3)
C=Z/T+C3
(4)
(5)
2.2 Thermochemical behavior of the dissolution in DMSO for BuAENA
C80 microcalorimetry is used in this work to study the behavior of the dissolution in DMSO for BuAENA. The dissolving process is shown in Fig.1. From Fig.1, we can see that the dissolving process under different temperatures for BuAENA in DMSO is endothermic.

Fig.1 Dissolution process of BuAENA in DMSO
The experimental and calculated values of molar enthalpies of the dissolution in DMSO for BuAENA are shown in Table 1, wherebrepresents molality of the solution and ΔdissHrepresents the molar enthalpy of the dissolving process.

Table 1 The dissolution enthalpies of BuAENA in DMSO at different temperatures and molality
The relationships between ΔdissHandb1/2for BuAENA dissolved in DMSO at four temperatures are shown in Fig 2. It can be seen that ΔdissHincreases as the temperature increases. On the other hand, the relationship between ΔdissHandb1/2changes regularly in the polynomial equations.
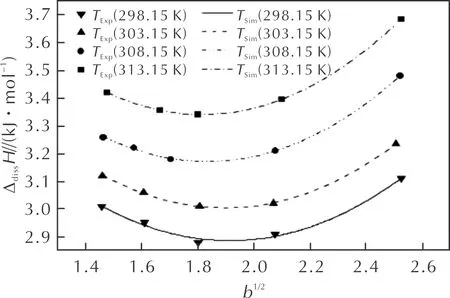
Fig.2 The relationship between ΔdissH and b1/2 of BuAENA in DMSO
ΔdissHandb1/2at the same temperature are fitted by polynomial equations and the empirical formulates of molar enthalpy at four temperatures are listed in Table 2.

Table 2 The values of A、B and C at four different temperatures
Based on the equations as listed in Table 2, other thermochemical data during the dissolving process, including ΔdissHpartial(the relative partial molar enthalpy of dissolution) and ΔdissHapparent(the relative apparent molar enthalpy of dissolution), is obtained by Eq.(6)—(7), respectively. The results are listed in Table 3.

Table 3 The values of ΔdissHpartial and ΔdissHapparent at different temperatures and molality
(6)
ΔdissHapparent=ΔdissH(b=b)-ΔdissH(b=0)
(7)
2.3 Method for predicting enthalpy of the dissolution for BuAENA in DMSO
The relationships betweenA,B,Cand temperature are shown in Fig.3, respectively. It can be seen thatA,BorCchange regularly as temperature changes in Fig 3. The empirical formulas describingA,B,Cversus 1/T, respectively, are shown in Eq.(8)—(10).
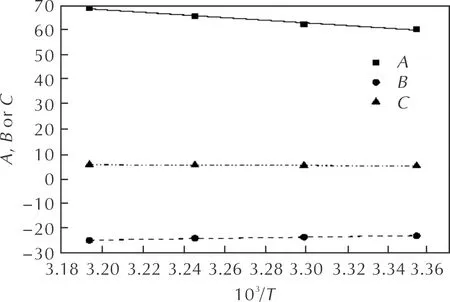
Fig.3 The relationships between A, B, C and 1/T
(8)
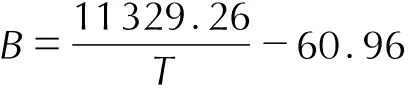
(9)

(10)
Based on Eq.(8)-(10),the molar enthalpy of the dissolution for BuAENA in DMSO at random temperature and molality can be predicted.
In order to verify the reliability of this method, the molar enthalpy of BuAENA dissolving in DMSO at 343.15K is measured by C80 microcalorimetry and the result obtained was 4.77kJ/mol(b=0.0632mol/kg). Besides, the predicted value of molar enthalpy is also obtained by this method and the result was 4.68kJ/mol. By comparing the experimental and predicted values, the result shows that the value obtained by this method is close to what is measured and the error is only about 1.9%. It demonstrates that it is reliable to predict the molar enthalpy of BuAENA dissolved in DMSO by using this method.
3 The Kinetic Behavior
Eq.(11)—(14)[23]are selected as the model function to study the kinetic behavior of the dissolving process for BuAENA in DMSO at different temperatures.
(11)
f(α)=(1-α)n
(12)
combining Eqs.(6) and (7) yields
(13)
Substitutingα=Q/Q∞into Eq.(13),
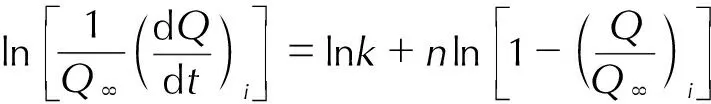
(14)
In these equations,αis conversion degree, f(α) is the kinetic equation,kis the reaction rate,nis the reaction order,Q∞is the enthalpy of the whole process for BuAENA dissolving in DMSO,Qis the enthalpy of dissolution at the time oft, soQ/Q∞can be described asα.
Substituting the data of BuAENA dissolved in DMSO at four temperatures into Eq.(14), and the values of the dissolution process at different temperatures are listed in Table 4. Based on the data, kinetic parameters such asnand lnkare obtained and the results are listed in Table 5.

Table 4 The origin data of dissolution process at four different temperatures

Table 5 The values of n, lnk and the correlative coefficient of BuAENA in DMSO at different temperatures
Arrhenius equation Eq.(15) is used to study the relationship between rate constant and temperature, and activation energy (E) and pre-exponential factor (A) can also be obtained by using the Arrhenius equation. The relationship of lnkversus 1/Tfor BuAENA dissolved in DMSO is shown in Fig.4. The value ofEis 3.35kJ/mol andAis 10-2.05s-1(R2=0.9971). It confirms that BuAENA can be easily dissolved in DMSO.

Fig.4 The relationship between lnk and 1/T
(15)
Ais a pre-exponential factor;Eis activation energy, kJ/mol.
4 Conclusion
(1) The dissolution behavior of BuAENA dissolved in DMSO is endothermic and the empirical formulate of dissolution enthalpies at 298.15, 303.15, 308.15 and 313.15K are ΔdissH=60.17b-23.02b1/2+5.09,ΔdissH=62.17b-23.58b1/2+5.24,ΔdissH=65.45b-24.01b1/2+5.37 and ΔdissH=68.57b-24.91b1/2+5.60,respectively.
(2) The method that predicts the dissolution enthalpy of BuAENA in DMSO is established by mathematical model and the empirical formulate for A, B, C versus 1/TareA=(-53080.91/T+237.81,B=11329.26/T-60.96 andC=(-3128.95)/T+15.57,respectively.The values ofA、BandCat 343.15K are predicted by this method and then the dissolution enthalpies for BuAENA dissolved in DMSO at 343.15K are obtained. The error between the calculated value and the experimental value is only 1.2%.
(3) The values of activation energyEand pre-exponential factorAfor the dissolution process are 3.35kJ/mol and 10-2.05s-1,respectively.

