Regulatory effect of acupuncture on electrical activity level of optic cortex in amblyopia model rats
HAO Xiaolu (郝小路), MA Chongbing (马重兵), ZHOU Na (周娜), SUN Yaling (孙娅玲), ZHU Tiantian (朱田田),SUN Yan (孙燕), WANG Jue (王觉), YAN Xingke (严兴科), LIU Anguo (刘安国)
1 The First Clinical Medical College of Gansu University of Chinese Medicine, Lanzhou 730000, China
2 Acupuncture and Massage College of Gansu University of Chinese Medicine, Lanzhou 730000, China
Abstract
Keywords: Acupuncture Therapy; Amblyopia; Vision Deprivation; Vision, Ocular; Neurons; Rats
Amblyopia is the most common curable ophthalmic disease in children and often occurs in the critical period of vision development. If children with amblyopia do not receive active and effective intervention treatment in the critical period of visual development, the damage to visual function will last lifelong and become one of the common causes of monocular visual damage in adults[1]. According to the latest epidemiological survey, the prevalence of amblyopia in the world is about 1.44%[2], its prevalence among children in China is about 0.93%[3], and there are more than 20 million children with amblyopia in China.Amblyopia affects the normal development of children’s visual function seriously. It is one of the urgent problems to be solved in clinical ophthalmology. A large amount of clinical evidence indicates many outstanding problems in the treatment of amblyopic children, such as long treatment cycles, poor dependence of children,and easily-induced decline of dominant eye vision. It is urgent to actively seek other effective treatment methods or find new targets of other anti-amblyopia drugs for children’s amblyopia treatment[4]. Fortunately,in recent years, acupuncture has shown great advantages and prospects in the treatment of amblyopia in children. The study by ZHOU Y,et al[5]has found that acupuncture can treat children’s amblyopia with 69% efficiency. Relevant evidence-based medical evidence also shows that acupuncture treatment for amblyopia in children has the advantages of a simple operation, a short course of treatment, and good effects. Infants can quickly adapt to this treatment and maintain a good dependence. However, the biological mechanism of acupuncture’s efficacy needs further study[6].
In recent years, with the help of micro-imaging technology, electrophysiological evaluation technology,and brain functional imaging technology, researchers have carried out a series of research on the morphological and functional changes of visual neurons and the remodeling of visual center function. The results have demonstrated that acupuncture treatment during the critical period of visual development can cause encouraging changes in synaptic structure and function of the optic nerve. For example, it can promote the repair of the morphological structure of neurons,enhance the efficiency of visual signal transmission between synapses, and antagonize the disorder of the visual environment[7-8]. However, whether acupuncture can correct the abnormal neuroelectric activity of the visual cortex caused by visual environment disorder needs further study. Therefore, this study was designed to detect the neuroelectric signal activity level in vision-deprived rats. This work will indicate the effectiveness of acupuncture in treating amblyopia from the perspective of neural electrophysiology.
1 Materials and Methods
1.1 Experimental animals and grouping
Sixty 14-day-old Sprague-Dawley rats with a body mass of 20±5 g were purchased from the Animal Center of Gansu University of Traditional Chinese Medicine(Batch No. 62001000000101). The rats were randomly divided into a blank group, a model group, an early-stage acupuncture group, a middle-stage acupuncture group, and a late-stage acupuncture group,with 12 rats in each group. This study was approved by the Animal Experimental Ethics Committee of Gansu University of Traditional Chinese Medicine (Review No.2016-58).
1.2 Main instruments and reagents
The main instruments included the eye electrophysiology diagnostic system (Roland Consult Stasche & Finger GmbH, USA); Cerebus multichannel data acquisition system (Blackrock Microsystem Inc.,USA); brain standard stereotaxic instruments (Shenzhen RWD Life Science Co., Ltd., China).
The main reagents used in this study included chloral hydrate (Lot No. 20130105, Tianjin Kaixin Chemical Industry Co., Ltd., China); atropine (Lot No. H31021175,Hubei Jiaxin Biomedical Co., Ltd., China); 4%paraformaldehyde (Lot No. 20141124, Beijing Solarbio Science & Technology Co., Ltd., China); erythromycin eye paste (Lot No. 131007, Nanjing Baijinyu Pharmaceutical Co., Ltd., China).
1.3 Model establishment
The model preparation of rats deprived of vision in one eye referred to the relevant literature[9]. The development and maturation of vision are closely related to the plasticity of the visual cortex. The sensitive period of visual development in rats begins from postnatal natural eye opening and lasts until the 45th day after birth. A monocular deprivation model was created on the 14th day after birth, and the rats were anesthetized by the intraperitoneal injection of 10% chloral hydrate [3 mL/(kg·bw)]. After the hair around the eyelid margin was shaved and the right eyelid was carefully disinfected, the upper and lower eyelid margins of the right eye were cut by 1.0-1.5 mm from the inner canthus to the outer canthus. Then, the upper and lower eyelids were sutured hierarchically.After the operation, the wound was disinfected again,and erythromycin eye ointment was applied to prevent infection. During the sensitive period of visual development, the right eye could not receive normal external light stimulation, resulting in the deprivation of amblyopia[10].
1.4 Acupuncture treatment
Rats in the early-stage acupuncture group began to receive acupuncture from the 3rd day after model establishment, rats in the middle-stage acupuncture group started the acupuncture treatment from the 12th day after model establishment, and rats in the late-stage acupuncture group from the 21st day after model establishment. The following acupuncture points were selected according to theExperimental Acupuncture Science[11]and theSectional AnatomicalAtlas of Sprague-Dawley Rat[12].
Points: Bilateral Jingming (BL1), Cuanzhu (BL2),Fengchi (GB20), and Guangming (GB37).
Method: The acupuncture needles of 0.28 mm in diameter and 5 mm in length were used. The depth of needle insertion into points Jingming (BL1), Cuanzhu(BL2), and Fengchi (GB20) was 5 mm, and that into Guangming (GB37) was 3 mm. A quick twisting method was performed, with a range of 180° and a frequency of 80 times/min. The operation lasted for 3 min per point.Then, the needles were retained for 15 min, during which the quick twisting method was performed for 3 min every 5 min. Acupuncture was given once a day for 9 d, and bilateral symmetrical points were stimulated every other day. The specific intervention time is shown in Figure 1.
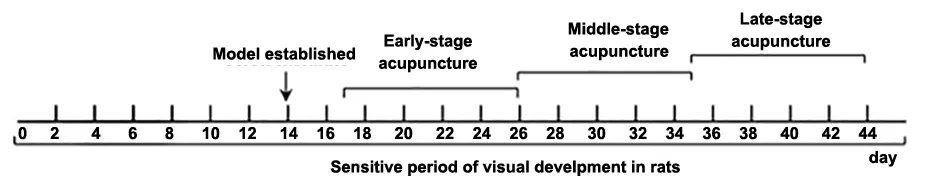
Figure 1. Acupuncture intervention time in the visual sensitive period
1.5 Pattern visual evoked potential (P-VEP)
Visual evoked potential (VEP) was represented by a typical pattern, as shown in Figure 2. The naming method of Harding was adopted. The waveforms recorded from each rat were relatively stable N-P-N forms. It showed the latency of N1, P100, and N2 waves and the amplitude of N1, P100, and N2 waves.

Figure 2. Schematic diagram of potential time and amplitude of P100 wave
1.5.1 P-VEP detection of the electrical activity in the primary visual cortex
The sutures on the eyelids of the model group and each acupuncture group were carefully cut open to expose the right eye, and then two drops of atropine eye drops were injected into the operative eye to dilate the pupil. Gold foil electrodes were inserted into the subcutaneous layer of the occipital protuberance,reference electrodes were placed subcutaneously at the midpoint of the binocular connection, and grounding electrodes were placed subcutaneously behind the right ear. P-VEP was detected after the rats adapted to the darkroom for half an hour. At the same time, the opposite eye was covered with a black patch.
1.5.2 P-VEP detection parameter settings
The binocular visual axis was parallel to the center of the screen. The stimulus mode of the black-and-white grid was selected. The distance between the recipient eye and the screen was 60 cm; the frequency was 2 Hz;the spatial frequency of the graph was 8×6; the upper limit was 50 Hz, and the lower limit was 1 Hz; the number of overlaps was 128 times; the magnification was 200 000 times, and the acquisition time was 300 ms. The recording electrode was placed 1.5 cm above the occipital protuberance, the reference electrode was placed at the middle of the forehead, and the impedance between the two was ≤5 Ω.
1.6 Microelectrode array detection in the primary visual cortex
First, anesthetics were injected into the abdominal cavity of rats in each group, and then the visual center was fully exposed. The primary visual cortex was marked with the help of the stereotaxic instrument(4.31-5.75 mm away from the anterior fontanel and 1.80-3.23 mm away from the midline). Then, a 16-channel nickel-chromium alloy microwire electrode was slowly inserted into the visual cortex by a depth of 1.5-2.0 mm using the instrument. With the computer recording system, the electrical signals of visual cortical neurons were collected through the Cerebus multichannel nerve signal acquisition system, and the data were analyzed with the Neuro Explorer software.
1.7 Statistical analysis
The P-VEP data and neuroelectric signal data of rats’visual cortical neurons were compared and analyzed by the P-VEP system software. For the rest of the data, if the data conformed to normal distribution, mean ±standard deviation (±s) was used for description, and one-way analysis of variance was conducted for statistical analysis; if the data did not conform to normal distribution, median (lower quartile, upper quartile) [M(QL, QU)] was used for description, and Kruskal-Wallis test was performed. The statistical analysis was performed with SPSS version 19.0 software, and the statistical inference was considered significant ifP<0.05.
2 Results
2.1 P-VEP in the visual cortex
P-VEP detection was employed. As shown in Figure 3,compared with the blank group, the latency of the P100 wave in the right eye of the model group was significantly prolonged (P<0.05), and the amplitude of the P100 wave was significantly shortened (P<0.05). The transmission efficiency of visual signals from the retina to the central nervous system was obviously lower in the model group, and there was a certain degree of visual pathway damage. The above test results indicated that the modeling was successful. In addition,compared with the model group, the latent time of the P100 wave in the three acupuncture groups was significantly shortened, while the amplitude increased to different degrees, with a very significant difference(P<0.05). The changes in the latency and amplitude of the P100 wave mentioned above indicate that early acupuncture intervention can restore the latency and amplitude of the P100 wave close to the normal level.Its characteristic performance is that the earlier the acupuncture intervention, the more obvious the recovery of the P-VEP action potential.
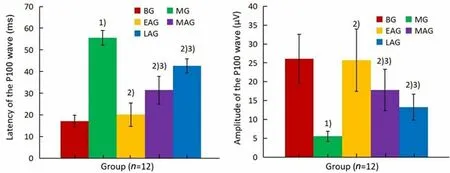
Figure 3. Latency and amplitude of P100 wave in different groups
2.2 Frequency and amplitude of optic neurons
Electrical activity levels of neurons in the primary visual cortex in each group were measured with the microelectrode array system (MAS).
As shown in Figure 4, neuronal discharge waveforms in the visual cortex were bidirectional, and the stage of these action potentials was obvious in the blank group.Compared with the blank group, the number of neurons involved in the normal neuroelectric activity in the visual cortex of the model group was significantly reduced, and the action potential waves detected showed disordered characteristics and even could not be distinguished from normal brain waves. Compared with the model group, in the early-stage acupuncture group, the number of active neurons increased significantly, and the stages of action potentials were more obvious, whereas the number of active neurons in the middle-stage acupuncture group was smaller than that in the early-stage acupuncture group, and the stages of action potentials were not obvious. In the late-stage acupuncture group, the number of active neurons was the smallest, and the stages of action potentials could not be distinguished.
From Figure 5, we can see that the discharge frequency and amplitude of optic neurons in the model group were significantly lower than those in the blank group (P<0.05). The above results suggest that during the critical period, form deprivation can cause abnormal electrical activities of all the optic neurons in rats.Compared with the model group, the frequency of electrical activity of the central visual nerve in the three acupuncture groups was significantly increased (P<0.05),and the average discharge amplitude was significantly increased (P<0.05), which was more obvious in the early-stage acupuncture group. However, the late-stage acupuncture intervention did not significantly promote the above electrical activities (P>0.05). This finding suggests that early- and middle-stage acupuncture intervention can promote discharge activity in optic neurons.

Figure 4. Action potential waveform of optic neurons in different groups
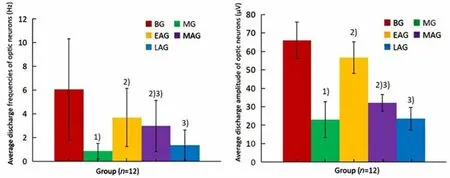
Figure 5. Comparison of the whole electrical activity level of the visual center in each group
2.3 Interspike interval (ISI) and power spectral density(PSD) of optic neurons
The ISI takes discrete points of time as the abscissa and pulse times as the ordinate. This measure can reflect the distribution of potential intervals. The PSD is the power carried by the frequency wave of each unit and obtained by multiplying the frequency density of the action potential signal of a neuron by an appropriate coefficient. Its significance lies in the synergy between the electrical activities of multiple optic neurons and the integration efficiency of nerve signals. The measured ISI and PSD data are as follows.
As seen in Figure 6 and Table 1, the ISI time of the optic neurons in each group was quite different and was distributed divergently. The ISI sequences in the blank group were concentrated below 0.3 s, those in the model group were below 15 s, those in the early-stage acupuncture group were below 1 s, those in the middle-stage acupuncture group were below 4 s, and those in the late-stage acupuncture group were below 10 s. Compared with the blank group, the ISI time in the model group was significantly prolonged (P<0.05). After acupuncture intervention, the ISI time in each acupuncture group was shortened to varying degrees(P<0.05), among which the most obvious change in the ISI was seen in the early-stage acupuncture group, while the smallest change was in the late-stage acupuncture group.
During the 120 s acquisition period, the PSD of the blank group mainly ranged from -105 dB to -100 dB, the PSD of the model group from -132 dB to -124 dB, the PSD of the early-stage acupuncture group from -115 dB to -110 dB, the PSD of the middle-stage acupuncture group from -120 dB to -115 dB, and the PSD of the late-stage acupuncture group from -129 dB to -122 dB.After the vision was deprived, the PSD interval of optic neurons increased with the deprivation duration.Compared with the PSD interval of the blank group, the PSD interval of the model group increased significantly(P<0.05). After acupuncture intervention, the PSD interval of the three acupuncture groups was shortened to varying degrees (P<0.05), and the most obvious effect was seen in the PSD interval of the early-stage acupuncture group, while the PSD showed the smallest change in the late-stage acupuncture group. It is concluded that acupuncture can significantly regulate the PSD of optic neurons in the critical period of visual development, and the earlier the intervention, the better the effect.
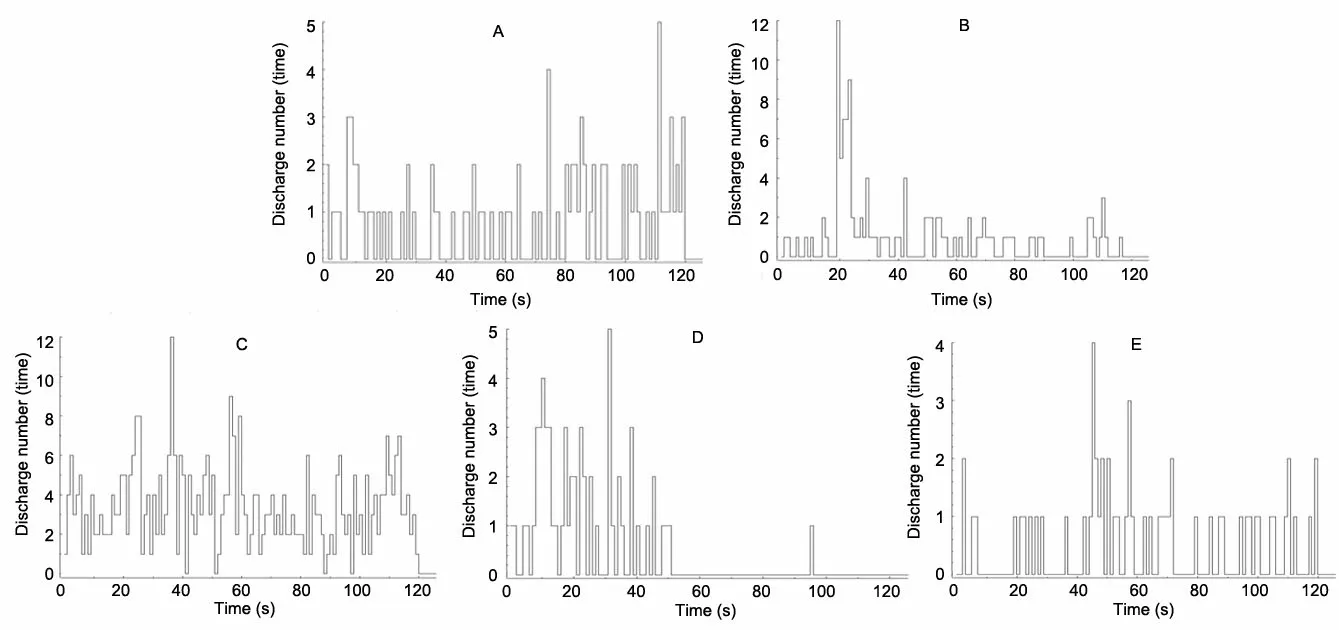
Figure 6. Discharge frequency of optic neurons in each group
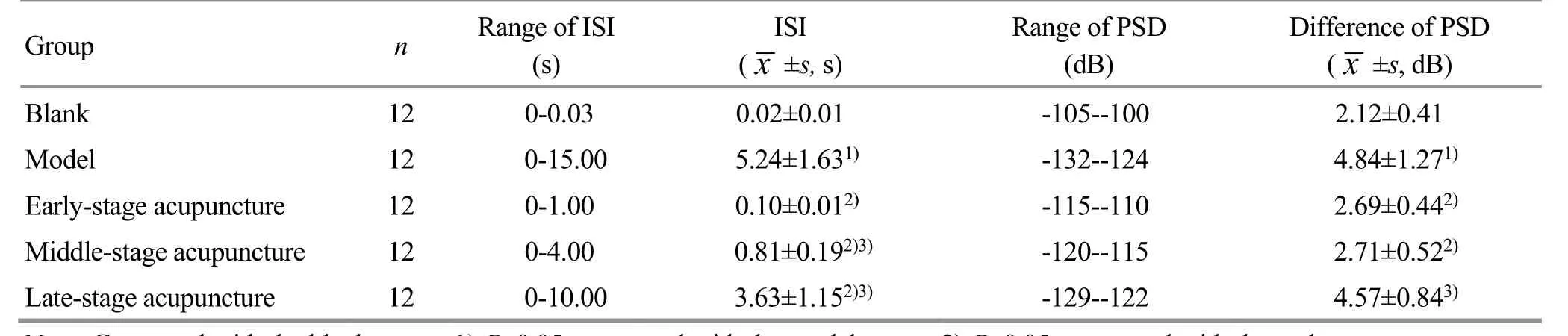
Table 1. Comparison of the average interspike interval (ISI) and power spectral density (PSD) of optic neurons in each group
3 Discussion
The visual system of mammals, including humans,has not yet matured at birth. This system has considerable plasticity during a certain period after birth. The original nerve connections and synaptic structure can change due to the stimulation from the external environment. This period is called the sensitive period of visual development[13]. The critical period of human visual development begins at the age of 3-4 years old and ends by the age of 6-12 years old. The 14th to 45th days after birth is the critical period of visual development in rats. According to the characteristics of visual development in rats, early-,middle- and late-stage acupuncture groups were set up based on the sensitive period. Amblyopia is classified in Chinese medicine under blurred vision, cross-eye concomitant esotropia, blue blindness, etc. Amblyopia is divided into two types: amblyopia due to spleen-stomach weakness and amblyopia due to constitutional insufficiency. It is attributed to nutritional deficiency of the eye caused by liver-gallbladder deficiency, insufficiency of kidney essence, or blood deficiency. Through preliminary clinical research,combined with recent research on the pathogenesis of amblyopia and the characteristics of clinical treatment,the points Jingming (BL1), Cuanzhu (BL2), Fengchi(GB20), and Guangming (GB37) were selected for amblyopia treatment in this study, which achieved satisfactory clinical results. Two points around the eyes,namely Jingming (BL1) and Cuanzhu (BL2), can be used to treat amblyopia based on their treatment effects on their nearby parts. Acupuncture at Guangming (GB37)can be used to unblock meridians and activate collaterals and dispel pathogenic wind to improve eyesight. Fengchi (GB20) is the crossing point of the Gallbladder Meridian and the Yang Link Vessel. It is beneficial to unblocking meridians and activating collaterals as well as nourishing blood for improving eyesight. The location of Guangming (GB37) and Fengchi (GB20) is based on their treatment effects on relatively distant parts from them. In addition, our previous work has illustrated that acupuncture treatment has a more notable effect on the ipsilateral visual cortex.
Previous studies have shown that changes in stimuli from the external environment can affect the connections between neurons in the visual cortex of mammals and the adjustment of the synaptic structure after birth[14]. P-VEP can be used to stimulate peripheral visual receptors and record visual signals from electrical conduction on the scalp above the occipital region of the brain. Additionally, P-VEP can reveal the conduction efficiency of electrical signals from neurons in the macular region to the visual cortex. At present, P-VEP is considered to be an effective evaluation index for ocular dominance column transfer and visual collateral nerve electrical signal transmission[15-16]. Neural coding is the rule used by single or multiple neurons to transmit and express information through discrete discharge sequences[17]. Relevant studies suggest that the action potentials produced by optic neurons and the ISI carry a large amount of coding information[18-19].
The encoding mode characterized by discharge frequency is called frequency encoding, and the encoding mode characterized by ISI is called time encoding[20]. REICH D S,et al[21]found that visual information is encoded in the ISI of neuronal action potentials in the V1 area of the visual cortex. Different ISIs represent different visual stimulus signals.CREWTHER D P,et al[22]found that the deprivation effect of amblyopia can reduce the projection of visual information in the primary visual cortex, and deprivation during the sensitive period of visual development can also change the visual field characteristics caused by the malfunction of the primary visual cortex. KIORPES L,et al[23]found that amblyopia can lead to a decrease in neurons in the bilateral primary visual cortex, a shift in the ocular dominance column, and a change in the appropriate frequencies of signals, and the spatial accuracy of the eyes by replicating the form deprivation model previously used in monkeys. These changes are closely related to the temporal and spatial specificity of nerve cells and indicate that the deprivation effect of amblyopia can lead to abnormal changes in optic nerve information coding[24].
In this experiment, we found that during the critical period of visual development, the reproduction of the monocular deprivation model can cause abnormal activities of P-VEP in the visual center of young rats, or rather the latency of P100 wave is significantly prolonged, and the amplitude is significantly reduced,suggesting that the vision deprivation in the sensitive period results in the damage of visual signal pathway,the delay of neuron response activity, and the decrease of the whole level of electrical activity. In addition, the electrical activity of optic cortical neurons collected by microelectrode array also showed that the average discharge frequency and PSD of optic neurons decreased significantly, the discharge waveform varied,the average amplitude decreased, and the ISI was prolonged significantly. All the changes in the electrical activity level of visual neurons confirmed the abnormalities of spatial and temporal coding of neurons,which led to the decline of the accuracy of the optimal spatial and temporal frequency, reduced the ability of the visual center to encode and process visual information, and ultimately affected the analysis of visual biological information, causing amblyopia.
In conclusion, after monocular vision deprivation in the sensitive period, the visual center of the rats had obvious abnormal neuroelectric activities, which was manifested as the decrease in the average spontaneous discharge frequency and discharge level of the primary visual cortex, the change in the shape of the discharge wave, as well as the shortening of amplitude, the prolongation of ISI, and the decrease of average PSD.These abnormal electrical activities from neurons seriously affect the carrying capacity, encoding, and decoding speed of electric signals from neurons in the primary visual cortex. Effective acupuncture treatment in the sensitive period of visual development can significantly repair the abnormal neuroelectric activity of the visual center, thus preventing further damage to visual function caused by form deprivation. The therapeutic effect of this acupuncture intervention is characterized by the fact that the earlier the intervention, the more obvious the effect. All the above results provide a new reference for the study of the electrophysiological mechanism of amblyopia and the mechanism of acupuncture in repairing visual function injury. At present, the limitations of present studies on the mechanism of acupuncture treatment for amblyopia lie in that most studies only explain the acupuncture effect with the evaluation method of functionalism from the perspective of morphological structure and functional change of optic neurons.Therefore, the molecular biological mechanism of the above-mentioned functional changes still needs further study.
Conflict of Interest
The authors declare that there is no potential conflict of interest in this article.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (国家自然科学基金项目,No. 81860879, No. 81260560, No. 81660816); Project of Chinese Medicine Inheritance Innovation Platform of Gansu Province (甘肃省中医药传承创新平台项目); Key Talent Project of Gansu Province (甘肃省重点人才项目);Science and Technology Capacity Enhancement Project for Middle-aged and Young Scholars at Gansu University of Chinese Medicine (甘肃中医药大学校级中青年科技能力提升项目, No. ZQ2015-15).
Statement of Human and Animal Rights
The treatment of animals conformed to the ethical criteria in this experiment.Received: 20 January 2021/Accepted: 27 September 2021
 Journal of Acupuncture and Tuina Science2022年4期
Journal of Acupuncture and Tuina Science2022年4期
- Journal of Acupuncture and Tuina Science的其它文章
- Study on the mechanism of moxibustion for rheumatoid arthritis based on liquid chromatography-mass spectrometry
- Study on the relationship between relieving energy crisis in myofascial trigger points with An-Pressing manipulation and AMPK/PGC-1α pathway activation
- Influence of Tuina plus oxiracetam on serum inflammatory factors and oxidative stress in mild vascular dementia patients
- Effects of acupuncture on nutritional status in patients in a persistent vegetative state:a prospective randomized controlled study
- Clinical observation of acupuncture plus acupoint sticking therapy for insomnia and its influence on subjective and objective sleep indicators
- Clinical observation of warm needling moxibustion plus lumbar traction for lumbar disc herniation
