Revisiting the role of delta-1-pyrroline-5-carboxylate synthetase in drought-tolerant crop breeding
Xuanjun Feng, Yue Hu, Weixiao Zhang, Rongqian Xie, Huarui Guan, Hao Xiong, Li Jia,Xuemei Zhang, Hanmei Zhou, Dan Zheng, Ying Wen, Qingjun Wang, Fengkai Wu, Jie Xu,Yanli Lua,,
a State Key Laboratory of Crop Gene Exploration and Utilization in Southwest China, Wenjiang 611130, Sichuan, China
b Maize Research Institute of Sichuan Agricultural University, Wenjiang 611130, Sichuan, China
Keywords:P5CS Proline Drought Tiller number Chalkiness
A B S T R A C T The delta-1-pyrroline-5-carboxylate synthetase (P5CS) gene exercises a protective function in stressed plants.However,the relationship between proline accumulation caused by P5CS and abiotic stress tolerance in plants is not always clear, as P5CS overexpression has been reported to repress plant growth under normal conditions in several reports. We re-evaluated the role of P5CS in drought-tolerant rice breeding by expressing the AtP5CS1 and feedback-inhibition-removed AtP5CS1 (AtP5CS1F128A) genes under the regulation of an ABA-inducible promoter to avoid the potential side effects of P5CS overexpression under normal conditions. ABA-inducible AtP5CS1 and AtP5CS1F128A increased seedling growth in a nutrient solution(under osmotic stress)and grain yield in pot plants.However,the evidently deleterious effects of AtP5CS1 on grain quality,tiller number,and grain yield in the field indicated the unsuitability of P5CS for drought-tolerance breeding.
1. Introduction
Proline was long considered an inert,compatible osmolyte that protects subcellular structures and macromolecules against drought, high salinity, heavy metals, and other stresses [1].Tobacco was first transformed with the delta-1-pyrroline-5-carboxylate synthetase(P5CS)gene isolated fromVigna aconitifoliaand driven by the 35S promoter [2]. These transgenic plants produced high levels of P5CS and consequently increased their proline synthesis by more than 10-fold compared to control plants and displayed increased root biomass under NaCl stress. A positive correlation betweenin vivoproline accumulation and osmotic,drought,salt, cold, and heavy metal tolerance has been reported in diverse transgenic plants;accumulated proline acts as a carbon and nitrogen source for plant recovery from stress conditions [1,3]. Thus,constitutive overexpression ofP5CShas been pursued as an effective strategy for improving plant tolerance to environmental stresses.
However, the effect ofP5CSoverexpression under normal conditions is not always consistent [1]. Because gene transcription and mRNA translation are energy-intensive processes,constitutive overproduction ofP5CSRNA and protein may drain energy,resulting in reduced plant growth and yield under normal conditions.Although a few studies have found thatP5CSoverexpression led to no adverse effects on plant growth under optimal conditions[2,4,5], most studies did not consider this possibility [6-9], and some clearly indicated a negative effect [10,11].
An enzyme activity assay usingEscherichia coli-expressedVignaP5CS indicated that a single amino acid substitution (F129A) ofVignaP5CS could reduce feedback inhibition by proline [12,13].Transgenic tobacco and chickpea expressingP5CSF129Aincreased their proline accumulation twofold compared to plants expressing the wild-typeVigna P5CSunder normal conditions or salt stress[2].These results allowed genetic manipulation ofP5CSand proline overproduction in crops by bypassingP5CSoverexpression. In plants,proline biosynthesis is closely associated with the metabolism of other amino acids [1]. Increased free proline may disturb amino acid metabolism, likely affecting the synthesis of storage proteins. Nitrogen or carbohydrate metabolism disturbance has received increasing attention because of its relationship with rice grain chalkiness [14,15]. Previous studies have shown that insufficient deposition of storage proteins and starch increase grain chalkiness in rice[14,15].Thus, proline overproduction may affect rice quality.
To minimize the adverse effect of constitutive overexpression,an inducible promoter that ensures the activation of target genes only under stressful conditions is a seemingly cost-effective strategy. Recently, an ABA-inducible promoter,ABRC321, was used to drive a late embryogenesis abundant gene,HVA1, in rice.ABRC321worked as a molecular switch and maintained a low basal level ofHVA1under normal growth conditions but induced the production of high levels under ABA, drought, salt, and cold stimuli. The expression ofABRC321:HVA1successfully increased tolerance to various abiotic stresses and did not cause yield reduction under normal field conditions [16].
Aroma is a cooking-quality trait in food grains that adds to their economic value [17]. The Maillard reaction is a key process in the enhancement of food aroma, particularly for cooked foods. Generally,meat-related aroma compounds are derived mainly from cysteine and ribose, while the aromas of bread, rice, and popcorn are typically derived from proline[18,19].Heating rice bran with proline at 95°C increased the intensity of corn snack-like,cracker-like,and milk powder-like aromas [20]. Specific reactions that occur at pH 6.0 increased the production of furaneol,while those occurring at pH 7.5 produced mainly furfural and vanillin [20].
In the present study, we set three goals. We aimed first to expressAtP5CS1andAtP5CS1F128Ain rice under the control of an ABA-inducible promoter; second, to evaluate drought tolerance and its application potential in transgenic plant breeding; and third, to assess quality changes in transgenic grains.
2. Materials and methods
2.1. Plant material and growth conditions
Rice was planted at Chongzhou in Sichuan province(30o32′37.56′′N, 103o39′10.53′′E) and Sanya in Hainan province(18o22′15.50′′N, 109o09′53.18′′E) in 2019 for normal seed reproduction. For drought-stress treatment, seedlings were planted in field soil in square plastic basins (length × width × depth = 60 ×40 × 15 cm) and in rain-protected fields at Wenjiang in 2020(March 5 to June 23) and 2021 (May 10 to August 19) with four replicate experiments each year. Approximately one month after planting, the drought-treated group was exposed to water deficiency for several days (15 and 10 days in 2020 and 2021, respectively) and then rewatered. The watering was halted again before the flowering stage.
2.2. Statistical analysis
All experiments were repeated three times,with three technical replicates.Differences between transgenic lines and control plants(GUS#3,a transgenic line with beta-glucuronidase geneuidA)were assessed by one-way analysis of variance (ANOVA).
More methods are described in Supplementary Methods.
3. Results
3.1. The ABA-inducible promoter worked well in rice
In our previous study of theAtP5CS1promoter [21], the minipromoter (approximately 460 bp) ofAtP5CS1activated low basal expression and was weakly induced by abiotic stress. We accordingly fused theAtP5CS1mini-promoter with an ABA response complex (3xABRC321) [14] to obtain an ABA-inducible promoter(Fig. S1A). TheuidA(also known as theGUSgene),AtP5CS1, andAtP5CS1F128Awere driven by this promoter and introduced into rice.UdiAgenes in most transgenic lines were highly induced by ABA treatment(Fig.S1B).Histochemical staining showed thatudiAhad low basal expression and was visibly induced by ABA in roots(Fig.S1B).Similarly,AtP5CS1andAtP5CS1F128Awere highly induced in most transgenic progenies upon ABA treatment(Fig.S1C).Thus,the synthetic ABA-inducible promoter was effective in rice.
3.2. ABA-inducible chimeric AtP5CS1 and AtP5CS1F128A promoted seedling growth under suitable and hyperosmotic conditions
We selected twoAtP5CS1(W#1 and W#8)and twoAtP5CS1F128A(M#2 and M#4) lines to compare the effects ofAtP5CS1andAtP5CS1F128Ain transgenic rice.Transgenic seedlings were exposed to 13% (w/v) polyethylene glycol (PEG6000)-mediated osmotic stress conditions to test their tolerance. Generally,AtP5CS1andAtP5CS1F128Atransgenic plants, especially their roots, grew better than transgenic control plants (GUS#3) under both normal and osmotic stress conditions(Fig.S2).AtP5CS1andAtP5CS1F128Asignificantly increased root volume, root diameter, and root dry weight under osmotic stress conditions (Fig. S2B, D, E). However, they increased the number of root tips under normal conditions but reduced it under osmotic stress (Fig. S2C).AtP5CS1F128Atended to show a stronger positive effect on shoot dry weight and root dry weight thanAtP5CS1(Fig. S2E, F). The higherP5CS1basal expression in W#8 and M#4 resulted in growth similar to that of W #1 and M #2 (Fig. S2B-F), suggesting that ectopic expression ofAtP5CS1andAtP5CS1F128Adid not impair seedling growth. This finding was consistent with most previous reports thatP5CS1overexpression rarely caused growth arrest[2,4,5].In addition,the proline content of transgenic plants was measured.AtP5CS1andAtP5CS1F128Aboth markedly increased the proline content under normal conditions. However, onlyAtP5CS1F128Atransgenic plants displayed significantly higher proline accumulation under osmotic stress conditions (Fig. S2G).
3.3.ABA-inducible chimeric AtP5CS1 and AtP5CS1F128A increased yield under drought stress in pots
Transgenic plants were planted in plastic square basins and grown in a greenhouse to investigate their drought tolerance.Generally,AtP5CS1F128Amarkedly increased vegetative biomass and yield under drought stress, whereas the beneficial effects ofAtP5CS1were significant only for W#8 (Fig. 1D-F).AtP5CS1F128Aalso increased yield under well-watered conditions. Thus, these phenotypes were consistent with the phenotype in the PEG6000 solution,whereAtP5CS1F128Amay have had greater efficacy thanAtP5CS1under water-deficient conditions (Figs. S2E, F, 1D-G).AtP5CS1F128AandAtP5CS1affected grain size, unfilled-kernel percentage,and thousand-kernel weight in a complex manner but displayed no significant effect on tiller number (Figs. 1G-I, S3A, B).AtP5CS1F128AandAtP5CS1transgenic plants showed a significantly lower percentage of unfilled kernels than control plants (GUS#3)under drought stress, a finding consistent with lower the grain yield per plant (Fig. 1F, I).
3.4. ABA-inducible AtP5CS1 and AtP5CS1F128A reduced yield by reducing tiller number in the field
The effects ofAtP5CS1andAtP5CS1F128Aon grain yield and tiller number differed between pot and field conditions (Fig. 2A-D).AtP5CS1F128AandAtP5CS1significantly reduced grain yield per plant in well-watered fields but did not affect this trait under drought stress in the field (Fig. 2C). Tiller number and thousandkernel weight variation was correlated with that of grain yield per plant (Fig. 2D, E).AtP5CS1F128AandAtP5CS1promoted grain-filling, resulting in a higher thousand-kernel weight under both well-watered and drought field conditions (Fig. 2E-G). The effects on grain size were undetermined under pot conditions(Fig.S3A,B).The development of tiller buds was inhibited and tiller number was consequently decreased(Fig.2B,D,J).Photosynthetic carbon assimilation rate (AR) and evaporation rate (ER) were investigated,as AR typically is associated with the grain-filling process. There was no significant difference in AR, ER, or water use efficiency (WUE) betweenAtP5CS1F128AorAtP5CS1transgenic plants and controls (GUS#3) (Fig. S3C-E).
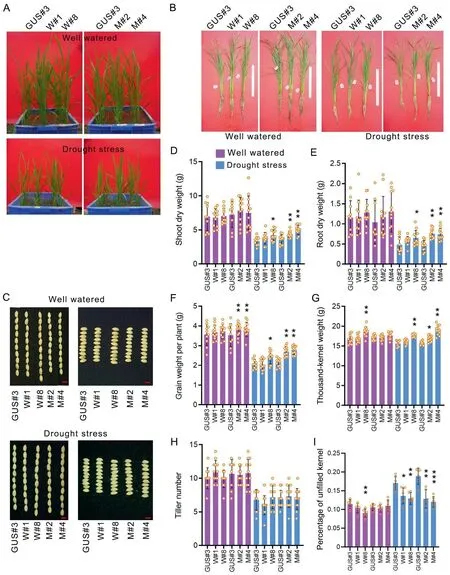
Fig.1. ABA-inducible AtP5CS1 and AtP5CS1F128A increased yield under drought stress in pots.(A)Phenotype of transgenic plants before flowering in pots under well-watered or drought-stress conditions.(B)Phenotype of transgenic plants after seed maturity in pots under well-watered or drought stress conditions.(C)Seed phenotype of transgenic plants.Red scale bars represent 5 mm.Statistics are of(D)shoot dry weight,(E)root dry weight,(F)grain weight per plant,(G)thousand-kernel weight,(H)tiller number,and(I)percentage of unfilled kernel.Each circle on bars represents a plant.Means were compared by one-way ANOVA,and the mean values of W#1,W#8,M#2,and M#4 were compared with GUS#3. Asterisks on bars denote significant differences (*, P <0.05; **, P <0.01; ***, P <0.001).
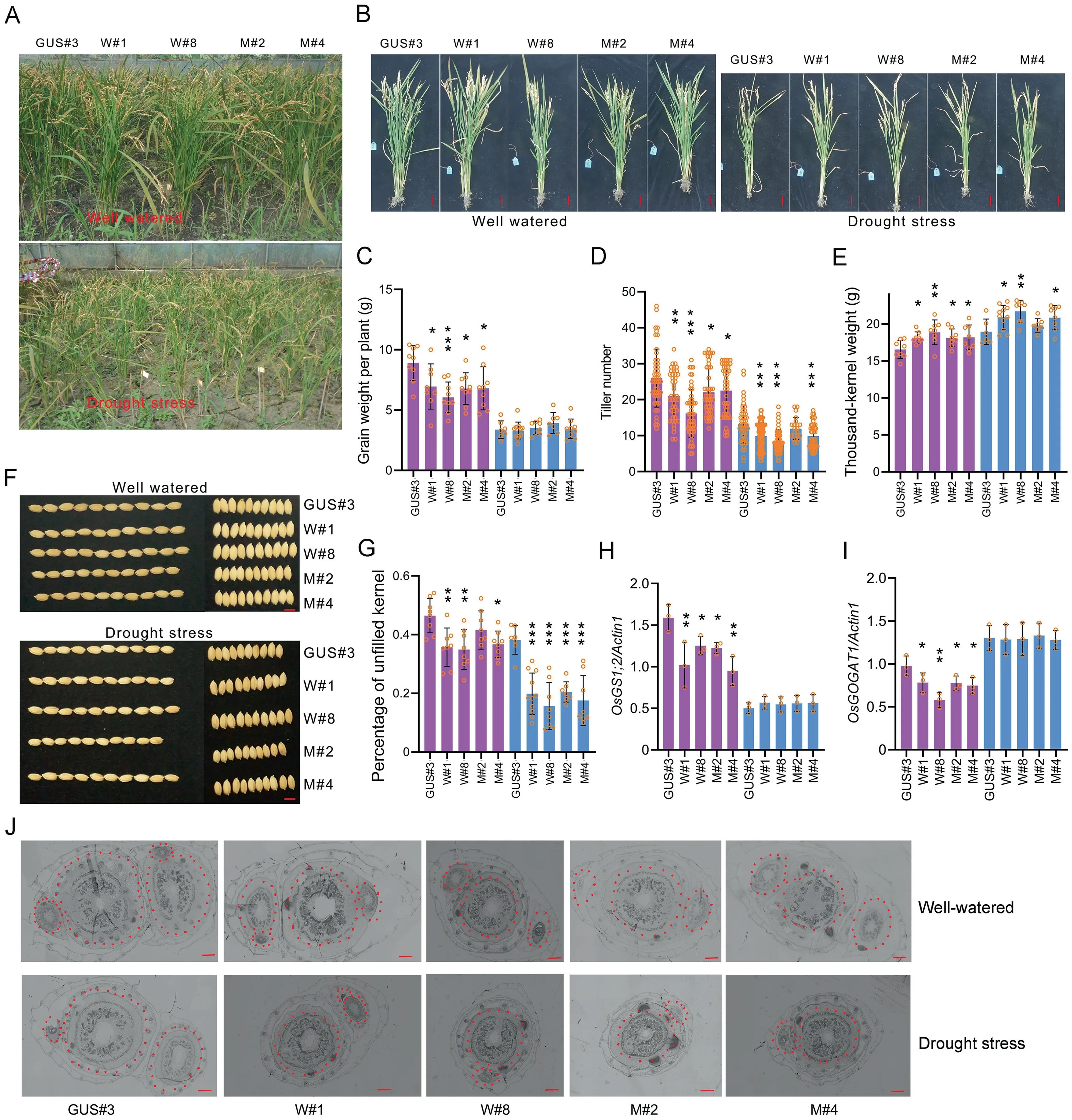
Fig.2. ABA-inducible AtP5CS1 and AtP5CS1F128A caused yield penalty by reducing tiller number in the field.(A,B)Phenotype of transgenic plants after seed maturity in fields under well-watered or drought stress conditions.(F)Seed phenotypes of transgenic plants.Statistics are of(C)grain weight per plant,(D)tiller number,(E)thousand-kernel weight,and(G)percentage of unfilled grain.Transcription level of(H)OsGS1;2 and(I)OsGOGAT1 in transgenic plants. Actin 1 and ubiquitin were used as reference genes. (J)Transverse section view of the junction part of root and shoot. Tiller buds are circled with red dotted lines. Red scale bars in B, F, and J represent 5 cm, 5 mm, and 500 μm,respectively. Each circle on bars represents a plant from (C) to (G). Three repetitions of gene expression are displayed in (H) and (I). Means were compared by one-way ANOVA,and the mean values of W#1,W#8,M#2,and M#4 were compared with GUS#3.Asterisks on bars represent the difference is significant(*,P <0.05;**,P <0.01;***,P <0.001).
In plants, proline biosynthesis is closely associated with glutamate metabolism,as proline is synthesized mainly from glutamate[1]. Glutamine, glutamate, and the associated genesglutamine synthetase(GS)andglutamate synthase(GOGAT)have been reported to affect tiller number [22-24]. Accordingly, increased proline synthesis (Fig. S2G) may interfere with glutamate metabolism,and the most frequently reported genes,OsGS1;2, OsGS1;1, OsGOGAT1,andOsGOGAT2,were detected at the root and shoot junctions by real-time qPCR. The basal transcription levels ofOsGS1;2andOsGOGAT1were lower inAtP5CS1F128AandAtP5CS1transgenic plants and comparable to those in GUS#3 under drought stress(Fig. 2H, I).OsGOGAT1andOsGOGAT2were slightly induced and repressed, respectively, by drought. However,AtP5CS1F128AandAtP5CS1had no apparent effect on the expression ofOsGS1;2,OsGS1;1, OsGOGAT1,andOsGOGAT2under drought stress(Figs. 2H, I, S3H, I).The physiological free amino acid content was measured in the same tissue,and 23 components were identified(Table S1).L-aspartic acid,L-threonine,L-serine,L-asparagine,L-glutamine, L-valine, L-histidine, 3-methyl-L-histidine, L-lysine,L-proline, and NH4increased under drought stress, and the accumulation of L-asparagine,L-glutamine,L-valine,L-proline,and NH4was higher inAtP5CS1F128AandAtP5CS1transgenic plants than in control plants (Table S1). However, the basal concentration of L-glutamine under well-watered conditions was lower inAtP5CS1F128AandAtP5CS1transgenic plants than in the control(GUS#3) (Table S1).
3.5.ABA-inducible AtP5CS1 and AtP5CS1F128A led to adverse effects on grain quality
Given that proline has been reported to increase desirable aroma intensity in foods via the Maillard reaction [18-20], we manually scored the desirable aroma intensity of cooked rice on a 0-10 scale (with a higher score corresponding to a stronger aroma). Kernels of W#8 and M#4 under drought stress were selected for aroma rating because of their high inducible expression ofAtP5CS1andAtP5CS1F128A. However, there was no increase of aroma in W#8 and M#4, and only the addition of 0.1% (w/w)proline in rice significantly improved the aroma level (Fig. S4A).This was probably because the increased proline content in transgenic seeds was insufficient to qualitatively improve the aroma.Accordingly, the proline contents of grain under well-watered and drought stress conditions were measured.The proline content was of approximately 20 μg g-1dry weight (approximately 0.002%)in dehusked rice(Fig.S4B),which may not result in a perceptible aromatic increase similar to that caused by adding 0.1%(w/w) proline. Additionally, drought stress did not cause a large increase in the free proline content of mature kernels (Fig. S4B).
Although there was no difference in free proline content in mature kernels, it was significantly increased in the vegetative organs at the seedling stage(Fig.S2G;Table S1).The increased free proline disturbed amino acid metabolism (Table S1), likely resulting in differences in storage proteins and rice quality. The perturbation of nitrogen or carbohydrate metabolism has received increasing attention because of its relationship with rice grain chalkiness [14,15]. Previous studies have shown that insufficient deposition of storage proteins and starch increases grain chalkiness in rice [14,15]. Accordingly, several general quality characteristics(chalky rice rate and chalkiness degree; crude protein, starch,and fat contents)and the amino acid profiles of dehusked rice were measured. Chalky grain rate and chalkiness degree were significantly increased byAtP5CS1andAtP5CS1F128Aexpression (Fig. S4).In contrast, the difference in the amino acid profile from hydrolyzed proteins in mature kernels was not significant (Table S2).Four types of storage proteins were quantified, and the contents of globulin, prolamin, or glutelin were sometimes lower inAtP5CS1F128AorAtP5CS1transgenic plants than in GUS#3. The difference of storage proteins may explain the increase in chalkiness rate in W#8, M#2, and M#4.
4. Discussion
P5CSand proline have been widely reported to impart stress tolerance in laboratory conditions [1-9]. However, their positive function has rarely been confirmed in the field[10].We found that ABA-inducibleAtP5CS1and feedback-inhibition-removedAtP5CS1(AtP5CS1F128A)increased drought tolerance under laboratory conditions. However, they exerted an evidently deleterious effect on field tiller number, grain yield, and grain quality, likely owing to the disturbance of amino acid metabolism.
ABA-inducibleAtP5CS1andAtP5CS1F128Amarkedly promoted seedling growth in PEG-mediated osmotic stress and soil drought stress in the laboratory (Figs. S2, 1), andAtP5CS1F128Ashowed greater efficacy (Figs. S2E, F, 1D-G). Thus, our findings are consistent with most previous reports stating that increased proline accumulation improved stress tolerance. However, seedling tiller number was markedly lower in pots than in the field (Figs. 1H,2D), in agreement with the finding that the phenotypes of seedlings varied between laboratory and natural field conditions[25,26]. The effect ofAtP5CS1andAtP5CS1F128Aon tiller number may have been masked by the negative effect of pot conditions(Figs. 1H, 2C). Thus, the displayed effect of ABA-inducibleAtP5CS1F128Aon grain yield was positive under pot and negative under field conditions(Figs.1F,2C).Why did pot conditions reduce the tiller number? We suggest two explanations. One is that the drought process was faster under pot than under field conditions.This observation is consistent with the earlier display of droughtstress-associated phenotypes in pot plants than in field plants.The other is that nutrient supply may be insufficient under pot conditions. ABA-inducibleAtP5CS1andAtP5CS1F128Apromoted grain filling and resulted in a larger thousand-kernel-weight under both pot and field conditions. We conclude thatAtP5CS1andAtP5CS1F128Aincrease drought tolerance but inhibit bud development (Figs. S2, 1, 2).
Proline biosynthesis is closely associated with glutamate metabolism and proline is synthesized mainly from glutamate in plants[1].Glutamine,glutamate,and the associated genesGSandGOGAThave been widely reported to affect tillering[22-24].OsGS1;2was reported to be responsible for ammonium assimilation and to affect bud development [24]. Tiller number and the contents of glutamine, glutamate, asparagine, and aspartate were markedly reduced in loss-of-function mutants [24]. Promoting proline synthesis byAtP5CS1orAtP5CS1F128Amay disturb the metabolism of amino acids, particularly that of glutamate and glutamine. The finding that transcription levels ofOsGS1;2and L-glutamine content were lower inAtP5CS1F128AandAtP5CS1transgenic plants may account for the decrease in tiller number (Fig. 2H, I;Table S1). Disturbances of amino acid metabolism may affect protein synthesis. The finding that the contents of globulin,prolamin,and glutelin were sometimes lower inAtP5CS1F128AorAtP5CS1transgenic plants is in accord with the increase in chalky grain rate and chalkiness degree (Fig. S4).
The Maillard reaction is of utmost importance for the aroma enhancement of foods during the heating process.Aromas of bread,rice,and popcorn are typically derived from proline[18,19].In the present study, we did not find an increase in the aroma level in cooked rice ofAtP5CS1andAtP5CS1F128Aplants because the free proline content did not increase in rice kernels (Fig. S4B). Exogenous addition of 0.1% proline (but not 0.01% proline) did increase the aroma(Fig.S4B).Thus,a higher level of free proline accumulation (approximately 1 mg g-1) in rice grain may indeed increase the aroma.However,this is difficult to achieve because the normal free proline content in rice grain is approximately 20 μg g-1(Fig. S4A).
To conclude, ABA-inducibleAtP5CS1andAtP5CS1F128Aincrease drought tolerance in rice but reduce grain quality, tiller number,and grain yield.For this reason,P5CSmay not be suitable for breeding drought-resistant rice cultivars.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China(U21A20209 and 31871640),the key Research Program of the Department of Science and Technology of Sichuan province, China (2021YFYZ0017, 2020YJ0350, and 2021YFH0053),and the Foundation of International Cooperation in Science and Technology of Chengdu, China (2020-GH02-00027-HZ). We thank James C. Nelson (Kansas State University) for his help about language editing.
Appendix A. Supplementary data
Supplementary data for this article can be found online at https://doi.org/10.1016/j.cj.2022.04.002.
- The Crop Journal的其它文章
- Brief Guide for Authors
- A new gain-of-function OsGS2/GRF4 allele generated by CRISPR/Cas9 genome editing increases rice grain size and yield
- Influence of seven levels of chemical/biostimulator protection on amino acid profile and yield traits in wheat
- Corrigendum to ‘‘De novo design of future rapeseed crops: Challenges and opportunities” [Crop J. 10 (2022) 587-596]
- Improving the resistance of the rice PTGMS line Feng39S by pyramiding blast, bacterial blight, and brown planthopper resistance genes
- Low N apparent surplus with higher rice yield under long-term fertilizer postponing in the rice-wheat cropping system

