Research progress on the divergence and genetic basis of agronomic traits in xian and geng rice
Linlin Jiang, Lian Wu, Yun Wang*, Quan Xua,*, Zhengjin Xua, Wenfu Chen
a Rice Research Institute of Shenyang Agricultural University, Shenyang 110866, Liaoning, China
b Agronomy College of Shenyang Agricultural University, Shenyang 110866, Liaoning, China
Keywords:Oryza sativa Differentiation between subspecies Genetic basis Plant architecture of geng rice
A B S T R A C T The Asian cultivated rice Oryza sativa can be classified into two major subspecies: japonica/geng and indica/xian. There are large physiological and phenotypic differences between the two subspecies, with each having its advantages and disadvantages. Understanding the differences between xian and geng could provide a foundation for cultivar improvement based on hybridization between subspecies in order to synthesize favorable traits. We review the origin and domestication of xian and geng rice, compare their differences in terms of physiological and phenotypical traits,and describe the molecular mechanism differences between the subspecies. Based on this knowledge, we propose an ideal plant architecture of geng rice varieties for northern regions.
Contents
1. Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 924
2. Origin of cultivated rice and differentiation ofxianandgengsubspecies . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 925
3. Differences in the genome, morphological, physiological, and anatomical traits. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 925
4. Differences in key quality and yield characters. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 925
5. Molecular mechanisms of trait differences. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 927
6. The model of ideal plant architecture forgengvarieties. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 929
7. Conclusion and perspectives. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 929
CRediT authorship contribution statement. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 929
Declaration of competing interest . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 929
Acknowledgments . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 929
Appendix A. Supplementary data . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 929
References . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 929
1. Introduction
Rice is the staple food crop of half of the global human population,including almost the entire population of southeast Asia[1].Asia is the largest rice-producing region in the world and exports morethan 90% of the product. Since the introduction of the semi-dwarf genesd1,the introductionof F1hybrids,and the super high-yield breeding project, the yield per unit area of rice in China has increased from 1890 kg ha-1in 1949 to 7044 kg ha-1in 2020,nearly 50%higher than the global average yield per unit area, according to the National Bureau of Statistics (http://www.stats.gov.cn/tjsj/zxfb/202012/t20201210_1808377.html). Asian-cultivated rice (Oryza sativaL.)was domesticated from the wild speciesO.rufipogonand can be further divided intoindica(xian) andjaponica(gengsubspecies),with the latter, distributed mainly in China, Japan, and the Republic of Korea,representing about 10%of cultivated rice.In China,gengandxianrice are equallyimportant inproduction,althoughxianaccounts for 67% of the total rice cultivation area. Rice is distributed across more than 35 degrees of latitude in China, from Sanya, Hainan(18°09′N)in the south to Mohe,Heilongjiang(53°27′N)in the north.Xianrice is grown mainly in southern areas at low altitudes,whilegengrice can be cultivated in high-altitude hilly regions.However,in the central region, both subspecies have historically been cultivated.Besides variation in geographical distribution,xianandgengalso have large genomic and phenotypic differences[2].
China has made remarkable achievements in the field ofxiangenghybrid breeding [3]. Indeed, according to pedigree analysis,most of the elite rice varieties in northern China, since the 1980s,have been bred from crosses betweenxianandgengrice,with most of the rice varieties planted in southern China also having pedigrees containing both [4].
This review summarizes the origin and domestication ofxianandgengrice,compares their differences in terms of physiological and phenotypic traits,and describes the molecular mechanism differences between the subspecies.Finally,we propose an ideal plant architecture ofgengrice varieties that is suitable for northern regions.
2. Origin of cultivated rice and differentiation of xian and geng subspecies
Cultivated rice have been domesticated from wild species thousands of years ago.Given that the differences betweenO.sativaandO. rufipogonare reflected in a wide range of morphological and physiological traits, the evolutionary and domestication process of cultivated rice have long been debated. Of special interest has been the question of whether the two subspecies of cultivated rice,xianandgeng, are derived from single or multiple domestications.In this context, a wide range of archaeological and genetic studies have been employed to examine the phylogenetic relationships between cultivated rice species as well as the demographic history of their domestication [5-14], with molecular phylogenetic analyses indicating thatgengandxianoriginated independently[5,15,16].However,well-characterized domestication genes in rice have been found [17-20] to be fixed in both subspecies with the same alleles, supporting a single domestication origin. A demographic analysis of single nucleotide polymorphisms (SNPs)detected in 630 gene fragments indicated a single domestication origin of rice [12]. In other studies [13,14], population-genetic analyses of genome-wide sequence data for wild and cultivated rice have suggested that thexianandgenggenomes are of independent origin,although many genomic segments bearing domestication alleles appear to have instead originated only once [13].Finally, a high-throughput sequencing analysis of 446O. rufipogonaccessions along with 1083 cultivatedxianandgengvarieties revealed thatO. sativa gengrice was first domesticated from a specific population ofO. rufipogon.Xianrice was subsequently developed from crosses betweengengrice and local wild rice as the initial cultivars spread into southeast and south Asia [21].However, archaeological and most population-genetic analyses suggest that key domestication alleles have a single origin ingengrice in east Asia,and that the spread ofgengto south Asia led to the introgression of domestication alleles into proto-xianor localO.nivarapopulations before the eventual emergence ofxianrice[22-24]. Recently, an analysis of gene, coding sequence (CDS),and haplotype diversity in rice helped to answer the longstanding question of the origin of rice[25].The results showed that approximately 29%-40% of the genes divergent betweenxianandgengwere retained during the differentiation ofO. rufipogonfromO. nivara, long before domestication, strongly suggesting thatxianandAuswere domesticated from differentO. nivarapopulations and thatgengandBaswere domesticated from differentO. rufi-pogonpopulations.These inferred proto-ancestors of local landrace populations reconstructed from conserved predominant (ancient)gene-coding sequence-haplotype (gcHaps) correlated strongly with wild rice accessions from the same geographic regions, supporting a multi-origin model [25]. The domestication and evolution of thexianandgengsubspecies of cultivated rice can be summarized in four ways based on the literature (Fig. 1).

Fig. 1. Models of rice domestication and evolution for the xian and geng subspecies.
3.Differences in the genome,morphological,physiological,and anatomical traits
After long-term natural selection and artificial selection, the genomes ofxianandgengrice have undergone continuous differentiation [1] and recent resequencing as well asde novogenome assembly have revealed that structural variants (SVs) have played key roles in influencing agronomic traits [26]. SVs have also contributed toxian-gengdifferentiation,with the total size of genome variations betweenxianandgengas great as 71 Mb.Thexianaccessions differed from the reference genome Nipponbare by 14,754 SVs on average,or 3.5 times as many as the SVs ingengaccessions[1].As already pointed out,these differentiated SVs are involved in the regulation of agronomic traits.For example,a region harboringSLB1andSLB2was selected ingengaccessions because it could enable rice to acquire more phosphorus under low-phosphorus conditions [27,28]. Similarly, an insertion at 643 bp upstream ofOsGLP2-1, found ingengbut not inxianaccessions [27], was associated with increased expression that could help to ensure appropriate seed dormancy ingengvarieties [29,30].
There are marked differences in the characteristics of grain shattering,palea hair and shell color,amylose content,grain shape(ratio of grain length to width),and phenol reaction,which can be used to differentiate betweenxianandgeng(Table S1). These genetic differences not only account for the ability of cultivated rice to adapt to a wide range of complex ecological environments around the world, but also form the basis ofxian-genghybrid breeding.However,despite being distinct,many characters ofxianandgengrice still show continuous variation, especially for the intermediate-type germplasm. This could have been because the breeding of crosses betweenxianandgenghas broken existing stable characteristics ofxianandgeng. Recent studies of genomewide variation in rice have provided a novel approach to addressing this question. A set of subspecies-specific InDel and intron length polymorphism (SSILP) markers (Table S2) was used to distinguish betweenxianandgengin our previous study [4]. A set of 100,529 subspecies-specific SNPs selected based on the diversity in 517 rice landraces [31], was successfully used to determine thexianpedigree of offspring from a cross betweenxianandgeng[32].These results reinforce the idea thatxianorgeng-specific SNPs will be useful tools for studying subspecies differentiation and classification.
4. Differences in key quality and yield characters
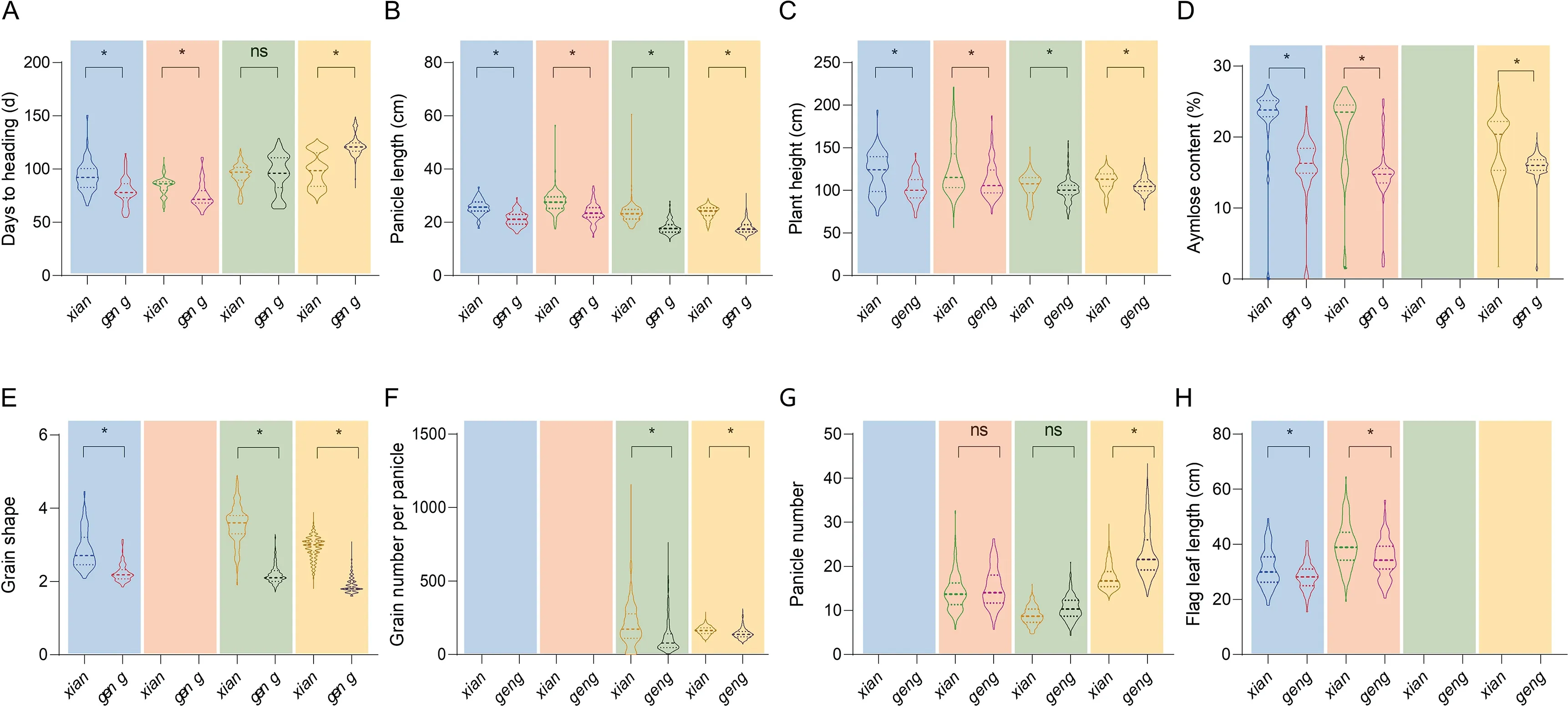
Fig.2. Comparisons between xian and geng rice for major agronomic and quality traits.Data were collected from Li et al.[27](654 varieties),Zhao et al.[28](183 varieties),Wei et al.[29](269 varieties),and Fei et al.[30](4449 varieties),shown with blue,pink,green and yellow backgrounds,respectively.(A-H)Differences between xian and geng in (A) days to heading, (B) panicle length (cm), (C) plant height (cm), (D) amylose content (%), (E) grain shape (ratio of grain length to grain width), (F) grain number per panicle, (G) panicle number, and (H) flag leaf length (cm). * and ns indicate respectively significant and non-significant differences between xian and geng at P <0.05.
Phenotypic data forxianandgengvarieties were collected from several genome-wide association studies (GWAS) as well as from Chinese national rice regional test data for the last two decades,with the aim of identifying differences in quality and yieldassociated traits between the two subspecies(Fig.2)[33-36].Both data sets indicated thatxianhad a greater flag leaf length, plant height, and panicle length thangeng.Xiangrain shape was also slimmer,butgengshowed a dramatic decrease in amylose content compared toxian. Although there were no marked differences in panicle number betweenxianandgengin the GWAS data set, differences were observed for this character in the national rice regional test data(Fig.2).Other tests focused on the quality of 8390 rice samples from 28 provinces of China from 1985 to 2002.Compared withxian,geng’s brown rice and head rice proportions were respectively 3.8% and 37.3% higher, while its chalkiness rice rate,chalkiness level, amylose content were respectively 24.5%, 46.1%,and 13.4% lower [37]. Our research group further examined national rice regional test data from 2004 to 2018[36],finding thatxianrice showed greater improvement in brown rice and head rice proportion thangeng.In particular,the head rice proportion ofxianincreased from 49.4% to 57.3%, narrowing the gap between the subspecies. or both species, the chalkiness rice proportion and chalkiness level decreased(thoughgengstill showed an advantage in these traits),along with amylose content,while the ratio of grain length to width increased. But overall, the relative difference betweenxianandgengremained unchanged. The slender grain ofxianrice is associated with improved appearance quality, while the short, rounded grain ofgengrice offer advantages in milling quality. There are also several large differences in the quality ofxianandgengrice planted in the middle and lower reaches of the Yangtze River[38-40],highlighting the influence of genetic factors.Although there was a slight difference in grain yield betweenxianandgeng,the latter displayed a clear advantage in head rice weight(Fig. 3) and this advantage confers ongengrice greater economic benefits in the consumer market. Overall, based on the summarized data describing changes in yield, quality, and related traits of new rice varieties in China over the last 15 years, our research team concluded [36] that the 1000-grain weight ofgengrice, as well as the grain density ofxianrice, should be increased within a certain range to achieve a combination of high yield and high quality.
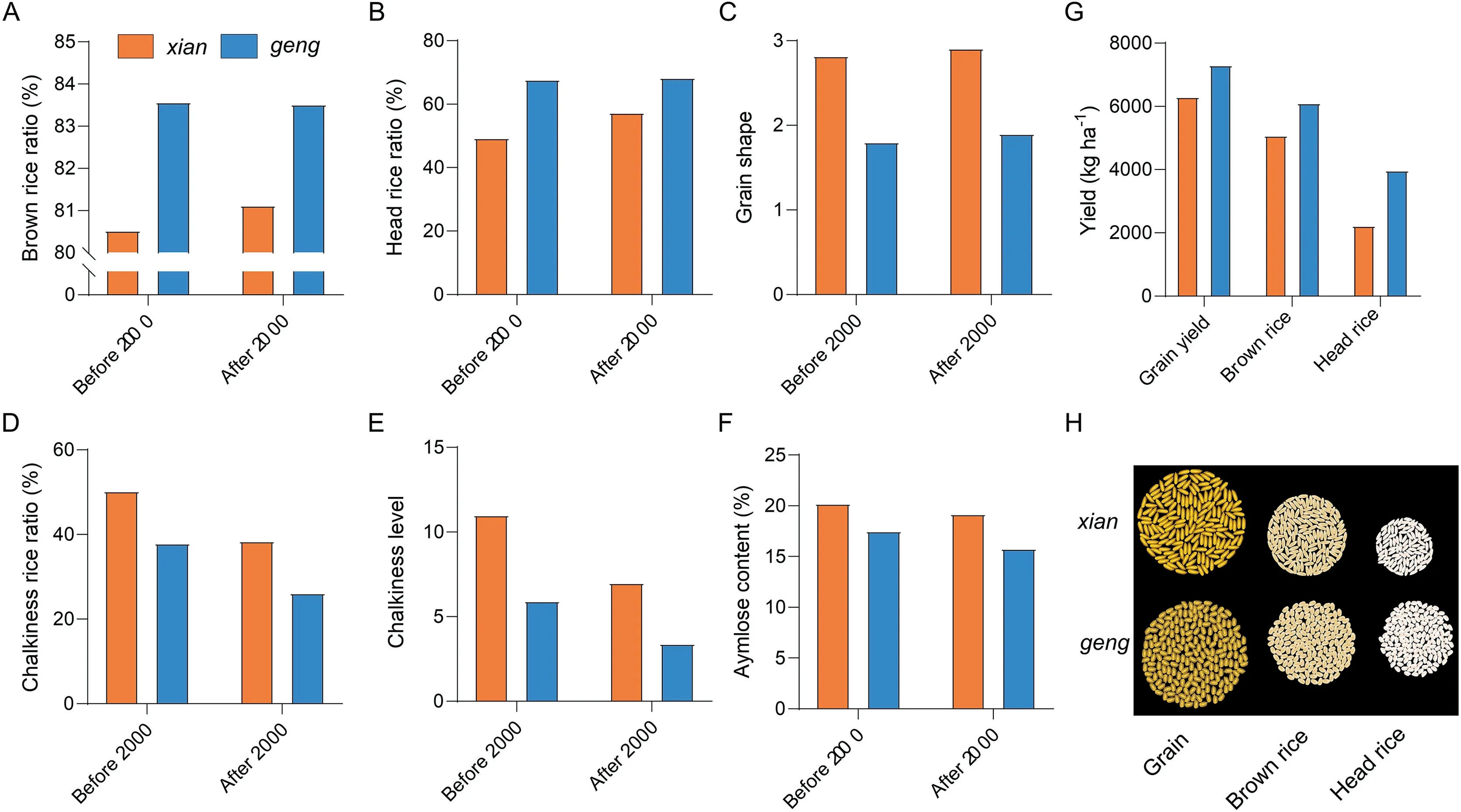
Fig.3. Comparisons between xian and geng rice for quality-and yield-associated traits.(A-F)Differences in quality traits between xian and geng varieties released before and after the year 2000.Yield-associated parameters in xian and geng(G).The diagram shows the differences between xian and geng in grain yield,brown rice,and head rice(H).
5. Molecular mechanisms of trait differences
Many genes associated with differences between the rice subspecies have been cloned.Wei et al.[35]listed 225 reported cloned genes that had been identified from natural variants,finding that at least 83 were involved in differentiatingxian-gengvarieties(Table S3).Among these differentiation genes,we focused on those that regulate traits closely associated with grain yield and grain quality. Since the 1980s, studies have shown that, compared withgeng,xianhas higher uptake of both nitrate and ammonium, with Hu et al. [41] showing that variation inNRT1.1B, a nitratetransporter gene, could have contributed to the differences in nitrate use. Inxian, these variations inNRT1.1Bhave been associated with increased nitrate uptake and root-to-shoot transport as well as upregulated expression of nitrate-responsive genes.Further evidence showed thatNRT1.1Bwas even linked with the recruitment of a large proportion ofxian-associated bacteria. An allelic variation ofOsNR2, a gene encoding an NADH/NADPH-dependent NO3-reductase, was also found to be associated with differences betweenxianandgengin the efficient use of nitrogen. Thexiantype allele ofOsNR2,encoding the structurally distinct OsNR2 protein,showed higher amounts of NO3-reductase than thegeng-type allele via feed-forward integration withOsNRT1.1B[42]. Natural variation in another gene,MYB61, were also responsible for differences in nitrogen use betweenxianandgeng, with MYB61 being directly regulated byGROWTH-REGULATING FACTOR4[43].Thexianallele of MYB61, in contrast to that ofgeng, displayed robust transcription,resulting in higher nitrogen-use efficiency and increased grain yield under reduced nitrogen supply [43] (Fig. 4).
Rice is sensitive to temperature and can be grown only in certain climate zones.The molecular basis of temperature adaptation betweenxianandgengdiffers correspondingly (Fig. 4).COLD1encodes a regulator of the G protein signaling pathway that is localized on the plasma membrane as well as the endoplasmic reticulum, and its overexpression ingengincreased chilling tolerance. In contrast, deficiency or downregulation ofCOLDgengcauses rice lines to be sensitive to cold. In a further study [44], an SNP inCOLD1, originating in the ChineseO.rufipogon,was responsible for this ability ofCOLDgeng/xianto confer chilling tolerance. A single functional polymorphism (G/A) inbZIP73(abZIPtranscription factor-encoding gene) between the two subspecies is also associated with cold-temperature adaptation. Indeed, the allelic version ofbZIP73ingenginteracts withbZIP1to modulate both abscisic acid (ABA) level and reactive oxygen species (ROS) homeostasis,thereby increasing adaptability to chilling[45,46].Finally,the need for low-temperature acclimation resulted in Tej-Hap ofCTB4abeing retained by artificial selection during the evolution ofgengin cold habitats. Upregulation ofCTB4awas correlated with increased ATP synthase activity through interactions with AtpB, a beta subunit of ATP synthase [47].
Owing to global warming,the breeding of rice varieties that are tolerant to high-temperature stress is desirable (Fig. 4).SLG1,which encodes the cytosolic tRNA 2-thiolation protein 2,functions in the response of rice plants to high-temperature stress,especially during the seedling and reproductive stages. This gene differs betweenxianandgengin both the promoter and coding regions,and these variations led to an increased level of thiolated tRNA as well as increased thermotolerance inxianrice varieties [48](Fig. 4).
High amounts of cadmium can be transferred from soil to rice tissues, andxianvarieties typically accumulate more of the heavy metal in the shoots and grain thangeng(Fig. 4). Sequence variations in theOsHMA3promoter were found to be a key determinant of differential accumulation of cadmium in grain, and based on this, the PA64s-type allele has been used to replace thexian-type allele in the super rice cultivar 93-11 to reduce cadmium concentration in grain without adverse effects on agronomic traits [49].Similarly,OsCd1, belonging to the major facilitator superfamily, is involved in root cadmium uptake and contributes to its accumulation in rice grain,but natural variation in this gene,along with the missense mutation Val449Asp, is responsible for differences in cadmium accumulation betweenxianandgeng[50] (Fig. 4).
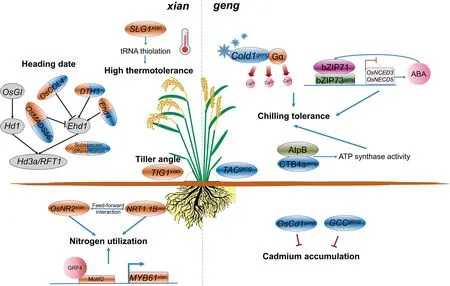
Fig. 4. A proposed model for the molecular mechanism underlying chilling tolerance, high thermotolerance, nitrogen utilization, cadmium accumulation, tiller angle, and heading date in xian and geng rice.
Artificial selection of an amino acid substitution in thePROG1protein during domestication led to a transition from the prostrate growth habit of wild riceO. rufipogonto that of domesticated rice,which has a relatively narrow tiller angle [18,19]. However, tiller angle,a key component of rice plant architecture,remains different betweenxianandgengrice varieties,withxianshowing a larger tiller angle as well as greater variation.Tiller angle control 1(TAC1)is a major quantitative-trait locus (QTL) influencing tiller angle. A variation at the 3′-splicing site of its 1.5-kb intron, from AGGA to GGGA,decreases the expression level ofTAC1,resulting in an erect plant architecture with a tiller angle close to zero. TheTAC1gene even allows subspecies differentiation, as its GGGA type is more common ingengaccessions, indicating that the GGGA type ofTAC1has been extensively deployed in densely planted rice grown in high-latitude temperate areas as well as at high altitudes wheregengrice varieties are widely cultivated [51]. Another major QTL influencing tiller angle,Tiller angle control 3(TAC3), was fixed ingengaccessions, but sits segregated inxiancould explain the wide variation in tiller angle observed inxiancompared withgengaccessions [52]. Finally, theTILLER INCLINED GROWTH 1(TIG1) gene which encodes a TCP transcriptional activator, inclined tiller growth in wild rice.PROG1,TAC1, andTIG1are three genes that not only are responsible for modifications in tiller angle during the domestication of plant architecture but also serve to differentiatexianfromgengrice (Fig. 4). Of these genes, theprog1allele,which confers the elimination of prostrate growth, was selected early in all cultivated rice, and only thereafter were thetac1and thetig1alleles selected ingengrice andxianrice respectively[53].
Heading date in crops is a critical determinant of the distribution and regional adaptability of rice accessions. Given that the geographical distribution ofxianandgenghave historically been different, the regulation of their heading dates also has sharp differences (Fig. 4). Rice is known as a short-day model plant, and itsOsGI-Hd1-Hd3aandEhd1-Hd3a/RFT1pathways,have been well characterized [54].DTH2gene encodes a CONSTANS-like protein that promotes heading by inducing the florigen genesHd3aandRFT1. But this gene also acts independently of the known floral integratorsHd1andEhd1[55]. In terms of differences between subspecies, haplotype analysis revealed that the types A1 and A2 ofDTH2were found inxianaccessions,whereas type A4 was found exclusively ingenglandraces grown in temperate areas. Another gene,Ehd4, encodes a novel CCCH-type zinc finger protein, upregulates the expression of theHd3aandRFT1throughEhd1[56].Differentiation is also possible based on this gene’s two major haplotypes:Hap_2,which is distributed mostly inxianaccessions,and Hap_3 which is the major haplotype ingeng. Finally,OsMADS51/DTH3is a short-day heading promoter that functions upstream ofEhd1, and based on indels as well as derived cleaved amplified polymorphic sequences (dCAPs) marker analysis, thedth3allele was found[57,58]to be present only in African rice accessions that tend to be similar togengcultivars. Other studies support subspecies differentiation based onOsCOL4andOsMADS56genes[35].
Grain quality is the main trait differentiatingxianfromgeng.Among genes controlling this character, theWaxy(Wx) gene,encoding granule-bound starch synthase I (GBSSI), controls amylose synthesis in the endosperm [59], and natural allelic variation in thisWxlocus is the main cause of the broad diversity in amylose content(AC)as well as in eating and cooking quality(ECQ)of modern rice [60]. In recent decades, there have been advances in the modulation of the AC for improving the ECQ of bothxianandgengrice using certainWxalleles, particularlyWxbandWxinwhich cause low to intermediate AC [61]. It is thus expected that mild regulation of AC can be achieved by fine-tuningWxexpression to improve rice ECQ. There is also obvious variation in grain appearance between the two subspecies, with grain shape, regulated by multiple loci, being a major differentiator betweenxianandgeng.Thegeng-type alleles ofqSW5/GW5,OsSPL16/GW8, andGS6increase the width of rice grain [62-67],while thexian -type ones ofGS5andOsSPL13/GW7increase grain length [68,69].
6. The model of ideal plant architecture for geng varieties
Rice plant architecture directly affects the number of effective tillers and kernels, both of which are key components of yield[70].To improve the yield potential of rice,the concept of an ideal plant architecture was proposed. It included several characters:moderately low tiller numbers with only a few unproductive tillers, more grains per panicle than currently cultivated varieties,thick and sturdy stems, and a well-developed root system [70-73]. Based on available knowledge, we propose an ideal plant architecture forgengvarieties for optimizing the yield potential while balancing the relationship between yield and quality(Fig.S1).In the northern regions where rice is cultivated,the plant height should be 95-105 cm,with the tiller compact and erect and the panicle number 3.3 × 106ha-1with only a few unproductive tillers.The panicle neck angle should be less than 40°,and the harvest index should be 0.55-0.60[74,75].The ideal panicle architecture requires the best combination of panicle traits associated with high yield and quality under specific ecological conditions[76].The number of kernels on the primary branch is approximately six,without being influenced by environmental conditions, or types and varieties. Therefore, the number of grain per panicle is determined mainly by the number of grain on the secondary branch.The seed setting rate negatively correlated with the number of kernels on secondary branches,and this had a direct effect on the seed setting of the large panicle ingengrice varieties [77]. The grain number on the secondary branch is greatly affected by the environment, but since its distribution is mainly controlled by genetics,there are differences in the secondary branch distribution betweenxianandgeng[78]. Finally, the varieties that had more secondary branches on the upper part of the panicle showed advantages in milling quality, appearance quality, setting rate, and yield[79,80]. Thus, increasing the grain number of secondary branches on the upper part of the panicle may be an effective way to coordinate yield and quality ingengrice grown in northern regions.
7. Conclusion and perspectives
Natural selection,artificial domestication,climate change,gene mutation, natural hybridization, and other factors have contributed to rice domestication. The domestication of wild rice to yield cultivated rice was a step-by-step process,with local varieties being the result of an evolutionary adaptation that involved the optimization of gene combinations under the influence of longterm natural and artificial selection. ‘‘De novodomestication” and‘‘re-domestication” offer novel approaches and methods for research on rice [81]. The heterosis of F1plants from crosses between subspecies has been shown to provide clear advantages compared to parental varieties.Variation in offspring derived from crosses betweenxianandgenghas facilitated the breeding process.A major obstacle to the application of heterosis between subspecies has remained hybrid sterility[82,83].With further research on the molecular mechanism underlying wide compatibility,a series of hybrid varieties, derived from crosses between subspecies,were bred in the middle and lower reaches of the Yangtze River,including Youyong, Chunyou, Zheyou, and Jiayouzhongke. These cultivars represent bright prospects for the application of heterosis between subspecies [84,85]. Based on pedigree analysis, it is also now known that most of the prevalent commercial rice varieties grown in Northern China since the 1980 s were bred from crosses betweenxianandgeng. In particular, the introgression of thexianpedigree significantly improved the yield potential ofgengrice varieties [4]. However, these varieties still maintainxianorgengsubspecies attributes, introgression among the subspecies is relatively low[86],and little is known about which excellentxianpedigrees were used in thegengbreeding process. In the future, it should be considered whetherxianandgenghybrid breeding strategies should break the subspecies boundary to achieve optimal combinations of thexianandgenggenome that can adapt to specific ecological conditions, or whether the genetic background of subspecies should be maintained by introducing only certain favorable genes ofxianorgeng. The adaptability ofxianandgengrice is the result of ecological conditions, natural selection, and artificial selection,with the latter’s application in the breeding process dependent largely on social factors such as consumption demand. The consumption demand is undoubtedly reflected in the recent years’ trend of expanding thegengcultivation area.The current geographical distribution ofxianandgengmight be improved to achieve maximum rice productivity, but whether the distribution ofxianandgeng, formed during the breeding history of China, can be partially adjusted or restructured remains a theoretical and practical issue that invites further investigation.
An increasing number of genes have been cloned in rice, especially those that are both involved inxian-gengdifferentiation and associated with important agronomic traits,and this approach has provided further possibilities for applying molecular biology and related tools to molecular-based breeding [87,88]. It is desirable to further study the relationships amongxianandgengdifferentiation genes and to realize the pyramiding of favorable subspecies genes, particularly genes associated with nutrient efficiency, quality, and biotic and abiotic stress resistance inxianandgeng.
CRediT authorship contribution statement
Linlin Jiang:Writing-original draft.Lian Wu:Writing-original draft.Yun Wang: Writing-original draft.Quan Xu:Writingoriginal draft.Zhengjin Xu: Conceptualization.Wenfu Chen:Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (32071982 and U1708231).
Appendix A. Supplementary data
Supplementary data for this article can be found online at https://doi.org/10.1016/j.cj.2022.02.006.
- The Crop Journal的其它文章
- Brief Guide for Authors
- Revisiting the role of delta-1-pyrroline-5-carboxylate synthetase in drought-tolerant crop breeding
- A new gain-of-function OsGS2/GRF4 allele generated by CRISPR/Cas9 genome editing increases rice grain size and yield
- Influence of seven levels of chemical/biostimulator protection on amino acid profile and yield traits in wheat
- Corrigendum to ‘‘De novo design of future rapeseed crops: Challenges and opportunities” [Crop J. 10 (2022) 587-596]
- Improving the resistance of the rice PTGMS line Feng39S by pyramiding blast, bacterial blight, and brown planthopper resistance genes

