Study on the mechanism of moxibustion for rheumatoid arthritis based on liquid chromatography-mass spectrometry
PANG Xiangtian (庞祥天), LENG Yufei (冷雨飞), YAO Yao (姚瑶), WANG Danwen (王丹文), LI Cheng (李程),XU Xiao (徐骁), SUN Zhiling (孙志岭)
1 School of Nursing, Nanjing University of Chinese Medicine, Nanjing 210023, China
2 Basic Medicine, Nanjing Vocational Health College, Nanjing 210038, China
3 School of Nursing, Zhejiang Chinese Medical University, Hangzhou 310053, China
Abstract
Keywords: Moxibustion Therapy; Arthritis, Rheumatoid; Arthritis, Experimental; Mass Spectrometry; Metabolomics; Rats
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease characterized by persistent synovitis, joint destruction, systemic inflammation, and even disability[1-2]. RA causes huge mental stress and Current treatment of RA mainly relies on economic burden to patients and their families[3].pharmacotherapy which may cause side effects and can only temporarily relieve or eliminate pain[4].
Moxibustion therapy is an important part of traditional Chinese medicine (TCM) and an internationally recognized complementary and alternative medicine treatment method[5]. It can reduce inflammatory reactions, repair synovial membrane,protect bones and cartilage[6], and thus effectively improve the symptoms in RA patients[7-9].
Metabolomics emphasizes holistic and dynamic concepts, consistent with the principle of TCM. It has been widely used to explore the mechanisms of complementary and alternative medicine, including moxibustion[10-11]. The significance and broad application prospects of metabolomics have been highlighted in modern biomedicine[12-15]. Metabolomic analysis of urine helps detect metabolic disorders in patients with autoimmune diseases[16]. It is noninvasive, and the necessary materials can be acquired conveniently. Liquid chromatography-mass spectrometry (LC-MS) is a highly sensitive analytical platform that can identify a wide range of metabolites,potential biomarkers, and metabolic pathways. In this study, LC-MS was performed to analyze endogenous metabolites of urine in rats with RA to explain the mechanism of moxibustion in treating RA.
1 Materials and Methods
1.1 Experimental animals and grouping
Twenty-four male Sprague-Dawley rats weighing 180-220 g were purchased from Qinglong Mountain Animal Breeding Farm, Jiangning District, Nanjing(Animal License No. SCXK-2014-0001). This experiment passed the ethical guidelines of the Animal Experimentation Committee, Nanjing University of Chinese Medicine (No. 201804A001). The animals were raised in a specific-pathogen-free environment at 20-24 ℃ with a relative humidity of 55%-57% and 12 h/12 h alternating light/dark cycles for one week.The rats were given free access to water and adaptive feeding. They were randomly divided into a control group, a model group, and a moxibustion group, with eight rats in each group. Those in the model and moxibustion groups underwent collagen-induced arthritis (CIA) modeling.
1.2 Main instruments and reagents
Bovine type Ⅱ collagen (Batch No. 20021), Freund’s complete adjuvant (Batch No. F5881), and Freund’s incomplete adjuvant (Batch No. F5506) were purchased from Chondrex, Inc., USA. Glacial acetic acid (Batch No. 15080553613, 99.5% purity) was purchased from Nanjing Chemical Reagent Co., Ltd., China. Smokeless moxibustion sticks (7 mm in diameter and 12 mm in length) were purchased from Henan Wanchuntang Co.,Ltd., China. Distilled water was purified using the Milli-Q purification system.
1.3 CIA model preparation[17]
First, 20 mg bovine type Ⅱ collagen was weighed with an electronic balance in shades and dissolved in 10 mL 0.05 mol/L glacial acetic acid to obtain a 2 mg/mL concentration. The configured solution was placed at 4 ℃ in a dark environment for 12 h and added with an equal volume of Freund’s complete adjuvant. The solution was stirred and emulsified in an ice bath using the Ultra-Turrax high-speed disperser (IKA® Works Guangzhou, China). This is the first model emulsion. To boost immune responses, the Freund’s complete adjuvant was replaced with Freund’s incomplete adjuvant to get the second model emulsion.
On day 0, the tail skin of the rats in the model group and the moxibustion group was disinfected, and 0.2 mL of the first model emulsion was subcutaneously injected into the terminal 1-2 cm of the tail for the initiate immunization. The rats in the control group were injected with 0.2 mL 0.9% normal saline in the same way. On day 7, the rats in the model group and the moxibustion group were reinjected with 0.1 mL of the second model emulsion for a booster immunization,while the rats in the control group were reinjected with 0.1 mL 0.9% normal saline. On day 14, the arthritis index (AI) was evaluated for rats in the model and moxibustion groups[18]. The AI score is the total points of the four limbs, and the highest AI score is 16 points.The CIA rat model was considered a success if the AI score was no less than 4 points.
1.4 Moxibustion treatment
Moxibustion intervention was performed after the modeling process was completed (on day 14).
Points: Bilateral Shenshu (BL23) and Zusanli (ST36).
Method: The hair around the points was shaved before moxibustion to prevent burns. The fixed rats received moxibustion at 1-2 cm above the points for 10 min. The treatment was given every morning for six consecutive days as one session. The whole intervention course consisted of three sessions with a one-day interval for a total of 21 d. The rats in the control and model groups were fixed by the same method at the same time but without moxibustion intervention.
1.5 Physiological parameters
The diet, fur condition, limb movements, and body mass of rats in the three groups were observed and recorded every seven days during the intervention. The body mass and hind paw volume of rats in the three groups were measured on day 0, day 14, day 21, day 28,and day 35. The hind paw volume was measured with a volume meter (PV-200, Chengdu Techman Software Co.,Ltd., China). The AI scores of the model group and the moxibustion group were evaluated on day 14, day 21,day 28, and day 35. The AI score was independently assessed by two experimenters and averaged.
1.6 Sample collection
Urine samples, serum samples, and the left ankle joint were collected after moxibustion intervention.After the last moxibustion intervention, 5 mL urine was collected from each rat housed in a metabolic cage. The supernatant was centrifuged at 3 000 r/min at 4 ℃ and frozen at -80 ℃. Blood samples were collected from the abdominal aorta. The serum samples were isolated by high-speed centrifuge at 3 500 r/min for 10 min at 4 ℃and then frozen at -80 ℃. The left ankle joints were removed and fixed in 10% formaldehyde solution to observe histopathological changes.
1.7 Serum cytokine level and histopathological examination
The levels of serum cytokines interleukin (IL)-6,tumor necrosis factor (TNF)-α, and prostaglandin E2(PGE2) were measured using the enzyme-linked immunosorbent assay following the manufacturer’s instructions (R&D Systems, USA). The optical density(OD) value was read by a multimode plate reader(PerkinElmer, Germany) at 450 nm. The primary concentration of each serum sample was calculated using the straight-line regression equation based on the OD value of the standard curve.
The tissue of the left ankle joints was fixed,decalcified, dehydrated in ethanol, cleared with dimethyl benzene, and embedded in paraffin blocks.Then it was sliced at 5 μm in thickness and stained with hematoxylin-eosin (HE). The tissue slices were observed under an optical microscope (Olympus, Japan).
1.8 Metabolite extraction
The sample of 100 μL urine was placed in an Eppendorf (EP) tube, and 400 μL 80% methanol aqueous solution was added for vortex oscillation. Then the EP tube was placed in an ice bath for 5 min. The solution was centrifuged at 15 000 g for 20 min at 4 ℃and diluted with mass-spectrum water until the methanol content reached 53%. Then, the diluted solution was centrifuged again at 15 000 g for 20 min at 4 ℃. Finally, the supernatant (2 μL) was obtained for LC-MS.
1.9 LC-MS analysis
The scan range was 100-1 500 m/z. Electrospray ionization source settings were as follows. Spray voltage:3.2 kV; sheath gas flow rate: 40 arb; aux gas flow rate:10 arb; capillary temperature: 320 ℃; polarity: positive and negative. MS/MS secondary scans were datadependent. The sequencing was conducted in Shanghai BIOZERON Co., Ltd., China.
1.10 Data preprocessing and annotation
MS raw data (.raw) files were converted into the mzML format using ProteoWizard and processed by the R package XCMS (version 3.2). The preprocessing results generated a data matrix consisting of retention time,mass-to-charge ratio (m/z) values, and peak intensity.OSI-SMMS (version 1.0, Dalian Chem Data Solution Information Technology Co., Ltd., China) was used for peak annotation after XCMS data processing with an in-house MS/MS database.
1.11 Data analysis
1.11.1 Statistical analysis of the general results
The measurement data conforming to normal distribution were presented by mean ± standard deviation (±s). The student’st-test was used to compare the two groups’ measurements.P<0.05 indicated that the difference was statistically significant.1.11.2 Metabolomics data analysis
The ProteoWizard software was used to convert the original data into the mzXML format for analysis. The R package XCMS was used for retention time correction,peak identification, peak extraction, peak integration,peak alignment, and other tasks. The OSI-SMMS software (version 1.0, Dalian Dashuo Information Technology Co., Ltd., China) in conjunction with a specific database was used for substance identification.
After the processed data were obtained, principal component analysis (PCA) was conducted to investigate the degree of aggregation of the samples. PCA can visualize metabolic differences between groups and variations of samples in the same group. The LC-MS data were converted by Paletto. The qualitative method for differential metabolites involved matching the exact molecular mass with the Merlin online database. The metabolites with aP-value of <0.05 were considered statistically significant. The metabolic pathways were determined using MetaboAnalyst 3.0. Significant metabolites were selected based on the fold change(control/model) >2.00 or ≤0.05, and the variable importance projection (VIP) >1.0[19-20].
2 Results
2.1 General condition
Before modeling, all the rats had smooth and colored fur, a regular diet, and normal limb movements. After successful modeling, rats in the model group and the moxibustion group generally showed reduced coat gloss,anorexia, and reduced mobility. Some rats even limped.The rats in the model group had severely restricted joint movements and even broken toes, whereas rats in the control group moved as usual. After three weeks of moxibustion intervention, the skin color of the rats in the moxibustion group was restored; their food intake gradually increased; the redness and swelling of their joints decreased, and the limb activity of some rats recovered.
2.2 Body mass
The body mass of rats in the model and moxibustion groups was not significantly different from that in the control group before modeling (P>0.05). Compared with the control group, the body mass gain of rats in the model group and the moxibustion group significantly slowed down after modeling (P<0.05). After one and two weeks of moxibustion treatment, the body mass of rats in the model and moxibustion groups was notably lower than that in the control group (P<0.05). After three weeks of moxibustion treatment, the body mass of rats in the model group was still lower than that in the control group (P<0.05), but the body mass of rats in the moxibustion group gradually recovered and was substantially higher than that in the model group(P<0.05). It is shown in Figure 1.

Figure 1. Body mass of the rats (n=8)

Figure 2. Hind paw volume of the rats (n=8)
2.3 Hind paw volume
The hind paw volumes of the three groups were not statistically different before modeling (P>0.05). After successful modeling, compared with rats in the control group, the hind paw volumes of rats in the model group and the moxibustion group substantially increased(P<0.05). After three weeks of moxibustion intervention,the hind paw volume of rats in the moxibustion group was lower than that in the model group (P<0.05). It is shown in Figure 2.
2.4 AI score
The AI scores in the model group and the moxibustion group were not remarkably different before moxibustion treatment (P>0.05), but were significantly different compared with the control group(P<0.05). After two and three weeks of moxibustion intervention, compared with the model group, rats in the moxibustion group had substantially lower AI scores(P<0.05), but were still significantly different from those in the control group (P<0.05). It is shown in Figure 3.

Figure 3. Changes in the arthritis index score of rats (n=8)
2.5 Serum cytokine levels
After successful modeling, compared with the control group, the serum IL-6, TNF-α, and PGE2levels substantially increased in the model group and the moxibustion group (P<0.05). After three weeks of moxibustion treatment, compared with the model group, the levels of serum IL-6, TNF-α, and PGE2in the moxibustion group considerably decreased (P<0.05). It is shown in Table 1.
Table 1. Comparison of serum IL-6, TNF-α, and PGE2 levels of rats in the three groups ( ±s pg/mL)

Table 1. Comparison of serum IL-6, TNF-α, and PGE2 levels of rats in the three groups ( ±s pg/mL)
Note: IL-6=Interleukin-6; TNF-α=Tumor necrosis factor-α; PGE2=Prostaglandin E2; compared with the control group, 1) P<0.05; compared with the model group, 2) P<0.05
TNF-α PGE2 43.58±14.90 33.01±3.01 129.58±56.451) 121.56±13.491)54.22±9.391)2) 46.72±16.201)2)
2.6 Histopathology
After moxibustion treatment, the rats’ left ankle joints in the three groups were randomly selected, and the histopathological changes in the ankle joints of rats in the three groups were observed (Figure 4). According to the pathological figures, the tissue structure of the rats in the control group was clear and did not change,whereas that in the model group had severe synovial swelling, hyperemia, edema, fibrous tissue proliferation,and a large amount of inflammatory cell infiltration. The rats in the moxibustion group had only mild joint synovial edema after the treatment, with a smoother surface and clearer layers than those in the model group.
2.7 LC-MS analysis
Data were collected from the test sample for LC-MS.The total ion chromatogram (TIC) of rats’ urine samples was analyzed by LC-MS (Figure 5). The components in the urine samples were well separated, and the baseline was stable. Moreover, statistical differences were observed among the three groups.
2.8 Pattern recognition of metabolites
The rats’ urine samples in the three groups were analyzed via PCA (Figure 6). Each point represented a sample in the experiment, and the PCA model was obtained. The quality control (QC) sample was relatively aggregated, proving that the experimental conditions were relatively stable. The PCA score charts were as follows: R2X=0.912, Q2=0.527 (Figure 6-A); R2X=0.905,Q2=0.639 (Figure 6-B). This result indicates that the urine samples in the three groups have a certain separation trend. The metabolites of rats in the moxibustion group tended to be the same as those in the control group. Still, they were different from those in the model group, indicating metabolic improvements.Furthermore, the results demonstrate that moxibustion is effective.
2.9 Differential metabolite identification and comparison
Differential metabolites (VIP>1,P<0.05, and fold change >2.00 or fold change ≤0.50) affected by RA(model groupvs. control group) were obtained. The differential metabolites that met these standards were considered the final differential metabolites. A total of 24 urine metabolites were obtained after three weeks of moxibustion intervention (Table 2).
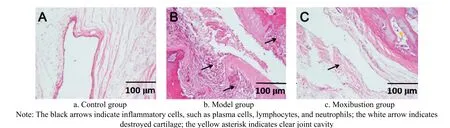
Figure 4. Histopathological changes of the left ankle joint of rats (hematoxylin-eosin staining, ×200)

Figure 5. The total ion chromatogram of the rats’ urine samples in the three groups

Figure 6. Principal component analysis diagram in each group

Table 2. Endogenous metabolites in the urine samples
2.10 Analysis of metabolic pathways involved in moxibustion intervention for RA
Metabolic pathway analysis was determined using MetaboAnalyst 3.0 (http://www.metaboanalyst.ca/).The main metabolic pathways (impact 0.1) included six metabolic pathways [alanine metabolism, taurine and hypotaurine metabolism, tricarboxylic acid (TCA) cycle,phenylalanine metabolism, tyrosine metabolism, and primary bile acid biosynthesis]. It was shown in Figure 7.The details of these metabolic pathways are described in Table 3. The network of the potential metabolic pathways associated with moxibustion treatment of CIA rats is shown in Figure 8.

Figure 7. Metabolic pathways in the urine samples

Table 3. The details of the metabolic pathways

Figure 8. Metabolic network of different metabolites in response to rheumatoid arthritis
3 Discussion
In this study, we monitored the body mass, AI score,swelling of the hind paw, cytokine levels, and histopathology of rats with RA. A total of 24 metabolites were identified in rats’ urine samples. The urine metabolite spectrum measured by LC-MS indicated that the changes in multiple metabolites and pathways were involved in RA. Analysis of metabolic pathways revealed that these differential metabolites were strongly related to six biological metabolic pathways.
3.1 Alanine metabolism
Alanine participates in alanine metabolism, which is related to muscle and energy expenditure associated with RA[21]. In this study, compared with rats in the control group, the RA rats had an abnormally high level of alanine and toe biting, which may be related to the severe pain caused by inflammation and erosion of bones and joints. After moxibustion intervention, the level of alanine in the urine of the rats was recovered,and the pain-induced behaviors decreased, suggesting that early intervention in rats with RA may prevent inflammation to a certain extent, delay disease progression, and relieve pain, consistent with the observations of SAFFARPOUR S,et al[22]. This result indicates that moxibustion may treat the pain caused by RA by regulating alanine metabolism.
3.2 Taurine and hypotaurine metabolism and primary bile acid biosynthesis
Taurine is not only involved in the taurine and hypotaurine metabolism but also in the primary bile acid biosynthesis. Taurine can protect lymphocytes and regulate cellular immunity[23-26]. In our study, the increase in taurine in the model group suggests that taurine may be an important factor in oxidative stress-related inflammation. After moxibustion treatment, the level of taurine decreased, consistent with the findings of WANG H,et al[27]. The biosynthesis of primary bile acids can promote the digestion and intestinal absorption of hydrophobic nutrients[28]. In this experiment, rats in the model group showed loss of appetite and dim coat color, suggesting that the intestinal circulation of bile acids may be disturbed.After moxibustion, the food intake in the rats increased,and the color of their fur and body mass were recovered. Therefore, moxibustion may increase appetite, protect immune cells, and protect the body.
3.3 TCA cycle
The TCA cycle is the strongest metabolite for diagnosing autoimmune diseases, and is correlated with disease activity[29-30]. In this study, the citric acid concentration of rats in the model group decreased.After moxibustion intervention, compared with the model group, the citric acid level in the moxibustion group increased, and the body mass, spirit, and coat color of the rats returned to normal. Oxoglutaric acid can reduce inflammatory factors (such as IL-6) and improve the exercise endurance of rat muscles[31-32]. In this study, the concentration of oxoglutaric acid increased after moxibustion intervention. The activity tolerance of the rats increased, and their AI scores and hind paw swelling improved remarkably. Therefore,moxibustion may improve the tricarboxylic acid cycle and promote changes in the hypoxic microenvironment in RA. These findings are consistent with the previous study[33].
3.4 Phenylalanine metabolism
Phenylalanine, 2-methyl hippuric acid, and phenethylamine are involved in the metabolism of phenylalanine. 2-methyl hippuric acid can be formed by phenylalanine and finally excreted in the urine. In this study, the metabolism of 2-methyl hippuric acid in the model group increased, which may be one of the reasons for the decline in phenylalanine. After moxibustion intervention, the levels of phenylalanine and 2-methyl hippuric acid decreased.Phenylethylamine provides energy and improves mood[34-35]. Compared with the control group, the concentration of phenethylamine increased in the model group, which impaired the mental state.Therefore, moxibustion treatment may improve the rats’ mental state by decreasing the concentration of phenylacetic acid.
3.5 Tyrosine metabolism
Hydroxylase catalyzes phenylalanine to oxidize to tyrosine, which is an important neurotransmitter and hormone[36-38]. In this study, the tyramine concentration of rats in the model group increased, and their activities decreased. However, the concentration of tyramine decreased after moxibustion intervention. This result indicates that moxibustion may improve tyrosine metabolism disorder. Indole-5,6-quinone is a tyrosine metabolite, and its metabolic disorder can cause melanin metabolism disorder in the body. The mechanism of this metabolite in RA has not been elucidated yet.
3.6 Possible mechanisms of moxibustion in treating RA
Moxibustion is one of the oldest external treatments in TCM. Moxibustion can warm meridians and dispel cold pathogens. This study showed that moxibustion treatment of RA affected various metabolic pathways.Therefore, moxibustion’s thermal stimulation can promote blood circulation, improve the cell growth environment, regulate metabolism, and improve the functions of the immune system.
In this study, the CIA model was successfully established. The results showed that moxibustion improved RA rats’ signs of fatigue, appetite, and activities, reduced toe swelling, lowered AI score,decreased serum cytokine level, and improved ankle pathology. Moxibustion has an antiarthritic effect. We further explored the mechanism by which moxibustion treated RA via metabolomic analysis of urine which suggested that moxibustion may regulate 24 metabolites and the related metabolic pathways.
The present study was limited to animal experiments.Thus, clinical research must be performed.Metabolomic studies of the efficacy of moxibustion treatment for RA patients are needed to explore the mechanism further. In this study, the metabolomic detection was performed via LC-MS. This experiment is the continuation and expansion of the early-stage studies of our research group. The experimental results need to be verified and explored in future studies.
Conflict of Interest
There is no potential conflict of interest in this article.
Acknowledgments
This work was supported by the Projects of National Natural Science Foundation of China (国家自然科学基金项目, No. 81774383, No. 81904274); Nursing Advantageous Discipline Construction Project in Jiangsu Universities (江苏省高校护理优势学科建设项目,No. 2019YSHL008, No. 2019YSHL021); Postgraduate Research & Practice Innovation Program of Jiangsu Province ( 江苏省研究生科研创新计划项目,No. KYCX21_1802).
Statement of Human and Animal Rights
The treatment of animals conformed to the ethical criteria in this experiment.
Received: 20 January 2021/Accepted: 27 September 2021
 Journal of Acupuncture and Tuina Science2022年4期
Journal of Acupuncture and Tuina Science2022年4期
- Journal of Acupuncture and Tuina Science的其它文章
- Research on clinical application of manual therapy to tumor-related adverse reactions
- Observation on efficacy of thumbtack needle combined with pediatric Tuina for constipation in children caused by liver depression and Qi stagnation
- Clinical study on Tuina plus physical agents for lateral collateral ligament injury of ankle in gymnasts
- Efficacy of knee-balancing manipulation plus heat-sensitive moxibustion for knee osteoarthritis and its influence on CTX-Ⅰ, TRACP-5b,ADAMTS-4, and MMP-3
- Clinical observation of warm needling moxibustion plus lumbar traction for lumbar disc herniation
- Clinical observation of acupuncture plus acupoint sticking therapy for insomnia and its influence on subjective and objective sleep indicators
