Advances in the functional study of glutamine synthetase in plant abiotic stress tolerance response
Huyn Yin, Fn Yng, Xioyn He, Xuye Du, Ping Mu,, Wujun M,,
aCollege of Agronomy, Qingdao AgriculturalUniversity, Qingdao 266109,Shandong,China
bFoodFutures Institute and College ofScience,Health,Engineering andEducation, Murdoch University, Perth , WA 6 150,Australia
cSchool of Life Sciences,Guizhou NormalUniversity, Guiyang 550001, Guizhou,China
Keywords:Glutamine synthetase Plant Abiotic stress Nitrogen metabolism
A B S T R A C T Plant glutamine synthetase (GS, EC6.3.1.2) catalyzes the synthesis of glutamine from glutamate and ammonium ions and acts as a key enzyme in the nitrogen metabolic pathway in organisms. Nitrogen is an essential element for plant growth and development and plays an important role in crop yield and quality formation.Therefore,GS is crucial in many physiological processes in plants.Currently,nitrogen regulation by GS in plants is well-studied in terms of its effect on plant growth and development.This article reviews the regulatory role of plant GS and its molecular mechanism in mitigating stress injury,such as low or high temperature, salinity, drought and oxidation. The function of plant GS in stress tolerance response is focused.The review aims to provide a reference for the utilization of plant GS in crop stress tolerance breeding.
Contents
2. Response of plant GS to abiotic stress . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 918
2.1. The biological function of GS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 919
2.2. GS activity as promising markers for determining abiotic stress tolerance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 920
2.3. Plant GS plays a key role in osmotic stress tolerance. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 920
2.4. GS activity is differentially affected in different tissues and species. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 920
2.5. GS activity can be affected by heavy metals. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 920
2.6. GS is an important enzyme for detoxification in plants. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 920
3. Stress tolerance mechanism analysis of plant GS genes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 921
4. Conclusions and future prospects. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 921
CRediT authorship contribution statement . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 921
Declaration of competing interest . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 921
Acknowledgments . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 921
References . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 921
1. Introduction
Abiotic stresses(e.g.,high or low temperature,drought salinity,etc.) can inhibit plant growth and accelerate plant senescence or even death[1].The frequency,extent,and duration of abiotic stress have increased with global climate change in recent years[2].This poses a serious threat to the maintenance of global food security[3]. Global atmospheric temperatures are expected to rise by 2-4.5°C by the end of the 21st century as greenhouse gas concentration increase [4]. Food and Agriculture Organization (FAO) data shows that crop yields will decrease by 15%-35% in Africa and West Asia and by 25%-35%in the Middle East when temperatures increase by 3-4 °C [5]. Drought also has a significant impact on agricultural production and is one of the major natural hazards to agricultural production as it negatively affects plant growth and causes crop yield losses of up to 40%[6].In addition,salinity stress is gradually becoming the main origin of abiotic stress that limits plant growth due to soil salinization[7].The total saline-alkali soil area worldwide has reached more than one billion km2,accounting for about 7.6%of the total land area,and this proportion continues to rise[8].Currently,annual crop production losses associated with saline and alkali environments range from 18% to 43% [9]. Therefore, improving plant resistance to abiotic stress is important to ensure good crop yield and food security.
The mechanism by which plants respond to abiotic stresses is complex. Plant photosynthetic organs are sensitive to abiotic stress, which mainly affects stomatal conductance, chloroplast photosynthetic electron transport, and enzyme activities related to carbon reaction processes, thereby affecting photosynthesis[10].Stress disrupts the balance of light energy uptake and utilization, leading to the accumulation of reactive oxygen species and thus oxidative damage to cells. Activation of the plant antioxidant system can scavenge reactive oxygen species, reduce damage due to cell membrane lipid peroxidation, and maintain cell membrane stability[11].In addition,stress-induced signaling substances(e.g.,hormones,Ca2+,and hydrogen peroxide),proteins,RNAs,and some metabolites react rapidly after plant stress,regulating downstream gene expression and physiological and biochemical processes.Epigenetic modifications,such as DNA methylation and histone modifications, are also involved in stress responses [12]. Therefore, an in-depth study on the physiological mechanisms of plant stress tolerance and an analysis of their genetic mechanisms can help improve crop resistance to abiotic stress and mitigate the adverse effects of abiotic stress on crop yield and quality, which is important for ensuring stable crop yield and food security.
Glutamine synthetase (GS) is ubiquitous in all organisms and catalyzes the synthesis of glutamine from ammonia and glutamate using the energy released from the hydrolysis of ATP to ADP and is a key enzyme involved in nitrogen metabolism in organisms [13].To date, more than 7900 GS enzymes have been identified in eukaryotes, bacteria, archaea, and viruses (NCBI database), which can be classified into 3 morphological categories: GS I, GS II and GS III [14]. Among these, GS I is commonly found in prokaryotes,GS II type is predominantly found in eukaryotes, and GS III is only found in a few bacteria[15].However,there are exceptions,such asArabidopsis thaliana[16],Saccharum officinarum[17],andMedicago truncatula[18], where GS I type genes were found. Similarly, GS II type genes have been found in some prokaryotes[19].Thus,it has been suggested that GS genes existed before the divergence of eukaryotes and prokaryotes and that GS I and GS II types evolved in parallel [16,20]. Emanuelsson et al. [21] and Bendtsen et al.[22] analyzed GS I peptide sequences and did not find a specific target signal or cleavage site,suggesting that the GS I type in plants is located in the cytoplasm. According to their phylogenetic relationships, GS I is encoded by a small multigene family [23]. The best-studied GS type in plants is GS II,which is encoded by a single gene and is active in chloroplasts and mitochondria[24].Based on the results of subcellular localization, GS present in higher plants can be broadly classified into two categories: one is localized in the cytoplasm and named GS1;the other is localized in the plastid and named GS2. In wheat, four subtypes of GS genes were reported, includingGS1,GS2,GSr, andGSe[25]. Habash et al. [26]used high performance liquid chromatography (HPLC) to separate GS and found two subtypes in leaves, including cytoplasmic subtype GS1 and chloroplast subtype GS2; the content and activity of both subtypes changes along with developmental processes.Both GSr and GSe are localized in the cytoplasm [25,27].
Plant growth is affected by many factors,among which nitrogen is an essential nutrient for plant growth and development.The key role of GS in the nitrogen metabolism pathway in plants is relatively well-studied. Numerous studies show that GS can regulate nitrogen content in plants and indirectly affect various aspects of photosynthesis and physiological metabolism, thus affecting growth and development as well as crop yield and quality [28-31]. However, the multiple status of GS isoforms suggests a complex role for GS in various aspects of abiotic stress tolerance(Fig. 1), e.g., it is a target of the herbicide glufosinate, which disrupts photosynthesis and nitrogen metabolism by inhibiting GS activity,which in turn leads to plant death[32].Since abiotic stress is an important factor limiting crop yield enhancement, and the frequency, extent, and duration of abiotic stress is significantly increasing with global climate change,research on the stress tolerance function of plant GS has attracted increasing attention [33].This article focuses on reviewing the progress of research on GS in plant stress tolerance responses.
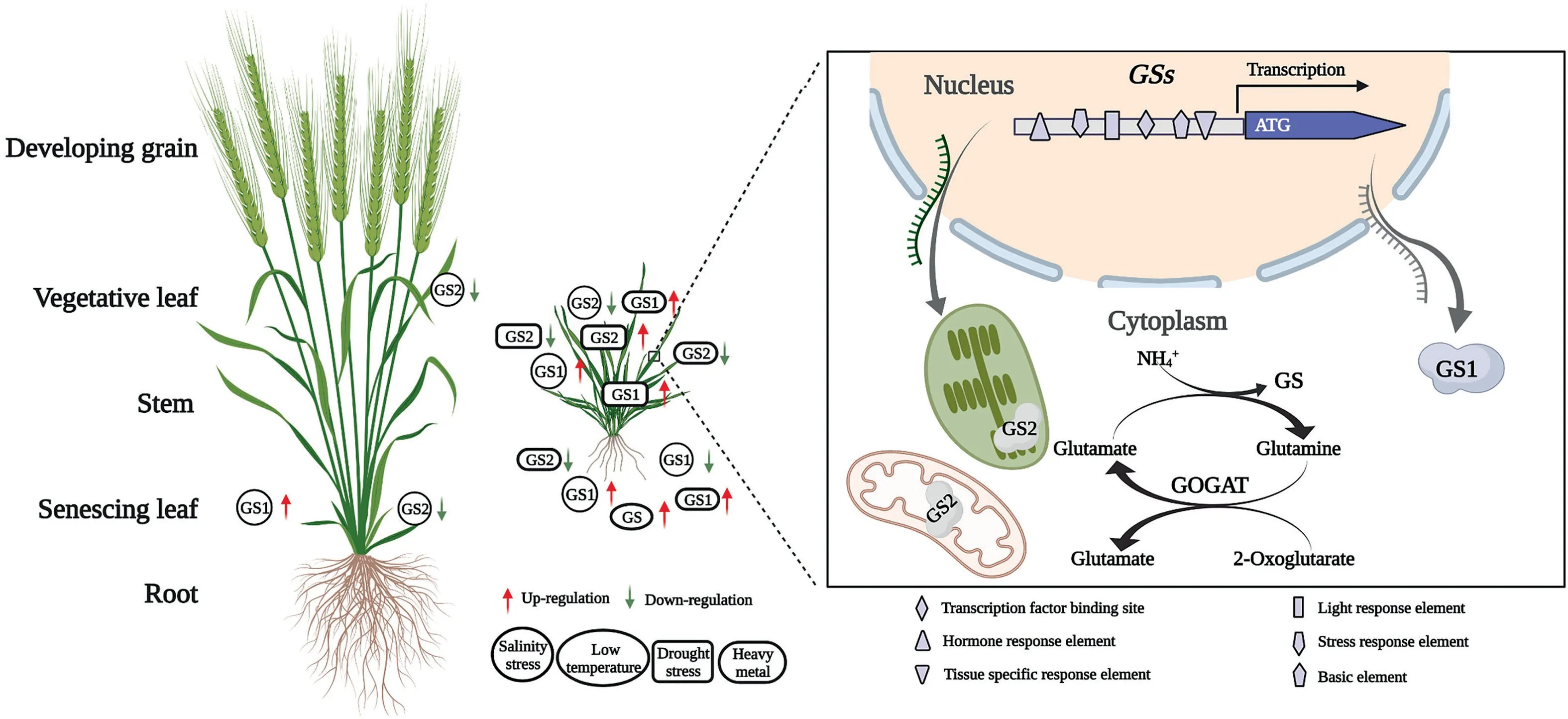
Fig. 1. The multiple status of Glutamine synthetase (GS) isoforms and associated roles in abiotic stress tolerance (original data source are summarized in Tables 1 and 2).
2. Response of plant GS to abiotic stress
Plant growth is often challenged by adverse environmental conditions, including high salinity, drought, extreme temperatures,and heavy metal pollution.In recent years,an increasing number of studies has found that plant GS can respond to various stresses(Table 1). Transgenic plants overexpressing the GS genes show increased tolerance to various abiotic stresses(Table 2).Therefore,in-depth studies on the stress tolerance function of GS and its molecular mechanisms can help improving crop resistance to abiotic stress and mitigating the adverse effects of abiotic stress on crop yield and quality formation.
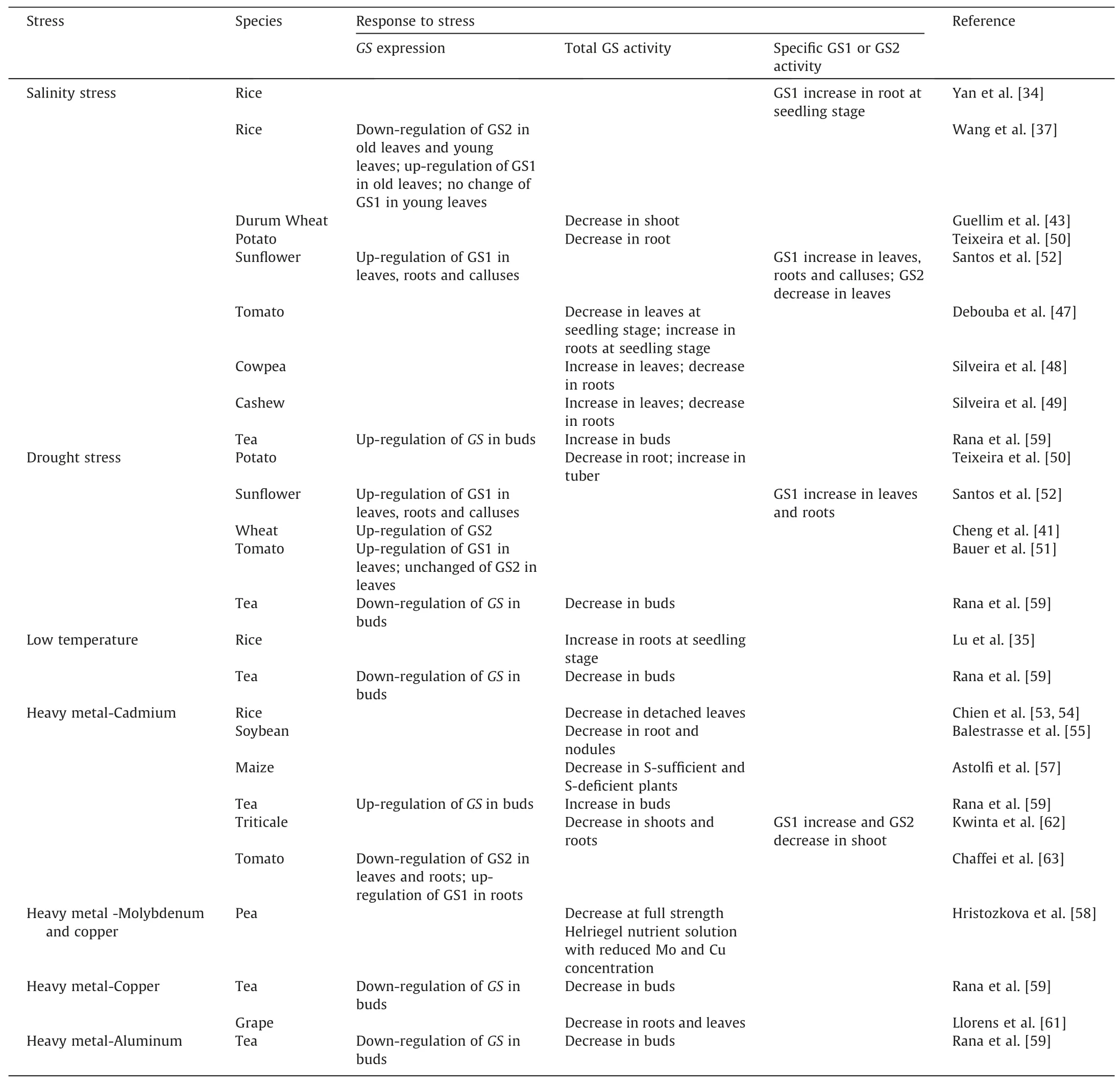
Table 1The role of GS in plant under different abiotic stress treatments.
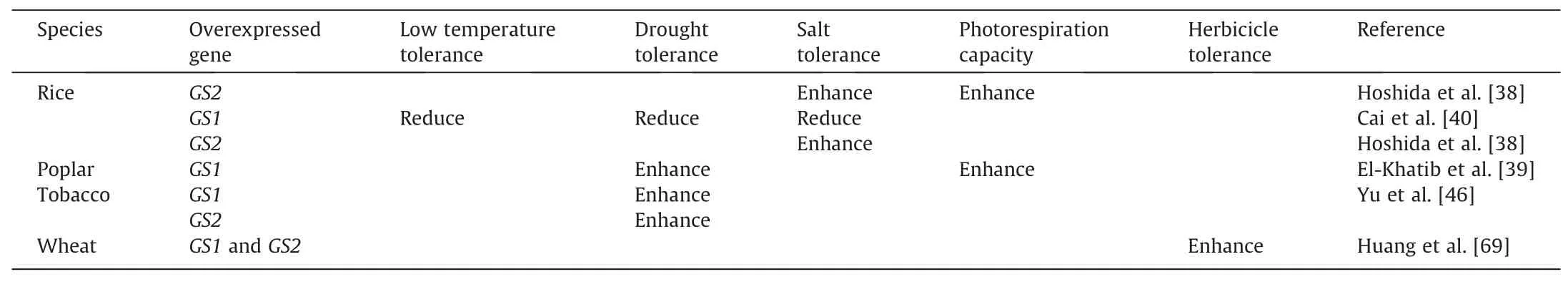
Table 2The role of GS overexpression in abiotic stress response in plants.
2.1. The biological function of GS
Studies in rice have found that salinity stress promotesGS1activity [34]. Lu et al. [35] demonstrated that GS activity was enhanced in rice roots after low-temperature treatment. Singh et al.[36]concluded that GS can be used to assess drought tolerant varieties in rice.Wang et al.[37]showed that the expression levels ofOsGS1;1,OsGS1;2, andOsGS1;3remained unchanged in young rice leaves under salinity stress, whereas the expression ofOsGS1was significantly up-regulated in older leaves.OsGS2expression was down-regulated in both young and old leaves. They believed that the down-regulation ofOsGS2might be due to reduced photorespiration after chloroplast damage under salinity stress. Further studies revealed that overexpression ofGS2gene in rice substantial increased photorespiration capacity and salt tolerance in transgenic plants.In addition,in the presence of isonicotinic acid hydrazide,a potent inhibitor of photorespiration,transgenic plants became sensitive to salinity as the control[38].Furthermore,ectopic overexpression ofGS1from pines in poplar trees resulted inenhanced photorespiration capacity and tolerance to drought stress in transgenic poplar trees[39].This suggests that GS overexpression increases the photorespiration capacity of transgenic plants to improve tolerance to abiotic stresses. However, Cai et al. [40] found that rice was sensitive to high salinity, low temperatures, and drought stresses afterGS1(GS1;2) overexpression,and they hypothesized that this may have occurred becauseGS1overexpression accelerates leaf senescence and disrupts the normal nitrogen metabolic process of the plant.Therefore,the biological function of GS must be further studied.
2.2. GS activity as promising markers for determining abiotic stress tolerance
Cheng et al.[41]analyzed the comparative proteomics of wheat under drought stress and found that GS2 was significantly upregulated in drought tolerant wheat varieties compared with that in sensitive varieties. In addition, Nagy et al. [42] found that GS activity was lower in older leaves than in flag leaves of both drought tolerant and drought sensitive wheat varieties under control conditions, while under drought conditions the GS activity in flag leaves of sensitive varieties was lower in flag leaves comparing with that of tolerant varieties. The researchers concluded that water deficiency led to accelerated leaf senescence and that premature senescence and increased cytoplasmic to plastid GS ratio in flag leaves are indicators of drought stress sensitivity;thus,they can be used as markers for determining drought stress tolerance.Moreover, salt tolerant wheat genotypes can be selected through the indicators of GS activity. Under 100 mmol L-1NaCl treatment,decrease of GS activity consequently increased the ammonium content in the shoot of a salt sensitive durum wheat cultivar while in a tolerant variety the ammonium content remained unchanged[43].
2.3. Plant GS plays a key role in osmotic stress tolerance
Szabados et al. [44] showed that plants accumulate proline to maintain normal metabolism and growth under drought stress conditions mainly by enhancing the GS-GOGAT cycle to promote the proline synthesis pathway with glutamate as a precursor.Cheng et al. [41] found thatGS2cexpression was up-regulated in drought tolerant wheat Xihan 2 under drought stress, leading to proline accumulation, which, in turn, enhanced the cellular osmoregulatory capacity. In addition, similar results were observed in lotusGS2mutant plants that had significantly lower proline content than wild type under drought treatment conditions. These mutants also showed multiple changes at the transcriptome and physiological levels, resulting in a reduced drought rehydration survival[45].Yu et al.[46]demonstrated that overexpression of wheatGS1andGS2genes,separately in tobacco,both enhanced the drought tolerance through increasing sucrose,proline, and chlorophyll accumulation capacity as well as reactive oxygen species scavenging capacity.These studies suggest that GS plays a key role in plant tolerance to osmotic stress.
2.4.GS activity is differentially affected in different tissues and species
Debouba et al. [47] found that, under salinity stress treatment at the seedling stage,the GS activity was inhibited in tomato leaves while enhanced in roots. Conversely, Silveira et al. [48,49] found that the total GS activity was increased in salt tolerant cowpea and cashew leaves and slightly decreased in roots under salinity stress. In potato, salinity stress inhibitedGS1activity in roots[50]. Bauer et al. [51] demonstrated that drought conditions induced higherGS1expression in leaves of drought-tolerant tomato but did not cause any change inGS2expression[51].Santos et al. [52] found the same pattern in sunflower, where bothGS1-type mRNA and protein content were increased in leaves after drought and salinity stress treatment butGS2activity decreased.Thus, GS activity was affected differentially in different tissues and crop species by stress treatment. In addition, different isoforms often appeared different trends of activity changes.
2.5. GS activity can be affected by heavy metals
Metal toxicity in the soil also affects the expression and activity of GS protein,which has been reported in many plants,such as rice[53,54], soybean [55,56], maize [57], pea [58], tea [59,60], grape[61], triticale [62], etc. In tea, Rena et al. [59,60] pointed out that cadmium,manganese,zinc,and nickel could all induce the cytosolic GS expression and activity whereas a contrary effect was found for copper treatment. Hristozkova et al. [58] reported that the GS activity decreased by 30% and 45% with twice-reduced treatment of molybdenum and copper concentrations in pea, respectively.After cadmium treatment, GS2 decreased and GS1 increased in tomato at protein and mRNA levels [63]. Despite substantial increases in GS1 activity was found in triticale seedlings under cadmium treatment, the total GS activity was significantly decreased due to the dramatic decrease of GS2, indicating a different response of GS isoforms in heavy metal stress [62].
2.6. GS is an important enzyme for detoxification in plants
Glufosinate ammonium, also known as PPT, is a widely used broad-spectrum and non-selective herbicide for post-emergence weed control [64]. PPT can competitively bind with the GS substrate, glutamate, on the basis of analogue structure, resulting in a rapid and substantial accumulation of ammonia and depletion of the amino acid glutamine [65]. It further causes inhibition of photosynthesis, disruption of chloroplastic structure, vesiculation of stroma,and accumulation of glyoxylate,resulting in severe damages to the plant and eventually death [65]. One strategy of increasing plant resistance to PPT is overexpressing GS genes,which has been confirmed by numerous researches on tobacco[66], alfalfa [67], rice [40,68], wheat [69]. On the other hand,target-site mutation in GS genes might also be effective for conferring plant resistance to PPT as reported in some cell cultures [70-73]. For example, some selected PPT-resistance cell lines of Maize have been detected with several mutations in GS genes [73] that may have changed the conformation and affinity of the GS binding site.
3. Stress tolerance mechanism analysis of plant GS genes
Plant GS genes exhibit significant tissue expression specificity,and their expression levels vary at different time courses in the same organ or tissue, implying that the function and metabolic processes of GS genes in the same organ vary in different tissues,cells, and organelles. It was reported that the GS gene expression is regulated at multiple levels, including genetic, posttranscriptional, and protein levels.
The promoter region of the GS gene contains MYB(v-myb avian myeloblastosis viral oncogene homolog), bHLH (basic helix-loophelix), Dof (DNA binding with one finger), NLP (nin-like protein),and other elements that specifically bind to transcription factors associated with resistance to abiotic stress [74-76]. El-Kereamy et al. [77] found that the amino acid content was significantly increased in rice transgenic forOsMYB55due to the binding ofOsMYB55to the promoter region of the target gene,directly activating the expression of genes such asOsGS1;2andGAD3, enhancing amino acid metabolism and thereby improving the plant’s tolerance to high temperatures. Similarly, Gómez-Maldonado et al.[78] found that the promoter of pine GS could be recognized by the MYB transcription factor, thereby enhancing GS expression.Wang et al. [79] measured the transcript levels of wheat GS genes using triple sequencing technology and analyzed their promoter sequences;they found thatTaGS1is mainly transcribed by chromosome 6B genes,TaGSeandTaGSrby chromosome 4D genes, andTaGS2by chromosome 2D genes.The distance of transcription start sites of different GS genes to the start codon ATG are different and the types,quantity,and arrangement order of cis elements of different GS gene promoters are also different. Among these genes, theTaGSepromoter on chromosome 4D has more stress-responsive transcription factors (MYB, MBS, LTR, etc.) binding elements. In addition,GSgene expression is somewhat regulated at the posttranscriptional level, presumably stemming from the 3′noncoding region of the GS gene that affects transcript stability[80].
GS proteins are modified at the posttranslational level. Finnemann et al. [81] and Lima et al. [82] showed that GS can interact with 14-3-3 proteins and alter the magnitude of response to 14-3-3 proteins by increasing or decreasing the level of GS phosphorylation. Maize GS proteins may undergo phosphorylation modifications that in turn affect GS enzyme activity [83]. In addition, studies in alfalfa found that light increased the phosphorylation level of GS1 in leaves but not GS2 in leaf chloroplasts, which suggests that light can regulate GS function at the posttranscriptional level and indicates differences between GS1 and GS2 responses to light stimulation [82].
4. Conclusions and future prospects
In summary, GS in plants can respond to a variety of abiotic stresses, such as drought, low temperature, and salinity. It also is an important physiological indicator of a plant’s ability to tolerate stress. Plants overexpressing different isoforms of GS do not have uniform phenotypes, suggesting that genes upstream or downstream of GS genes may affect the expression of GS and its biological functions to some extent. However, studies on GS genes are still preliminary, and some aspects require further in-depth analysis.
Firstly, most studies on GS function in plants focused on two subtypes, cytoplasmic GS and plastid GS, and the same subtype GS often has multiple genes encoding it. For example, wheat GS is divided into two categories, cytoplasmic and plastid, where the cytoplasmic GS is further divided into GS1, GSr and GSe. Wheat is a heterozygous hexaploid species, consisting of chromosomes A, B and D, with each set of chromosomes having a set ofTaGSgenes. Along with the refinement of genome sequences in several species, genome-wide mining ofGSgenes has become a streamlined task, it is essential therefore to identify which GS isoforms or gene alleles that play major roles in abiotic stress tolerance as well as their genetic pathways and regulatory mechanisms. Secondly, GS is involved in plant adaptation to abiotic stresses and respond at the levels of transcription, protein expression, enzyme activity, and post-translational modifications, but little is known about the mechanisms of its stress tolerance regulation in plants.In conclusion, the study of the above questions will not only help to elucidate the biological status of GS genes in plants but also provide new clues to elucidate the molecular mechanisms in response to abiotic stresses, which are potentially valuable in breeding and innovating plant stress-tolerant germplasm. Finally, the rapid development of modern gene-editing technology for target gene modification,e.g.,CRISPR-Cas9,has cast a light on improving plant abiotic stress tolerance through modifying GS genes. Based on the reported herbicide resistance of plant cell line mutants, CRISPRCas9 method may potentially be utilised to develop drought, high or low temperature, salinity, and/or heavy metal tolerate plants through modifying the GS genes.Notably,all GS genes can be taken into consideration through such an approach due to their diverse encoding genes, isoforms, and functions.
CRediT authorship contribution statement
Huayan Yin, Fan Yang, Xiaoyan He and Xuye Du:Writing -original draft.Wujun Ma and Ping Mu:Writing-review&editing.Wujun Ma, Huayan Yin and Ping Mu:Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by Qingdao Agriculture University Internal Strategic Research Fund,National Natural Science Foundation of China (32101715), the Foundation of Research and Application of Whole Genome Selection in Wheat (2019LZGC016),the High-Level Talents Project of Qingdao Agricultural University(663/1119057), and the State Key Laboratory of Crop Biology at Shandong Agricultural University (2020KF03). We acknowledge Biorender(©BioRender-biorender.com)since figure of this manuscript was made using this software.
- The Crop Journal的其它文章
- Brief Guide for Authors
- Revisiting the role of delta-1-pyrroline-5-carboxylate synthetase in drought-tolerant crop breeding
- A new gain-of-function OsGS2/GRF4 allele generated by CRISPR/Cas9 genome editing increases rice grain size and yield
- Influence of seven levels of chemical/biostimulator protection on amino acid profile and yield traits in wheat
- Corrigendum to ‘‘De novo design of future rapeseed crops: Challenges and opportunities” [Crop J. 10 (2022) 587-596]
- Improving the resistance of the rice PTGMS line Feng39S by pyramiding blast, bacterial blight, and brown planthopper resistance genes

