Foliar applications of various nitrogen (N) forms to winter wheat affect grain protein accumulation and quality via N metabolism and remobilization
Xiaokang Lyu, Yang Liu, Na Li, Liban Ku, Yuting Hou, Xiaoxia Wen
College of Agronomy, Northwest A&F University, Yangling 712100, Shaanxi, China
Keywords:Nitrogen remobilization Source-Sink Gluten protein Grain filling Quality formation
A B S T R A C T Foliar nitrogen (N) application is an effective strategy to improve protein content and quality in wheat kernels, but the specific effects of N forms remain unclear. In a two-year field study, foliar application of various N forms (NO-3, urea, NH+4) at anthesis was performed to measure their effects on wheat grain protein accumulation, quality formation, and the underlying mechanisms. Foliar application of three N forms showed varying effects in improving grain gluten proteins and quality traits. Under NH+4 application, there was more post-anthesis N uptake for grain filling, with relatively strong increase in enzyme activities and gene expression associated with N metabolism in flag leaves at 8-20 days after anthesis(DAA), whereas its promotion of grain N metabolism became weaker after 20 DAA than those under NO-3 and urea treatments. More N was remobilized from source organs to grain under treatment with foliar NO-3 and urea. Genes controlling the synthesis of gluten protein and disulfide bonds were upregulated by NO-3 and urea at 20-28 DAA,contributing to increased grain protein content and quality.Overall,foliar applications of NO-3 and urea were more effective than those of NH+4 in increasing grain N filling.These findings show that manipulating the source-sink relationship by reinforcing grain N metabolism and N remobilization is critical for optimizing grain protein accumulation and quality formation.
1. Introduction
Wheat(Triticum aestivumL.)is one of the most important staple cereals worldwide, providing approximately 20% of calorie and protein supply[1].Over 85%of the harvested grain of wheat is processed into flour derivatives for consumption because of the unique viscoelasticity of flour dough [2]. The product quality of grain flour can be evaluated using parameters such as Zeleny sedimentation,maximum resistance,and development time[3].With the gradual improvement of human living standards, the demand for high-quality wheat has increased. Thus, there is a need to increase wheat grain quality to increase farmers’ income and wheat processing quality.
The quality of wheat flour is closely associated with the content and composition of grain proteins[4].In mature grain,storage proteins (gliadin and glutenin)make up over 80% of the total protein,constituting gluten proteins that play essential roles in flour quality. After blending with water, gluten proteins can produce a continuous three-dimensional network by forming disulfide bonds through intermolecular and intramolecular forces, resulting in the specific properties of viscosity and elasticity [5]. The glutenin macropolymer (GMP) is an indicator of the processing quality of flour dough.
A series of complex biochemical processes are involved in N metabolism during grain filling [6]. Nitrate reductase (NR) is the first key enzyme in N assimilation [7]. Glutamine synthetase (GS)and glutamate pyruvate transaminase (GPT) function in N assimilation and biosynthesis of amino acids not only in source organs but in kernels, with the genes involved reported [8,9] to be positively associated with N remobilization. Previous studies [10,11]have indicated that the genesTaASN1,TaWCP2,andTathiol proteaseregulate proteolysis and conversion among amino acids, which supply substrates for protein synthesis. The expression ofTaPDIL2-1, encoding protein isomerase (PDI), regulates the formation of disulfide bonds,the bridges determining the polymerization of gluten proteins, contributing to their synthesis and functional properties [12,13]. Thus, manipulation of N metabolism and protein synthesis is a promising strategy for improving wheat protein accumulation and quality formation.
Nitrogen (N) is a structural component of metabolic enzymes and grain protein of wheat.The level of N supply influences wheat grain protein and quality [14]. Aside from a small amount of N taken up from the environment during grain filling, almost 60%-90% of grain N at maturity is remobilized from vegetative organs[6,15]. The supply capacity of N from vegetative organs (source)and the strength of protein synthesis in kernels (sink) contribute to grain protein accumulation collaboratively, but which is the dominant factor remains in dispute [9,16]. Thus, the source-sink relationship in grain N filling and the regulatory role of exogenous N supply,because they affect grain protein accumulation and quality formation, merit study.
Nitrogen fertilizers are widely applied to sustain crop production. Urea (amide N), nitrate (N) and ammonium (N)are major forms of N fertilizer. The acquisition of nitrate, ammonium, and organic N (e.g., urea) by crops is mediated by different gene families,with specific high-affinity and low-affinity transport systems [17,18]. Besides being absorbed by crops, urea degrades into ammonium in the environment [19]. Nitrate must be converted to ammonium for N assimilation in crops, as ammonium is the final form of inorganic N for amino acid synthesis. Owing to their distinct chemical and biological characteristics, various forms of N exert different effects on crop growth. For wheat, the form of N has been found [20-23] to affect many physiological characteristics relevant to organic growth and nutritional metabolism. Recent research [24] has shown that wheat kernels treated with urea and nitrate have a stronger ability to synthesize starch under drought stress than those treated with NH+4. However, the way in which the various N forms influence grain protein accumulation and quality formation in wheat remains unclear.
Foliar application of fertilizers is an effective strategy for rapidly addressing crop undernutrition at later growth stages, when the availability of soil nutrients may decrease owing to root senescence and dry weather conditions [25]. Late-season foliar N application is beneficial for reducing N loss and environmental risk caused primarily by denitrification and N leaching [4,26]. There are few reports of the effect of late-season foliar application of N on wheat grain protein and quality.
In the present study, foliar application of urea, nitrate, and ammonium was conducted at anthesis, with the aim of determining how various N forms influence N assimilation, grain protein,and quality in wheat.Our approach was to measure the accumulation of various protein components, the amount of N remobilization and uptake, and the expression of genes and enzymes involved in N metabolism and protein synthesis during grain filling.
2. Materials and methods
2.1. Experimental design and treatments
The study was conducted from 2017 to 2019 at Northwest A&F University, Yangling, Shaanxi province, China (34°18′N, 108°01′E),at an elevation of 527 m. The soil in the experiment station is an Eum-Orthrosol (Chinese soil taxonomy), with mean bulk density of 1.35 g cm-3. The concentrations of available N, phosphorus,and potassium were 56.97,22.46 and 115.47 mg kg-1,respectively.The pH and organic matter content in the top 0-20 cm in the field were 7.37 and 12.33 g kg-1. The mean daily temperature and precipitation during the two years of wheat growth are shown in Fig. S1.
Two wheat cultivars, Xinong 20 and Xiaoyan 22, were sown on October 18, 2017, and October 4, 2018, respectively, at a sowing density of 150 kg ha-1. Before sowing, urea (nitrogen content:46%) and diammonium orthophosphate (phosphate content: 46%)were applied as basal fertilizers, whose contents of nitrogen and phosphate were 209 kg ha-1and 150 kg ha-1respectively.
At anthesis,foliar application treatments were applied for three successive days. For foliar application of nitrate nitrogen (),urea, and ammonium nitrogen (), solutions of sodium nitrate,urea,and ammonium chloride were sprayed at equal amounts of N(10.35 kg N ha-1day-1). Deionized water was used as a control(CK). All solutions and deionized water contained 0.01% (v/v)Tween-20,sprayed evenly at the rate of 750 L ha-1with a backpack sprayer. The foliar application was performed at sunset to avoid salt damage from strong sunlight and high daytime temperatures.A randomized complete block design was used. Each treatment had three replicates, with a plot size of 3 m × 3 m.
2.2. Sampling and measurement
At anthesis, 400 spikes that flowered on the same day were tagged for sampling in each experimental plot. During the anthesis-to-maturity stage,samples were prepared from 30 wheat plants,which were cut from the stem base at 4-day intervals.Half of the samples were dried to constant weight at 60°C after heating for 15 min at 105 °C. The dried samples were separated into stem+sheath,leaf,glume+rachis,and grain,for determining characteristics associated with N.Grain and flag leaves were separated from the other half of the samples and stored at-80°C after freezing in liquid N for 3 min, for measurement of enzyme and gene expression.
2.3. Measurement of yield and yield components
At maturity,wheat plants in three 1-m2areas were harvested to measure yield and yield components(number of spikes per square,number of spikelets per spike, and grain weight). Twenty spikes were randomly selected from the harvested plants to calculate the spikelet number for three replicates. The wheat was naturally dried to 13%grain moisture content before grain weight and quality parameters were measured.
2.4. Near-infrared reflectance (NIR) analysis of grain
A Diode Array 7200 NIR spectrometer (Perten Instrument AB,Stockholm, Sweden) was used to measure grain quality: wet gluten,Zeleny sedimentation,maximum resistance,absorption,development time, and stability time. The data collection mode was reflectance, with wavelengths ranging from 950 to 1650 nm. Each sample was evaluated in triplicate.
2.5. Extraction of protein components and glutenin macropolymer(GMP) of grain
Grain samples at maturity and 4, 8, 12, 16, 20, 24, and 28 days after anthesis (DAA) were milled into powder and sifted through an 80-mesh sieve for the determination of protein. Extraction of protein components was performed as described by Amagliani et al.[27]with modifications.Grain flour(0.2 g)was blended with 5 mL deionized water by vortexing and extracted at 50 °C for 30 min.After centrifugation at 5000×gfor 30 min,the supernatant was collected for determining albumin.The residue was extracted with 5 mL of 5%NaCl at 25°C for 30 min.The homogenate was centrifuged at 5000×gfor 30 min to obtain globulins.The residue was blended with 75% ethanol at 25 °C for 30 min and centrifuged at 5000×gfor 30 min to extract the gliadin. Finally, the residue was extracted with 0.2% NaOH (pH 11) at 30 °C for 30 min. After centrifuging at 5000×gfor 30 min,glutenin was obtained.Each extraction was repeated twice.GMP was extracted as described by Zhang et al. [28]. Grain samples (0.05 g) were blended with 1 mL 1.5%sodium dodecyl sulfate solution at 37 °C for 2 h and centrifuged at 15,500×gfor 15 min to obtain GMP.
2.6. Analysis and calculation of plant N and grain protein
Plant parts (including stem + sheath, leaf, glume + rachis, and grain) at anthesis and maturity were weighed and ground into powder for N determinations. N concentration was determined by the Kjeldahl method [29].
The contents of total protein, protein components and GMP were also determined by the Kjeldahl method, by multiplying the N concentration by a conversion coefficient, 5.7.
The N amount of an organ (NAO), the amount of N remobilization from an organ to grain(ANR)and the amount of post-anthesis N uptake(APN)were calculated following previous studies[30,31]:

where NAOanthesisand NAOmaturityare NAO at anthesis and maturity respectively. NAOgrainmeans NAO of the grain at maturity. ANRtotalis the sum of the ANR in stem + sheath, leaf, and glume + rachis.
2.7. Determination of free amino acid and activities of enzymes involved in N metabolism in flag leaves and grain during grain filling
The activity of nitrate reductase (NR) (EC 1.6.6.1) in flag leaves was determined following Hu et al.[32].First,0.3 g of fresh leaves was ground into powder with liquid N and phosphate buffer.After centrifugation at 12,000×gfor 20 min, the supernatant was transferred into a reaction mixture containing a solution of nicotinamide adenine dinucleotide (NADH) and KNO3. In the control solution,NADH was replaced with sodium phosphate.The mixture was incubated at 25 °C for 30 min in the dark. Sulfanilamide and N-1-naphthylethylenediamine dihydrochloride were added to terminate the reaction. After centrifugation at 12,000×gfor 15 min,the absorbance of the supernatant was measured at 540 nm and used to calculate NR activity.
Following Ding et al.[33],0.3 g fresh samples in grains and flag leaves were ground into powder to determine the activities of glutamine synthetase (GS) (EC 6.3.1.2) and glutamic-pyruvic transaminase (GPT) (EC 2.6.1.2). After centrifugation at 26,000×gfor 20 min at 4 °C, the supernatant was retained for reaction. The reaction mixture for GS was a solution containing imidazole-HCl,MgSO4, and Na adenosine triphosphate. After incubation at 25 °C for 5 min, hydroxylamine was added to the mixture and it was incubated at 37°C for 30 min.The mixture was then stained using a mixed reagent (10% FeCl3·6H2O, 50% HCl, 24% trichloroacetic acid) for 25 min, before centrifugation at 15,000×gfor 15 min.The absorbance of the supernatant was measured at 540 nm for the calculation of GS activity.The solution of the GPT reaction mixture included alanine, 2-oxoglutarate, and phosphate buffer. After incubation at 37 °C for 30 min, the reaction was stopped by addition of dinitrophenylhydrazine solution and allowed to stand for 30 min. The solution was then mixed with NaOH and held for 10 min and absorbance was measured at 500 nm to calculate GPT.
Free amino acid content (FAA) in grains and flag leaves was determined by extraction with acetic acid and ninhydrin treatment[34].All analyses of FAA and enzymes were performed in triplicate.
2.8.Measurement of gene expression in flag leaves and grains by qRTPCR
RNA was extracted from leaves and grain at 4, 12, 20, and 28 DAA using the E.Z.N.A.Plant RNA kit(Omega Bio-Tek Inc.,Norcross,GA, USA). cDNA was synthesized from RNA samples using the PrimeScriptTMRT reagent kit (TaKaRa Bio Inc., Shiga, Japan). cDNA was mixed with primers and SYBR premix Ex Taq II kit (TaKaRa)to perform quantitative real-time PCR(qRT-PCR)using the QuantStudio 3 real-time PCR system(Applied Biosystems,Foster City,CA,USA). The primers (based on previous work [11,35,36]) are described in Table S1.qPCR was initiated at 95°C for 30 s,followed by 40 cycles of 95°C for 3 s,60°C for 30 s,95°C for 15 s,60°C for 1 min,and 95°C for 15 s.Three biological replicates were used for each treatment. The relative expression of genes was determined by the 2-ΔΔCTmethod [11], where the CK of Xiaoyan 22 at 4 DAA was used as the reference to calculate ΔΔCT, withTAβ-actinas internal control.
2.9. Statistical analysis
All data were subjected to one-way analysis of variance(ANOVA). Samples were analyzed in triplicate and mean values were used for comparison. Means were compared using least significant difference (LSD) at the level of 0.05. The analysis of variance was conducted by SPSS 17.0 (IBM, New York, NY, USA).
3. Results
3.1. Yield and yield components
As shown in Table 1, foliar application of N increased the grain yield of Xinong 20 and Xiaoyan 22 in 2017-2018 and 2018-2019.The grain yield of Xinong 20, as a new cultivar, was higher than that of Xiaoyan 22. The grain yield responses to the three N forms varied in the following order:urea >NO-3>NH+4.No difference was found between the foliar N application and control for spikes per hectare and spikelets per spike. Grain weight was increased by foliar N application.
3.2. Grain quality traits
Grain quality traits(Table S2)differed in 2017-2018 and 2018-2019, although the effects of cultivar and N form on quality traits were similar for each year. Xinong 20 showed significantly higher values than Xiaoyan 22 for all grain quality traits irrespective of the growing season. In both seasons, the highest values of wet gluten,Zeleny sedimentation, maximum resistance, absorption, and stability time were found undertreatments, whereas there was no significant difference in development time betweenand urea.Application of urea significantly improved most of the quality traits of two cultivars compared withand control in both growing seasons. However, urea showed no significant effects on Zeleny sedimentation and stability time of Xiaoyan 22 in 2017-2018 and stability time of Xiaoyan 22 in 2018-2019. For thetreatment, wet gluten, maximum resistance, absorption, and stability time of Xinong 20 were significantly improved relative to the control in both growing seasons, but were weaker than those underand urea treatments. The quality traits of Xiaoyan 22 were not significantly affected byapplication, irrespective of the growing season.Overall,the regulatory effect of foliar N application on the grain quality traits of Xinong 20 was stronger than that on Xiaoyan 22. Treatment withled to greater improvement of grain quality in both cultivars,but the improvement effect ofwas weaker than those underand urea treatments.
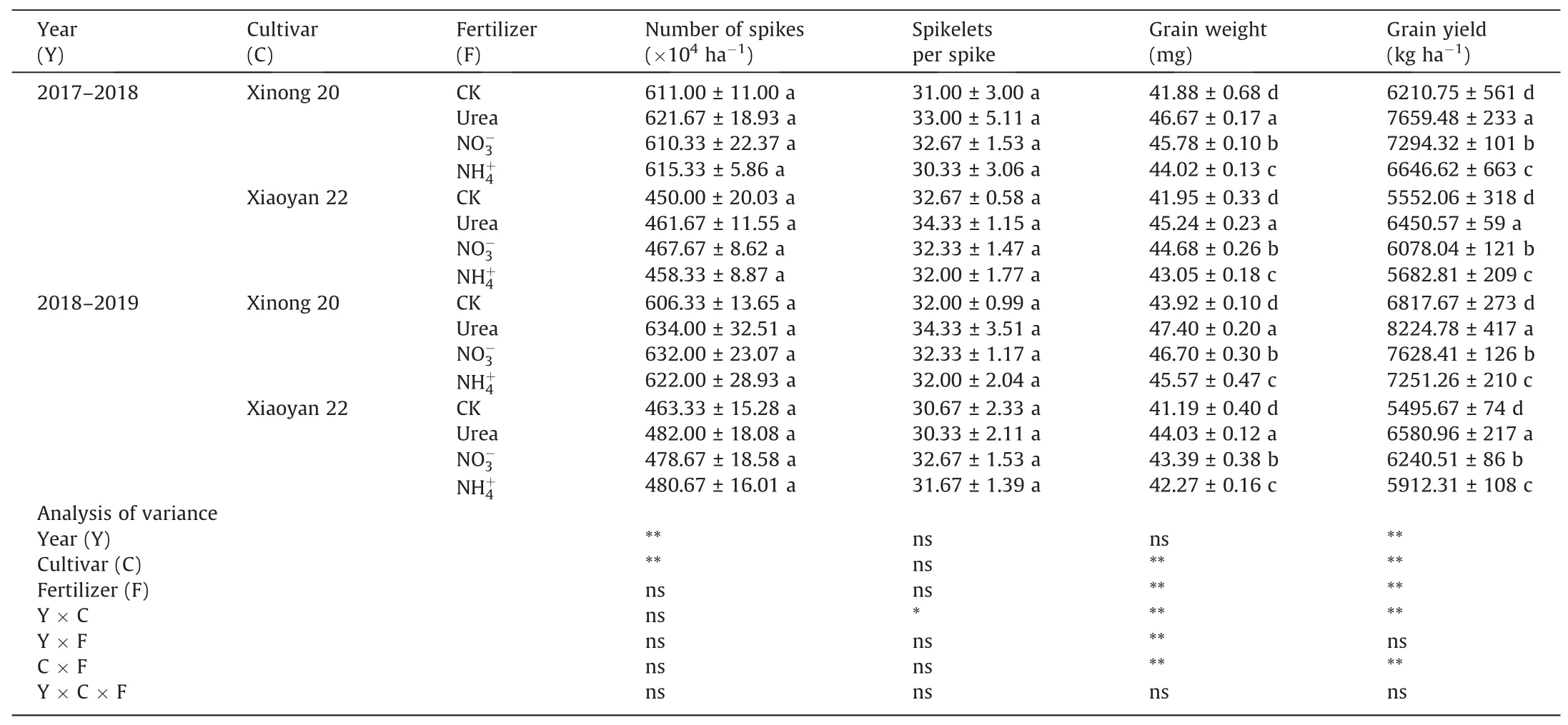
Table 1Effects of foliar application of three N forms on grain yield and yield components.
3.3. Contents and dynamic accumulation of total protein, GMP, and protein components
Contents of total protein and gluten proteins(gliadin and glutenin) were higher in Xinong 20 than in Xiaoyan 22 (Fig. 1 A, B).Foliar N application significantly increased the contents of total protein, gliadin and glutenin. Application ofshowed a stronger effect on total protein and gluten proteins,compared with urea andtreatments. However, the difference between urea andwith respect to gliadin in Xiaoyan 22 was not significant. No significant effect was observed for albumin and globulin, except for albumin in Xiaoyan 22 undertreatment. The effects of the three N forms on GMP content were similar to those on glutenin (Fig. 1 C, D), except forin 2017-2018.
Effects of cultivars and N forms on dynamic changes in total protein,GMP,and protein components in 2017-2018(Fig.S2)were consistent with those in 2018-2019(Fig.S3).Accumulation of protein in Xiaoyan 22 was weaker than that in Xinong 20. Total protein content declined until 16 DAA, and then increased continuously. The contents of GMP, gliadin and gluten accumulated rapidly beginning at the middle grain filling stage. The albumin and globulin contents remained at low levels from 20 DAA.In comparison with the control,the contents of albumin and globulin were not significantly affected by N application. In contrast, foliar N application promoted the accumulation of total protein, GMP,and gluten proteins.The differences in the application of the three N forms on the accumulation of total protein,GMP and gluten proteins became significant mainly from the middle grain filling stage.Furthermore,and urea treatments showed higher promotion effects on the accumulation of total protein, GMP and gluten proteins thantreatments.
3.4. N remobilization and post-anthesis N uptake during grain filling
More than 60% N in mature grain came from the leaf,stem+sheath,and glumes+rachis(Fig.1 E,F).Foliar N application increased the N content in grain, with the three N forms showing
3.5. Changes in activities of NR, GS, GPT, and free amino acid content(FAA) in flag leaves and grain during grain filling
Results from the 2017-2018 season (Figs. S4, S5) were similar to those in 2018-2019(Figs.2,3).In flag leaves,NR activity continued to decrease,and GS activity peaked at 4 DAA before decreasing.During the decline in GPT activity,there was a slight increase at 8-12 DAA.FAA content in flag leaves attained a maximum at 12 DAA,and dropped subsequently.Foliar N application slowed the decline of activities in NR, GS and GPT and elevated FAA levels during the early and middle grain filling periods, whereas no such effect of foliar N application in the late grain filling stage was found.Underapplication, flag leaves had much higher activities of NR and GS at 8-20 DAA, with higher activities of GPT at 8-16 DAA, followed by urea and, although the difference between urea andwas slight. In contrast, the elevation of FAA level was lower underthan under the other two N forms.
In grain, the activities of GS, GPT and FAA content were higher in Xinong 20 than in Xiaoyan 22. GS activity showed a constant decrease during grain filling. GPT activity peaked at 20 DAA and then decreased steadily. For the FAA level, the peak value was achieved at 20 DAA in Xinong 20 and at 16 DAA in Xiaoyan 22.Foliar N application significantly elevated activities of GS, GPT,and FAA levels in grains until the late grain filling period. Treatment withresulted in higher activities of GS from 4 to 8 DAA, with higher activities of GPT at 4-8 DAA. Treatment withresulted in greater improvement in GS and GPT at 20-24 varying effects. Alhough N uptake was promoted after anthesis,and urea treatments resulted in a much higher amount of grain N remobilized from vegetative organs, especially in leaves supplied with.The amount of post-anthesis N uptake supplied bywas significantly higher than that supplied by the other treatments, whereas its promotion of N remobilization in vegetative organs was weaker than that of theand urea applications,even with no significant difference in stem + sheath compared with the CK entirely.DAA and 16-24 DAA respectively,followed by urea.Grain supplied withreached a much higher peak value of FAA,with urea andnext.

Fig.1. Effects of foliar application of three N forms on contents of grain protein,protein components(A and B),GMP(C and D),N remobilization,and post-anthesis N uptake(E and F).ANR,amount of N remobilization from the vegetative organ to grain.APN,amount of post-anthesis N uptake.Vertical bars represent standard deviation.Values with a different letter for the same cultivar and same type of bar are significantly different at P <0.05. For CK, deionized water was sprayed on leaves at anthesis.
3.6. Expression of genes involved in N metabolism and protein synthesis in flag leaves and grain
In flag leaves, expression ofTaGS1remained relatively stable from 4 to 20 DAA, peaking at 12 DAA before dropping sharply at 28 DAA,while the expression level ofTaGS2continued to decrease,with a higher level at 4-12 DAA than that ofTaGS1(Fig.4 A-D).The expression level ofTaAlaATreached a peak value at 12 DAA before declining rapidly from 20 to 28 DAA (Fig. 4 E, F). Foliar N application increased the expression of these genes from 4 to 20 DAA.The expression levels forand urea treatments were much higher at 4-20 DAA, withTaGS2expression forbeing significantly higher than that for urea.TaASN1andTaWCP2were highly expressed at 20 and 28 DAA, whereas the high-level expression ofTathiol proteaseoccurred only at 28 DAA (Fig. 5). When these genes were highly expressed, their expression levels underand urea applications were much higher than under thetreatment.
In grain, expression ofTaGS1andTaGS2decreased with grain filling, changing gradually (Fig. 6 A-D). The expression levels ofTaAlaATandTaASN1peaked at 20 DAA before declining markedly(Fig. 6 E-H). Foliar N application significantly promoted the expression ofTaGS1,TaGS2,TaAlaATandTaASN1during 4 to 20 DAA. The expression levels ofTaGS1,TaGS2andTaAlaATunderand urea treatments were much higher at 4 DAA, whereas their expression levels underand urea were slightly higher than underand control at 20 DAA when differences among the three N forms inTaAlaATwere more pronounced. Similarly,underand urea treatmentsTaASN1showed higher expression levels after 20 DAA.
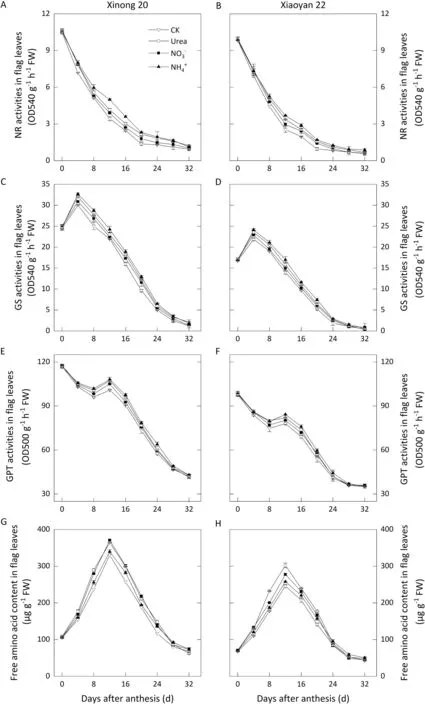
Fig.2. Effects of foliar application of three N forms on changes in enzymes and free amino acids in flag leaves in 2018-2019.Vertical bars represent standard deviation.For CK, deionized water was sprayed on leaves at anthesis.
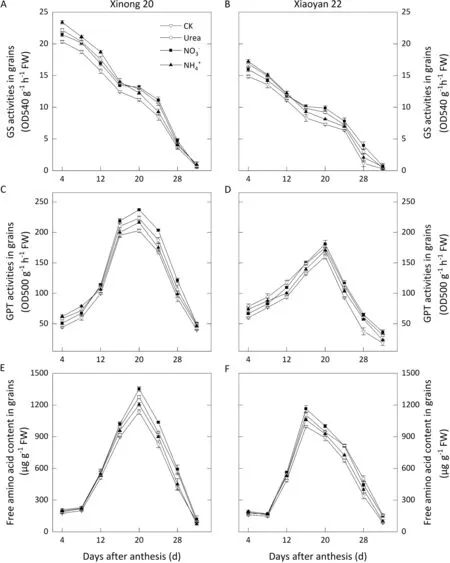
Fig. 3. Effects of foliar application of three N forms on changes in enzymes and free amino acids in grain in 2018-2019. Vertical bars represent standard deviation. For CK,deionized water was sprayed on leaves at anthesis.
The relative expression levels ofthe Tatransmembrane amino acid transporter protein gene(TaTAATP),TaPDIL2-1,Taγ-Gliadin,TaHMW-Bx7in grain were measured (Fig. 7).TaTAATPexpression remained at a high level at 12-20 DAA before dropping at 28 DAA.Expression ofTaPDIL2-1andTaγ-Gliadinshowed similar patterns, increasing markedly from 12 to 20 DAA. GeneTaHMW-Bx7was expressed later thanTaγ-Gliadin,peaking at 28 DAA.Foliar N application increased the expression ofTaTAATPat 4-20 DAA. The expression level ofTaTAATPunderwas the highest at 20 DAA,followed by urea.Foliar application ofand urea promoted the expression ofTaPDIL2-1,Taγ-Gliadin, andTaHMW-Bx7during 20-28 DAA, especially when they reached peak values, where the expression level under urea was significantly lower than that underand higher than that under.The significant promotion effect ofapplication on their expression occurred only at 20 DAA forTaPDIL2-1andTaγ-Gliadinand at 28 DAA forTaHMWBx7, respectively.
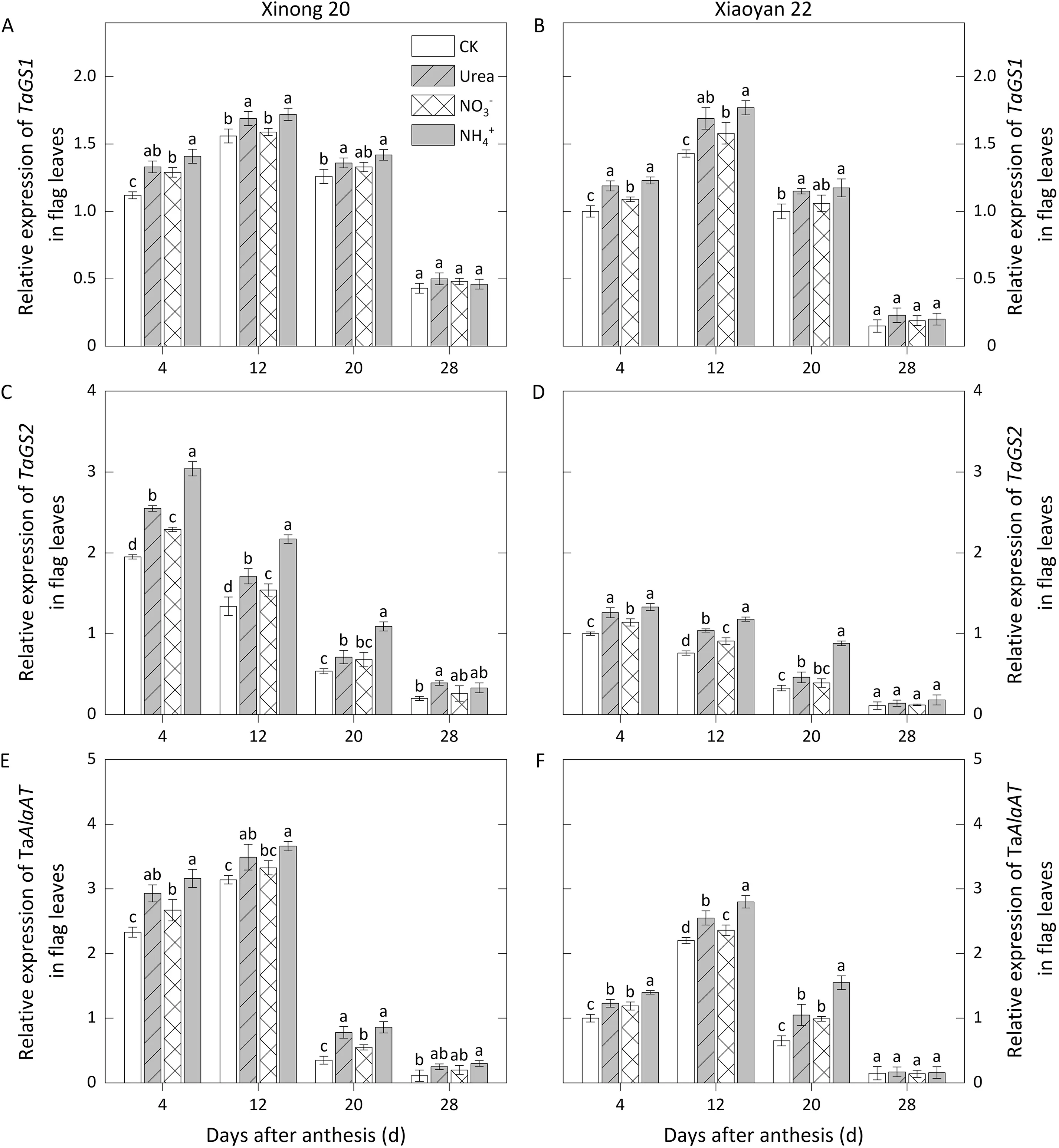
Fig. 4. Relative expression levels of TaGS1, TaGS2, and TaAlaAT affected by foliar application of three N forms in flag leaves during grain filling in 2018-2019. Values with a different letter for the same cultivar and same group of columns are significantly different at P <0.05.Vertical bars represent standard deviation.In CK,deionized water was sprayed on leaves at anthesis.
4. Discussion
In the present study, the two-year’s results showed the same trends, as the climate characteristics of the wheat growing season in 2017-2018 and 2018-2019 were similar.Foliar N application at anthesis has been reported to affect grain quality positively[37].In our study, the NO-3treatment showed the strongest effect on the improvement of grain quality traits. In contrast,application improved grain quality traits slightly, and significantly only for Xinong 20. Moreover, our results showed that the grain quality traits of Xinong 20 were superior to those of Xiaoyan 22. The promotion of foliar N application on grain quality was more effective for Xinong 20 than for Xiaoyan 22,indicating that the good-quality cultivar Xinong 20 has a higher potential for improved quality.
In the present study, foliar application of N forms showed almost no significant effects on albumin and globulin contents,indicating that albumin and globulin are regulated mainly by genotype instead of external N supply, which was in accordance with the report by Raymbek et al.[38].Proteins in wheat grain are considered the major factors that determine grain quality characteristics [39]. In this study, contents of total grain protein, gluten proteins and GMP in mature grain also showed strong positive correlations with most quality traits(Table S3).Moreover,different N forms promoted the accumulation of gliadin, glutenin, and GMP during the middle and late grain filling periods,with the strongest effect obtained forapplication.From these results we can conclude that foliar application of different N forms increased the accumulation of gliadin, glutenin, and GMP to different extents,which may be one of the primary causes of the differing responses of grain quality to N form.
N remobilization from vegetative organs is the primary origin of grain N filling, followed by N uptake during this period [6]. In the present study,the leaf was the largest source of N for grain,a finding consistent with those of previous studies[9,31].At the onset of grain filling,N stored in vegetative organs is remobilized,gradually leading to protein hydrolysis and N deficiency in vegetative organs,and in turn to senescence[6].Our results showed that foliar application of the three N forms promoted N remobilization and N uptake after anthesis, thus improving the overall level of grain N,although the specific effects differed.Theapplication showed a greater effect on increasing N uptake after anthesis, possibly delaying senescence by relieving N deficiency in source organs.Lv et al.[24]also showed thatextended grain-filling duration.However, dry weather, low availability of soil mineral nutrients,and lower activity in the root system determined the limited potential of N uptake at the later period of wheat growth, especially in comparison with N remobilization [40]. N remobilization was aslo reported[41]to be positively associated with grain quality. In the present study,and urea treatments increased N remobilization in higher level compared with, which may be the reason why the promotion of grain protein accumulation and quality byand urea treatments was stronger than that by.
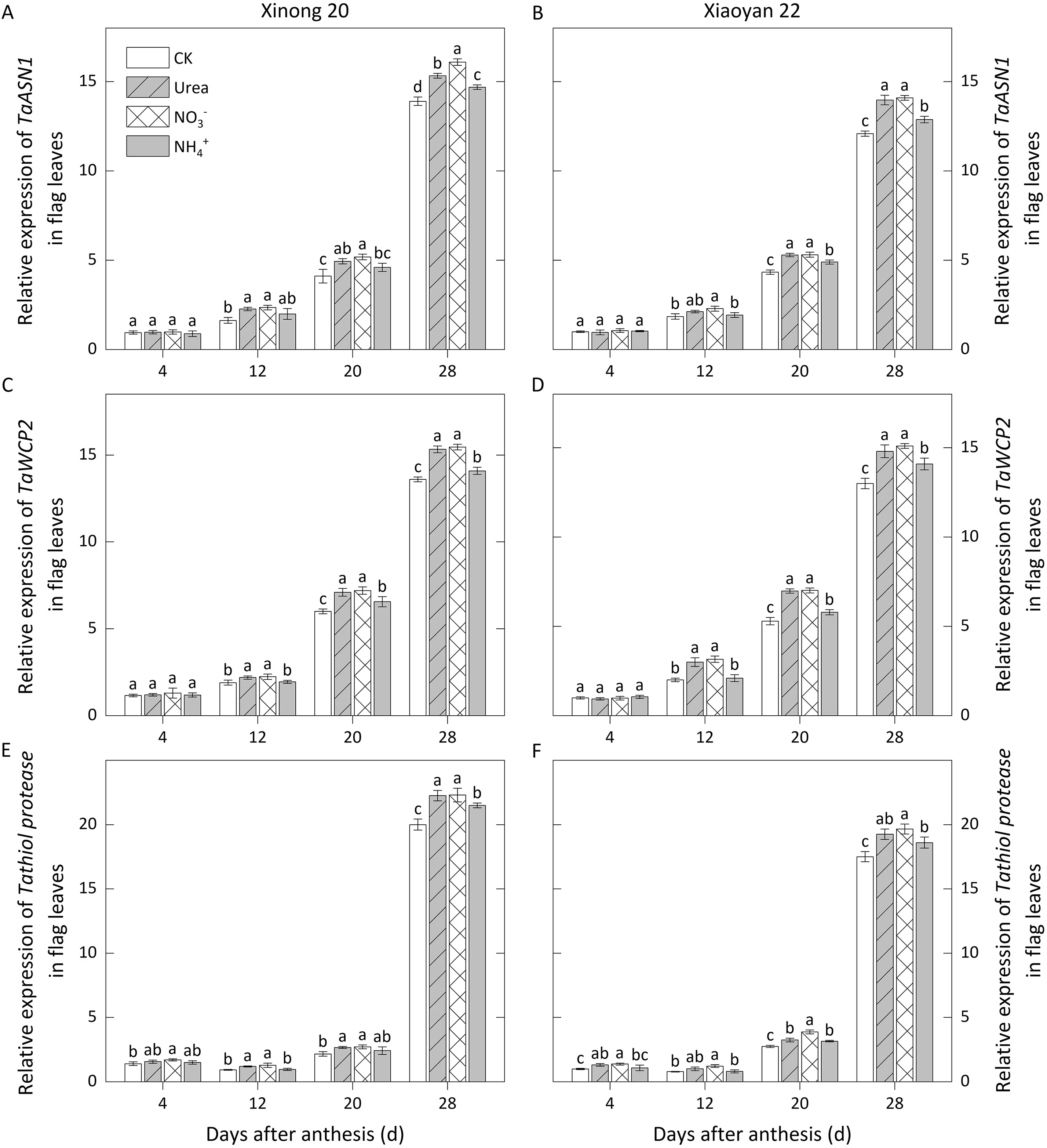
Fig.5. Relative expression levels of TaASN1,TaWCP2,and Tathiol protease affected by foliar application of three N forms in flag leaves during grain filling in 2018-2019.Values with a different letter for the same cultivar and same group of columns are significantly different at P <0.05.Vertical bars represent standard deviation.In CK,deionized water was sprayed on leaves at anthesis.
NR plays an essential role in N assimilation. In the present study, theapplication showed a stronger effect on increasing NR activity, and possibly caused more post-anthesis N uptake. At the early and middle grain filling stages, the treatments withand urea improved the activities of GS and GPT more markedly.GS and GPT function in N metabolism[10].Thus,the increase of N metabolism in source organs was stronger forand urea treatment than forapplication. This result was confirmed by the expression ofTaGS1,TaGS2, andTaAlaAT. In source organs,FAA content depends on the hydrolysis of protein macromolecules and the metabolism of amino acids,regulated byTaASN1,TaWCP2,andTathiol protease,the key genes involved in leaf senescence[41].Gene expression analysis in flag leaves showed thatand urea application upregulated the expression levels ofTaASN1,TaWCP2,andTathiol proteaseto a greater extent thantreatment at 20 and 28 DAA, which could explain the higher level of FAA in leaves underand urea treatment.Amino acids function in N remobilization, with asparagine and glutamine being most involved [9].Overall, we may conclude that application ofand urea has a stronger effect on leaf senescence and N remobilization thanapplication.
Higher levels of GS and GPT in grain contribute to a stronger metabolism and demand for grain N [12,36]. In the present study,regulatory effects of various N forms on GS and GPT activities showed patterns similar to those of protein accumulation in grain.After the early grain-filling stage,the increase in GS and GPT activities was less undertreatment than underand urea treatments,indicating that the promotion bytreatment of grain N metabolism and protein accumulation began decaying.In contrast,the finding that treatments withand urea maintained higher GS and GPT activities during the middle and late grain filling periods suggests a higher demand for grain N filling. The increase by foliar N application of the expression of these enzymes occurred as a consquence of the upregulation of key genes encoding Nmetabolism enzymes,evidenced by the expression ofTaGS1,TaGS2,andTaAlaAT. Moreover, plants underand urea applications showed higher grain FAA contents at the middle and late grain filling periods, in agreement with the expression ofTaASN1andTaTAATP. The expression ofTaTAATPplays a central role in amino acid transport, indicating the strength of N remobilization and the N demand of grain[11].Once transferred to grain,amnio acids participate in N metabolism and protein synthesis. Therefore, we may conclude that foliar application ofand urea increased demand for grain N filling and accelerated N remobilization.
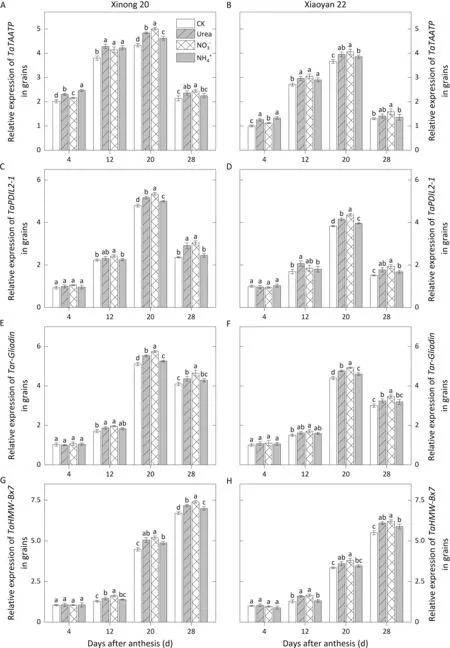
Fig.7. Relative expression levels of genes involved in protein synthesis affected by foliar application of different N forms in grains during grain filling in 2018-2019.Values with a different letter for the same cultivar and same group of columns are significantly different at P <0.05.Vertical bars represent standard deviation.In CK,deionized water was sprayed on leaves at anthesis.
The genesTaPDIL2-1,TaHMW-Bx7andTaγ-Gliadinremained expressed at high levels during the middle and late grain filling periods, as evidenced by the accumulation of gluten proteins.HMW-GSsand γ-gliadin proteins, encoded byTaHMW-Bx7andTaγ-Gliadin, are rich in cysteine residues and play dominant roles in forming the gluten network structure and contributing to gluten quality,whereas disulfide bonds act as bridges to stabilize the gluten network, regulated by protein disulfide isomerase encoded byTaPDIL2-1[1]. Thus, the middle and late grain-filling periods are crucial for gluten protein accumulation and quality formation in wheat grain. The increase by foliar application of, urea andof expression of these genes also focused on this period, possibly the main reason for the differing effects of various N forms on grain protein and quality.It has been reported that treatments of N fertilizers may have certain effects on the signals originating from specific tissues,which regulate gluten protein gene promoters[31].In future studies, the use of omics technology would shed further light on the regulatory mechanisms of various N sources in gluten protein accumulation.
N remobilization is regarded [35] as the critical factor for grain N filling,closely associated with N demand of sink(e.g.,grain)and the source-sink relationship, as the majority of grain N originates from remobilization from source organs. However, there exists debate whether the potential of grain N filling is limited by the sink side or source side[41-43].In our study,there was relatively high activity in the source under treatments with different N forms,strongest for,whereas promotion byapplication of grain N metabolism and protein accumulation began decaying at middle and late grain-filling periods, exactly the crucial period for gluten protein synthesis and quality formation.In contrast,foliar application of the other two N forms (especially) increased grain N metabolism and gluten protein synthesis, resulting in a more sufficient supply of amino acids.We surmise that the different extents of the regulatory effect on sink activity is the main reason for the differences between the effect of each N source on grain N accumulation and quality.
5. Conclusions
Under our experimental conditions, the good-quality cultivar Xinong 20 showed higher potential for grain quality improvement.Foliar N application increased grain N accumulation and quality,but the effect for each N form differed. Underapplication,there was more post-anthesis N uptake for grain filling, with greater enhancement of N metabolism in the source. In contrast,foliar application ofand urea at anthesis increased the activities of grain N metabolism and protein accumulation especially during middle and late grain-filling periods, in turn increasing grain N demand and N remobilization and leading to higher grain protein content and grain quality. Our results showed that foliar application ofand urea at anthesis could be an effective strategy for improving grain N filling.For optimizing grain protein accumulation and quality formation, it is critical to manipulate the source-sink relationship by elevating grain N demand and N metabolism activity, resulting in higher N remobilization.
CRediT authorship contribution statement
Xiaokang Lyu:Writing - original draft.Yang Liu:Writing -review & editing.Na Li:Formal analysis.Liban Ku:Investigation.Yuting Hou:Visualization.Xiaoxia Wen:Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (31971860).
Appendix A. Supplementary data
Supplementary data for this article can be found online at https://doi.org/10.1016/j.cj.2021.10.009.
- The Crop Journal的其它文章
- Research progress on the divergence and genetic basis of agronomic traits in xian and geng rice
- From model to alfalfa: Gene editing to obtain semidwarf and prostrate growth habits
- The chloroplast-localized protein LTA1 regulates tiller angle and yield of rice
- Genome-wide association study and transcriptome analysis reveal new QTL and candidate genes for nitrogen-deficiency tolerance in rice
- Advances in the functional study of glutamine synthetase in plant abiotic stress tolerance response
- Identification of microRNAs regulating grain filling of rice inferior spikelets in response to moderate soil drying post-anthesis

