From model to alfalfa: Gene editing to obtain semidwarf and prostrate growth habits
Lihu Zheng, Jingqi Wen, Jinling Liu, Xingzho Meng, Peng Liu, N Co, Jingli Dong,To Wng,
a State Key Laboratory of Agrobiotechnology, College of Biological Sciences, China Agricultural University, Beijing 100193, China
b Institute for Agricultural Biosciences, Oklahoma State University, Ardmore, OK 73401, USA
c Department of Plant and Soil Sciences, Oklahoma State University, Stillwater, OK 74078, USA
Keywords:Medicago sativa Medicago truncatula Plant architecture Gibberellins GA 3-oxidase
A B S T R A C T Alfalfa (Medicago sativa L.) is a nutritious forage crop with wide ecological adaptability. The molecular breeding of alfalfa is restricted by its heterozygous tetraploid genome and the difficult genetic manipulation process.Under time and resource constraints, we applied a more convenient approach. We investigated two MtGA3ox genes, MtGA3ox1 and MtGA3ox2, of Medicago truncatula, a diploid legume model species,finding that MtGA3ox1 plays a major role in GA-regulated plant architecture.Mutation of neither gene affected nitrogenase activity. These results suggest that MtGA3ox1 can be used in semidwarf and prostrate alfalfa breeding. Based on the M. truncatula MtGA3ox1 sequence, MsGA3ox1 was cloned from alfalfa,and two knockout targets were designed.An efficient CRISPR/Cas9-based genome editing protocol was used to generate msga3ox1 mutants in alfalfa. We obtained three lines that carried mutations in all four alleles in the T0 generation.Fifteen clonal plants were vegetatively propagated from each transgenic line using shoot cuttings. The plant height and internode length of msga3ox1 null mutants were significantly decreased.The number of total lateral branches,leaf/stem ratio and crude protein content of aerial plant parts of msga3ox1 mutants were significantly increased. Thus, we obtained semi-dwarf and prostrate alfalfa by gene editing.
1. Introduction
Alfalfa(Medicago sativaL.)is the most commonly grown perennial forage legume in the world and is known as the‘‘Queen of Forages” because of its high biomass yield, wide adaptability,excellent forage qualityz and capacity for symbiotic nitrogen fixation [1-3]. The ability of alfalfa to fix atmospheric N2allows it to grow in a wide range of soil types and provide N2to surrounding crops when alfalfa is included in a crop intercropping scheme[4,5]. Planting alfalfa can reduce the use of nitrogen fertilizer,which is very important for sustainable agriculture [6,7]. Alfalfa is an obligate outcrossing tetraploid crop.Seeds from an individual alfalfa plant are genetically heterogeneous [8,9]. For this reason,breeding progress in alfalfa has been particularly slow compared with that in other major field crops. In particular, few studies of alfalfa plant architecture have been reported. With the decoding of alfalfa genome information and the rapid development of genome editing technology, it is possible to study the regulation of alfalfa architecture.
Medicago truncatulahas been chosen as a model species for legumes.It is diploid,self-fertilizing,regenerable from cell culture lines, and transformable byAgrobacterium; has a short generation time;and produces many seeds[10,11].Various genetic and genomic resources have been developed inM. truncatula, including a small,deeply sequenced,well-annotated genome and various ESTs and theMedicago truncatulaGene Expression Atlas [12-14].CRISPR/Cas9-based gene editing has been used for single- or multiple-target editing inM. truncatula[15,16]. These resources permit the efficient study of gene function inM. truncatula, especially when different genes in the same family must be examined in genetic functional analyses, and ultimately the application of the resulting knowledge to more complex species, such as alfalfa.
Gibberellin (GA) is an important hormone regulating growth and development in higher plants.Its synthesis can be divided into three stages involving a series of oxidation-reduction reactions in plastids,the endoplasmic reticulum,and the cytosol[17].The first stage is the synthesis ofent-kaurene from geranylgeranyl diphosphate. This reaction is catalyzed byent-copalyl diphosphate synthase (CPS) andent-kaurene synthase (KS) in plastids [18,19].During the second stage,ent-kaurene is converted to GA12in the endoplasmic reticulum by sequential oxidation: in the first three steps,ent-kaurene oxidase (KO) catalyzes the transformation ofent-kaurene intoent-kaurenoic acid, which is oxidized byentkaurenoic acid oxidase (KAO) in the last three steps [19-21]. In the third stage, the conversion of GA12into bioactive forms involves the action of GA 20-oxidase (GA20ox) and GA 3-oxidase(GA3ox) [22,23]. These enzymes are located in the cytosol [24].Almost all of the genes encoding key enzymes in GA synthesis have been cloned in many species, and their corresponding mutants have been obtained [18,25-33]. These mutants showed a dwarf phenotype that could be restored by exogenous application of bioactive GAs. This achievement demonstrates that we can optimize plant architecture by manipulating the expression of genes involved in GA biosynthesis.
The breeding of semidwarf alfalfa by modifying genes involved in GA biosynthesis has not been reported.In this study,we identified a semidwarf mutant from theM. truncatula Tnt1mutant population. Sequencing and PCR analysis revealed that the semidwarf phenotype was caused by the deletion of 8 bp ofMedtr2g102570(MtGA3ox1). Further study showed that MtGA3ox1 is associated with GA-regulated plant architecture. Subsequently, alfalfaMsGA3ox1was cloned, andmsga3ox1null mutants were obtained.msga3ox1mutants showed phenotypes of reduced plant height and internode length and increased numbers of lateral branches,leaf/stem ratio, and aboveground crude protein. In summary, we bred a semidwarf and prostrated alfalfa new material by gene editing ofMsGA3ox1.
2. Materials and methods
2.1. Plant materials, growth conditions, and generation of transgenic plants
TheM. truncatulaR108 ecotype, which served as the WT, was used as the genetic background forAgrobacterium-mediated transformation as previously described [34]. Generation of theM. truncatula Tnt1insertional mutant population was as described previously[35].A tetraploid cultivated alfalfa(M.sativa)genotype,‘Baoding Alfalfa’, was used forAgrobacterium-mediated transformation to produce transgenic plants. Fully developed leaves were collected from 4-week-old alfalfa plants and sterilized.Agrobacterium tumefaciensstrain EHA105 carrying the binary vector was used to transform calli as follows. EHA105 cells were cultured overnight until reaching an OD600=0.4 and were then resuspended in 200 mL SAM4 liquid medium(Murashige&Skoog Basal Medium with Vitamins (Phyto Technology, M519), 30 g L-1sucrose, 4 mg L-12,4-D, 0.2 mg L-16-benzylaminopurine, pH 5.85) containing 100 μmol L-1acetosyringone. Leaves were then submerged in the suspension in a covered wide-mouth bottle.The bottle was evacuated to ≥0.09 MPa for 5 min. It was then placed in an ice-water mixture for ultrasonic treatment for 2 min and evacuated again for 5 min.The leaves were placed on sterilized filter paper in a Petri dish and then transferred to SAM4 medium containing 100 μmol L-1acetosyringone and 3 g L-1Phytagel, followed by incubation in a growth chamber at 24 °C for 3 days. They were then transferred to SAM4 medium containing 200 mg L-1cefotaxime,200 mg L-1timentin,10 mg L-1hygromycin B,and 3 g L-1Phytagel for 28 days (with replacement of the culture medium every 7-10 days). After selection cultivation, all calli were transferred to MSBK medium (Murashige&Skoog Basal Medium with Vitamins,30 g L-1sucrose, 1 mg L-1kinetin, 0.2 mg L-16-benzylaminopurine, 200 mg L-1cefotaxime, 200 mg L-1timentin,10 mg L-1hygromycin B, 3 g L-1Phytagel, pH 5.85) for 28 days(with replacement of the culture medium every 7-10 days). Next,all calli were transferred to SH9 medium (SH basal salts and vitamins, 100 mg L-1myo-inositol, 20 g L-1sucrose, 8 g L-1BD agar,200 mg L-1cefotaxime,200 mg L-1timentin,10 mg L-1hygromycin B,pH 5.85)for at least 30 days(with replacement of the culture medium every 15-20 days),and the regenerated plants were then transferred to 1/2 MS root induction medium (MS basal salts and vitamins, 15 g L-1sucrose, 8 g L-1BD agar, pH 5.85). Finally, the plants were transferred to soil and grown to maturity in a greenhouse. Both transgenic and WT alfalfa plants were vegetatively propagated from shoot cuttings.
Seed sterilization was based on the procedure described in Duan et al.[36].Plants were grown in a greenhouse at 22°C under a 16-h light/8-h dark cycle and 60%-70% relative humidity.
2.2. Exogenous application of GA4
Bioactive GA4(Sigma,CAS: 468-44-0)was dissolved in ethanol(0.01 mol L-1). A solution of 0.01 mol L-1GA4was diluted to 0.1 mmol L-1with 2 mL ddH2O and sprayed on the surfaces of all plants in a pot.The control plants were sprayed with a mixture of 20 μL 99.8% ethanol and 2 mL ddH2O. The plants were sprayed first at 14 days after planting and then once every four days. Photographs were taken after three sprays.
2.3. Phylogenetic analysis
The sequences of GA3ox proteins were obtained from the JCVI(https://medicago.toulouse.inra.fr/MtrunA17r5.0-ANR/) and NCBI databases (http://www.ncbi.nlm.nih.gov). A phylogenetic tree was generated using the neighbor-joining method implemented in MEGA X (https://www.megasoftware.net/)with 1000 bootstrap replications.
2.4. RNA extraction and RT-qPCR
Total RNA was extracted from various tissues using TRIzol reagent (Invitrogen, 15596018), and two micrograms of total RNA was used for reverse transcription (Promega, catalog no.M1701). RT-qPCR was performed on a CFX-96 real-time system(Bio-Rad) with SYBR Premix Ex-Taq (TaKaRa, catalog no.RR420A).Gene expression was calculated using the 2-ΔΔCTmethod[37].MtACTINandMtGAPDHwere selected as reference genes[38-39]. All primers used in this study are listed in Table S1.
2.5. Constructs for GUS reporter lines and GUS staining
The 2.507 kb upstream ofMtGA3ox1ATG and 2.286 kb upstream ofMtGA3ox2ATG were cloned as promoter regions and ligated into the pCAMBIA1381 vector to drive the expression of the GUS (β-glucuronidase) gene. These constructs were transformed into R108 via a process mediated byA. tumefaciensstrain EHA105, which resulted in the generation of stable transgenic lines. The transgenic plants were stained as previously described[16].
2.6. Mutants generated via the CRISPR/Cas9 approach
Generation of stableMtGA3ox1andMtGA3ox2mutants was based on the CRISPR/Cas9 system [16]. The target sites ofMtGA3ox1andMtGA3ox2were designed using CRISPOR (CRISPOR(tefor.net))(Fig.S1).The MtU6-5 and MtU6-6 promoters were used to drive the expression of sgRNAs [16]. The PCR fragment(T1T2-PCR) was amplified from p5CBC-DT1T2 and the purified PCR fragment was combined with the binary vector pHSN401 by restriction-ligation reactions as previously described [40]. The constructs were separately transformed into R108 with resistance to hygromycin byA.tumefaciensstrain EHA105, and the regenerated seedlings were tested by PCR using primer pairs for fragments of transferred T-DNA. The specific mutations were confirmed by amplifying the genomic fragments flanking the target sites,ligating them into PLB vectors (TIANGEN, catalog no. VT205), and then sequencing randomly selected positive clones.
2.7. Bacterial materials and cultivation conditions for nodulation
Sinorhizobium melilotistrain 1021 (Sm1021) was used for symbiosis-associated experiments,and rhizobia cultured overnight were resuspended in water at OD600= 0.05 for inoculation. Plants were grown in a vermiculite:perlite (5:2, v/v) mixture saturated with nitrogen-depleted Fåhraeus liquid medium. Seven-day-old seedlings were injected with 15 mL(per plant)of rhizobial suspension in the matrix [41].
2.8. Acetylene reduction assay
In legume nodules, nitrogenase reduces acetylene to ethylene during symbiotic nitrogen fixation. Therefore, the acetylene reduction assay was used to measure the nitrogenase activity of wild-type plants andMtGA3oxmutants [42]. Fresh underground samples of wild-type andMtGA3oxmutants were placed in 10-mL rubber-capped tubes with 500 μL ddH2O. Next, 200 μL of acetylene was injected into the tubes and reacted for 2 h at room temperature. Following the reaction, 100 μL of the reaction gas was separated by gas chromatography (Shimadzu, Nexis GC-2030).The peak area of ethylene was used to calculate nitrogenase activity,and the fresh nodule weight was measured.Ethylene generated per unit nodule weight per unit time represented the activity of nitrogenase.
2.9. Determination of leaf-to-stem-ratio
The cutting height was 2 cm above ground level.The dry weight of leaves (including leaf blades and petioles) and stems was obtained after oven drying for 72 h at 60°C.The leaf-to-stem ratio was calculated as the dry weight of leaves divided by that of stems[43-45].
2.10. Determination of crude protein
Crude protein was determined as described previously [46].Approximately 0.2 g of dry powder samples of WT andmsga3ox1mutants were weighed and placed in a digestion tube. Then,2.5 g catalyst (K2SO4: CuSO4: selenium powder = 100:10:1) and 5 mL concentrated sulfuric acid were added to the digestion tube,followed by boiling at 410 °C for 1 h. The volume of hydrochloric acid standard solution required for sample titration was determined with a Kieldahl Azotometer (Hanon Instruments K1100F).The resulting data were used to calculate the crude protein content.
3. Results
3.1. MtGA3ox1 mutants show a semidwarf phenotype
NF20764 plants were obtained from theTnt1retrotransposon-taggedM. truncatulapopulation at the Noble Research Institute (Ardmore, OK, USA). Some NF20764 plants in the segregating progeny showed a semidwarf phenotype.The observed phenotypic segregation ratio of 8:37 (χ2= 1.252<χ20.05(1) = 3.841) suggested that the mutation was caused by the loss of function of a single recessive nuclear gene. To identify the gene underlying this phenotype, NF20764 plants were investigated for all possible defective genes. Based on a search of theMedicago truncatulaMutant Database, PCR identification and analysis of 106 genes were performed. However, no insertion sites associated with the semidwarf phenotype were identified. With the aim of identifying the causative mutation,whole-genome deep sequencing was performed with the Illumina HiSeq 2500 System. Genomic DNA from 20 individual mutant plants was purified and sequenced to 40X coverage at the Oklahoma Medical Research Foundation. Sequencing reads were cleaned and mapped to the reference genome. One of the homozygous mutations identified was an 8-bp deletion in the geneMedtr2g102570(MtGA3ox1) (Fig. 1a).MtGA3ox1encodes a GA3ox enzyme belonging to the class of 2-oxoglutarate-dependent dioxygenases, which catalyzes the final step in the biosynthetic pathway of bioactive GAs. In 14-dayoldMtGA3ox1mutant plants treated with 0.1 mmol L-1GA4,the heights of the plants were restored relative to the control(Fig. 1b, c). This result indicated thatMtGA3ox1mutant plants lack bioactive GAs, in turn affecting plant architecture inM.truncatula.
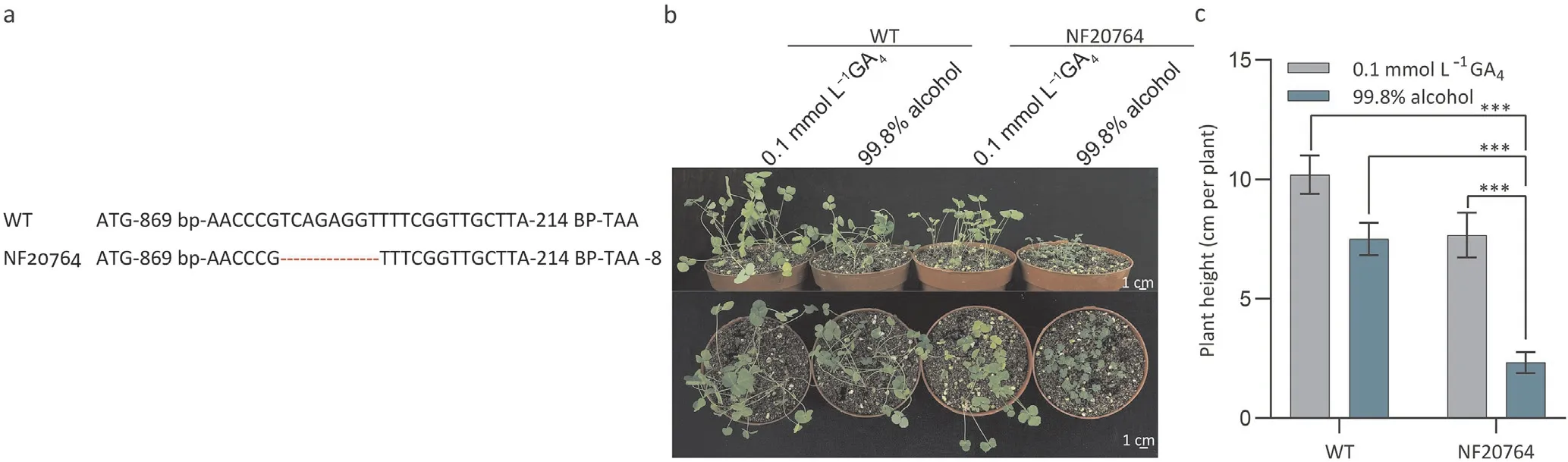
Fig. 1. Effects of exogenous application of GA4 on wild-type (WT) and NF20764 Medicago truncatula. (a) NF20764 carried a deletion of 8 bp. (b) Phenotypes of WT and NF20764 mutant seedlings treated with 0.1 mmol L-1 GA4 in comparison with untreated controls. (c) The height of plants in the experiment shown in (b). The experiment was repeated three times, and one representative set of results is shown. Values are means ± SD, and significant differences were determined by the Kruskal-Wallis nonparametric test (***, P <0.001, n ≥15).
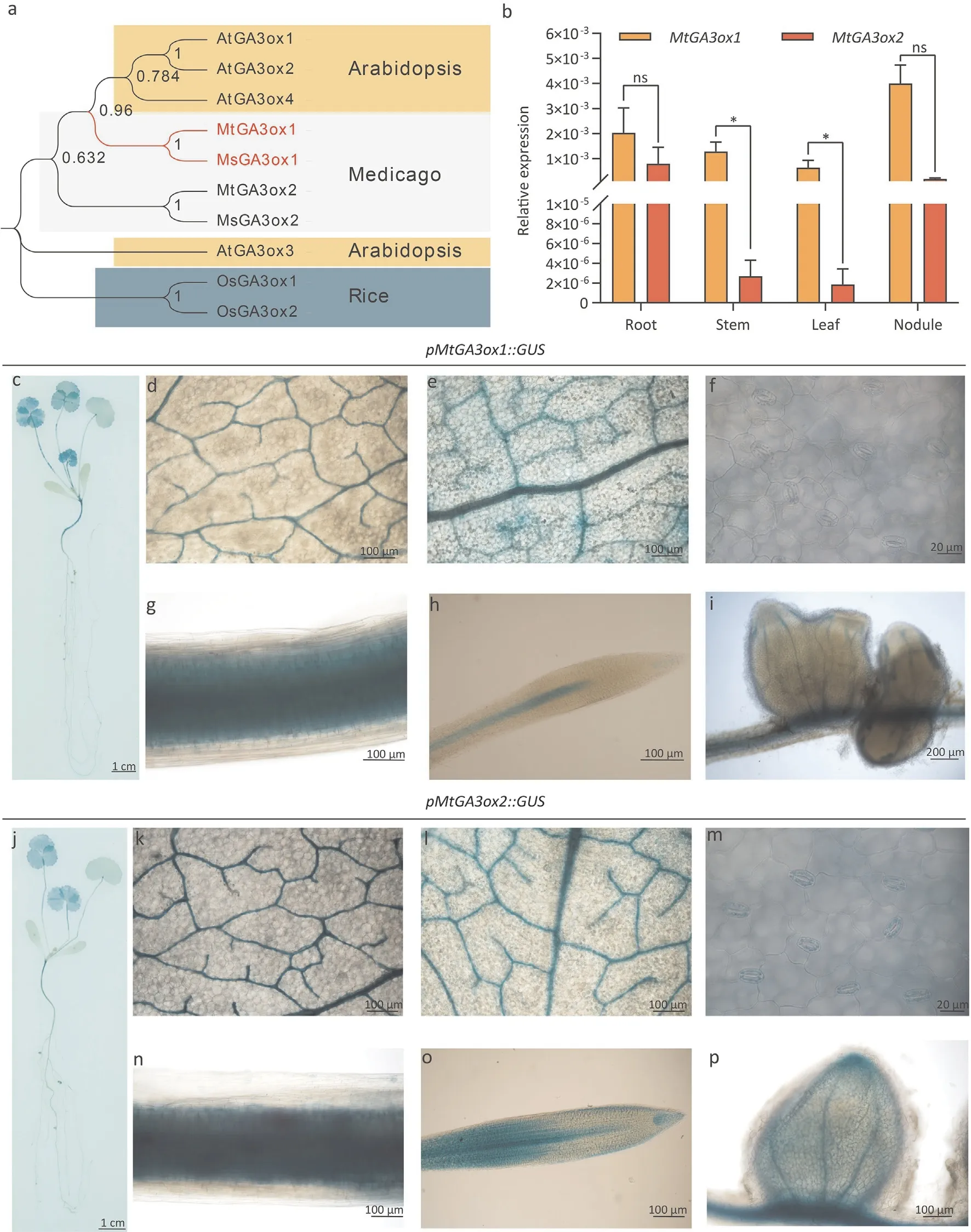
Fig. 2. Phylogenetic tree of GA3oxs and the expression patterns of the MtGA3ox1 and MtGA3ox2 genes. (a) Phylogenetic tree of GA3oxs. Medicago truncatula: MtGA3ox1(Medtr2g102570),MtGA3ox2(Medtr1g011580).Medicago sativa:MsGA3ox1(MsG2A10011279),MsGA3ox2(MsG1A10000633).Arabidopsis thaliana:AtGA3ox1(At1g15550),AtGA3ox2(At1g80340),AtGA3ox3(At4g21690),AtGA3ox4(At1g80330).Rice(Oryza sativa):OsGA3ox1(AB054084),OsGA3ox2(AB056519).(b)Relative expression levels of MtGA3ox1 and MtGA3ox2 in roots,stems,leaves,and nodules(WT plants at 14 dpi with Sinorhizobium meliloti 1021)were measured by RT-qPCR using MtACTIN and MtGAPDH as reference genes.Values are means±SD of n=3 pooled tissue samples,each of which consisted of 3-5 plants.Significant differences were determined by Student’s t-test(*,P <0.05).GUS staining of MtGA3ox1 and MtGA3ox2 expressed in M.truncatula.Expression of MtGA3ox1 in the whole plant(c),cotyledons(d),compound leaves(e),stoma(f),stems (g), root and root tip (h), nodules (i). Expression of MtGA3ox2 in the whole plant (j), cotyledons (k), compound leaves (l), stoma (m), stems (n), root and root tip (o),nodules (p).
3.2. MtGA3ox1 and MtGA3ox2 showed different expression patterns
The protein sequence of MtGA3ox1 was searched with BLASTp against the JCVIMedicago truncatulaprotein database. MtGA3ox2,another member of the GA3ox family inM. truncatula, showed the highest similarity (45.5%) with MtGA3ox1. MtGA3ox2 was encoded byMedtr1g011580. In a phylogenetic tree, MtGA3ox1 was close to AtGA3ox1 (Fig. 2a). To determine the tissue expression patterns ofMtGA3ox1andMtGA3ox2, the transcript levels ofMtGA3ox1andMtGA3ox2in various tissues were measured by RT-qPCR, andMtACTIN(Medtr3g095530) andMtGAPDH(Medtr4g103920) were selected as reference genes.MtGA3ox1showed relatively high expression in stems and leaves, and the expression showed no significant difference betweenMtGA3ox1andMtGA3ox2in roots and nodules(Fig.2b).TheGUSreporter gene driven by the promoter ofMtGA3ox1orMtGA3ox2was also transformed into theM.truncatulaR108 ecotype byAgrobacterium tumefaciens-mediated transformation. A histochemical analysis of GUS staining in the T1 generation of stable transgenic lines was performed after three weeks of growth.According to the GUS staining results,MtGA3ox1andMtGA3ox2were expressed in the vascular bundles of cotyledons (Fig. 2d, k), compound leaves (Fig. 2e, l),stems (Fig. 2g, n), roots (Fig. 2h, o), and leaf veins. The staining ofMtGA3ox2was stronger than that ofMtGA3ox1in the stoma and root tip (Fig. 2f, h, m, o). BothMtGA3ox1andMtGA3ox2were expressed in nodules, andMtGA3ox2was expressed in the meristem of the nodules (Fig. 2i, p). These results indicated that the expression patterns ofMtGA3ox1andMtGA3ox2differed, suggesting further study of their effects on plant architecture.
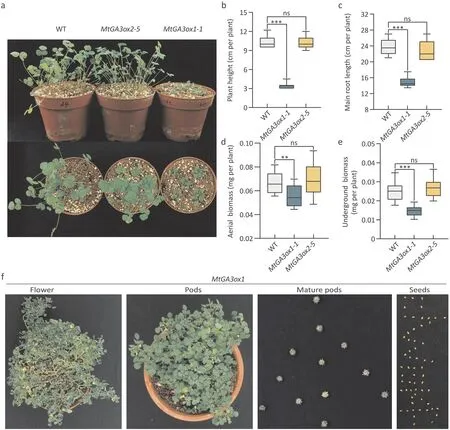
Fig. 3. Phenotypic identification of the MtGA3ox1-1 and MtGA3ox2-5 mutants under normal conditions. Five-week-old plants were photographed and measured under normal conditions, n ≥15. (a) Photographs of WT and MtGA3ox. (b) Plant height. (c) Main root length. (d) Aerial biomass. (e) Underground biomass. The experiment was repeated three times, and one representative set of results is shown. An asterisk indicates a significant difference between MtGA3ox1-1 and WT (**, P <0.01; ***, P <0.001,Kruskal-Wallis nonparametric test). (f) MtGA3ox1 show normal flowering and seed formation phenotypes.
3.3. MtGA3ox1, rather than MtGA3ox2, acts in GA-regulated plant architecture regulation
To study the effect of MtGA3ox1 and MtGA3ox2 on plant architecture, a CRISPR/Cas9 strategy based on two target sites was implemented to generate stableMtGA3ox1andMtGA3ox2mutants.Four T0MtGA3ox1-knockout mutants were identified (Fig. S1a).Two (MtGA3ox1-1andMtGA3ox1-7) were homozygous, showing early termination of translation due to a single base-pair insertion.The other two (MtGA3ox1-3 and MtGA3ox1-10) harbored biallelic mutations that caused translation frameshifts.In the T0 generation transgenic material ofMtGA3ox2,four homozygotes (MtGA3ox2-5,MtGA3ox2-9,MtGA3ox2-11,MtGA3ox2-13)were identified, and translation ofMtGA3ox2was prematurely terminated in all four mutants(Fig.S1b).T1 homozygotes ofMtGA3ox1-1andMtGA3ox2-5were retained for subsequent experiments. The phenotypes of wild-type(WT)plants andMtGA3oxmutants that had been grown for five weeks under normal conditions are shown in Fig.3a.There was no difference in plant height,main root length,aerial biomass,or underground biomass between theMtGA3ox2-5mutant and WT plants (Fig. 3b-e). TheMtGA3ox1-1mutant showed a semidwarf phenotype relative to the WT phenotype, with its height reduced by approximately 70% (Fig. 3b). The aerial biomass ofMtGA3ox1-1mutants was decreased by approximately 20% (Fig. 3d). Both the main root length and the underground biomass ofMtGA3ox1were reduced by approximately 40% relative to the WT (Fig. 3c,e).Continuous observation of the phenotypes ofMtGA3ox1mutant plants showed that their lateral branches grew horizontally. The mutants were capable of flowering and setting seeds, but their flowering time was approximately three weeks later than that of the WT (Fig. 3f).
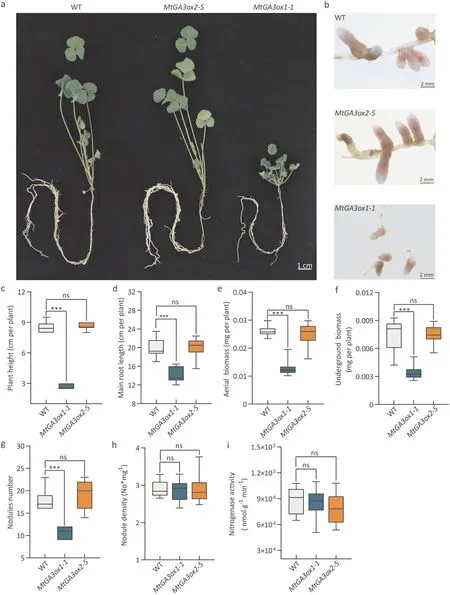
Fig. 4. Phenotypic characterization of MtGA3ox and WT under nodulation conditions. Medicago truncatula plants were inoculated with Sm1021 after 7 days of nitrogen deficiency treatment.Photographs were taken at 28 DPI.(a)Photographs of WT and MtGA3ox.(b)Morphology of nodules.(c-i)Seedlings were measured at 28 DPI,n ≥15.(c)Plant height.(d)Main root length.(e)Aerial biomass.(f)Underground biomass.(g)Number of nodules.(h)Nodule density(No.mg-1 dry weight of underground plant parts).(i) Nitrogenase activity. The experiment was repeated three times, and one representative set of results is shown. An asterisk indicates a significant difference between MtGA3ox1-1 and WT (***, P <0.001, Kruskal-Wallis nonparametric test).
Thus,MtGA3ox2 showed negligible effect on the plant architecture ofM. truncatulaunder normal conditions. TheMtGA3ox1mutation caused dwarfism,but showed no adverse effects on seed setting.
3.4. MtGA3ox1 and MtGA3ox2 did not affect nitrogenase activity
Legumes are key components of sustainable agriculture because they can fix nitrogen (reduce N2to NH3) in symbiotic associations with bacteria called rhizobia, providing crops with a free and renewable source of nitrogen [6]. Therefore, we wished to determine whether the mutation ofMtGA3oxgenes had any effects on the symbiotic nitrogen fixation ofM. truncatulaunder nitrogenfree conditions. WT andMtGA3oxplants were grown in low-N medium for seven days and then inoculated withSinorhizobium meliloti1021 (Sm1021) for four weeks (Fig. 4a, b). The differences in plant height, main root length, aboveground biomass, and underground biomass betweenMtGA3ox1-1,MtGA3ox2-5and WT under nitrogen-free conditions were the same as those under normal conditions(Fig.4c-f).There was no difference in nodule number, nodule density or nitrogenase activity betweenMtGA3ox2-5and WT (Fig. 4g-i). Nodule number was significantly reduced inMtGA3ox1-1plants, but nitrogenase activity did not differ from that in WT plants (Fig. 4g, i). According to the nodule density observed inMtGA3ox1-1plants, the decrease in nodule numbers was due to the abnormal development of roots. Thus, although bothMtGA3ox1andMtGA3ox2were expressed in nodules, their effects on nodules were not significant.
3.5. Genetic modification of MsGA3ox1 alters plant architecture in cultivated alfalfa
Based on the above results, we concluded that MtGA3ox1,rather than MtGA3ox2,influences GA-regulated plant architecture.Moreover,MtGA3ox1mutants still possess nodulation,nitrogen fixation, flowering and seed formation abilities. MtGA3ox1 shows promising potential in the breeding of semidwarf alfalfa.Two studies [47,48] provided genome information for cultivated alfalfa in 2020. This information provides a foundation for the molecular breeding of cultivated alfalfa. Accordingly, we decided to editMsGA3ox1with CRISPR/Cas9 technology to obtain prostrate growth of cultivated alfalfa.
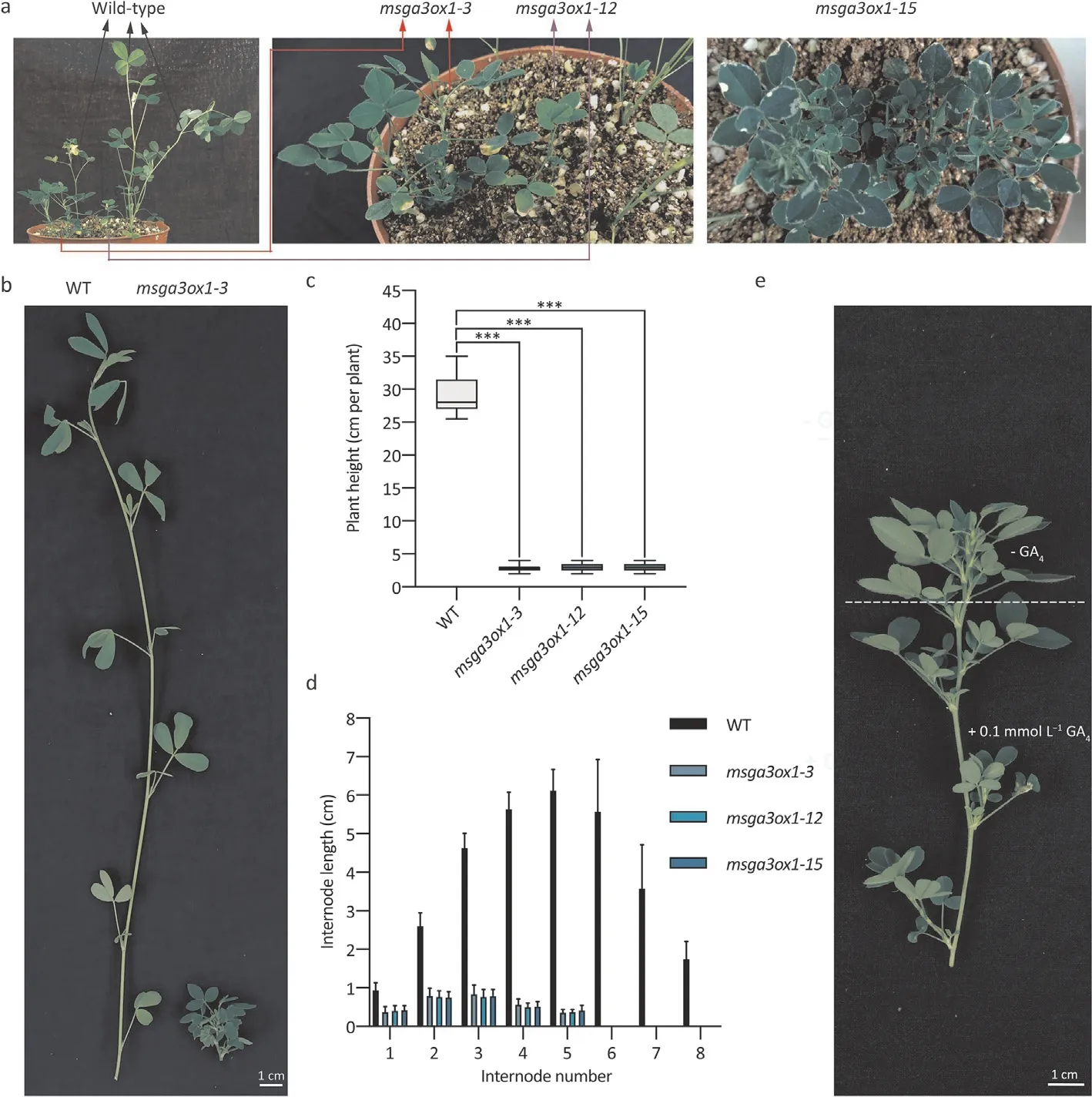
Fig.5. Genetic modification of MsGA3ox1 altered alfalfa architecture.(a)Photos of three representative msga3ox1 mutants(msga3ox1-3,msga3ox1-12,and msga3ox1-15)and three mutants exhibiting a dwarf phenotype. Clonal plants vegetatively propagated from shoot cuttings were photographed and measured at 6 weeks of age, n = 15. (b)Photographs of WT and msga3ox1-3. (c) Plant height. (d) Internode length. The experiment was repeated three times, and one representative set of results is shown. An asterisk indicates a significant difference between msga3ox1 and WT (***, P <0.001, Kruskal-Wallis nonparametric test.). (e) The msga3ox1 mutants were treated with 0.1 mmol L-1 GA4, and a representative plant is shown three weeks later.
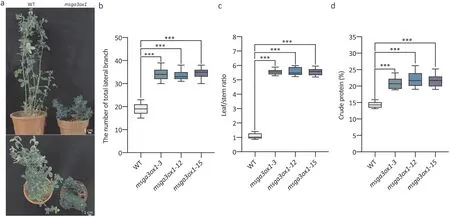
Fig.6. Genome editing of MsGA3ox1 improves the number of lateral branches and the ratio of leaf to stem biomass.Clonal plants vegetatively propagated using shoot cuttings were photographed and measured at 12 weeks of age,n=15.(a)Photographs of WT and msga3ox1.(b)Number of lateral branches.(c)Leaf/stem ratio.(d)Percentage of crude protein.The experiment was repeated three times,and one representative set of results is shown.An asterisk indicates a significant difference between msga3ox1 and WT(***,P <0.001, Kruskal-Wallis nonparametric test).
A plant binary transformation vector referred to as p6401-5CBC was constructed to stably transform alfalfa cultivars usingA. tumefaciens. In this vector, the CaMV 35S promoter drives the expression of zCas9 and the hygromycin phosphotransferase selectable marker gene (Hyg), and the MtU6-5 and MtU6-6 promoters drive the expression of sgRNAs. Using theM. truncatula MtGA3ox1sequence as a query, a BLASTn search against theMedicago sativagenome database (https://figshare.com/articles/dataset/Medicago_sativa_genome_andannotation_files/12623 960) was performed. Four nearly identicalMsGA3ox1alleles were identified. Two guide sequences located in the conserved region of exon 1 ofMsGA3ox1were chosen and were then synthesized and integrated into the p6401-5CBC vector. After transformation,30 plants were regenerated from 150 transformed calli,three(designatedmsga3ox1-3,msga3ox1-12andmsga3ox1-15) of which exhibited the anticipated dwarf phenotype, while the remaining plants showed no significant difference from the wild type(Fig. 5a). To confirm the CRISPR/Cas9-inducedmsga3ox1mutants,PCR amplicons encompassing the target site ofMsGA3ox1were subcloned from candidate mutants, and 10 positive recombinant clones were randomly sequenced. This step confirmed thatmsga3ox1-3,msga3ox1-12, andmsga3ox1-15harbored four mutated alleles at the target site (Fig. S2).
To perform further characterization,15 clonal plants were vegetatively propagated from each transgenic line using shoot cuttings. These propagated transgenic plants exhibited dwarfism and shorter internode lengths (Fig. 5b-d). Exogenous application of GA4partially recovered the plant height ofmsga3ox1mutants(Fig.5e).When the propagated transgenic plants grew to 12 weeks,the number of total lateral branches ofmsga3ox1mutants was significantly higher than that of the control(Fig.6a,b).The leaf/stem ratios ofmsga3ox1mutants were more than fivefold greater than the control (Fig. 6c). As expected, increased leaf/stem ratios also correlated with increased crude protein(Fig. 6d). Thus, semidwarf and prostrated alfalfa lines were obtained by gene editing ofMsGA3ox1, which increased the number of total lateral branches,leaf/stem ratio and crude protein content of aerial plant parts.
4. Discussion
Plant height is a key factor in the architecture of crop plants and affects yield and quality [49,50]. The ‘‘Green Revolution” of the 1960 s realized spectacular increases in cereal crop yields because farmers adopted new cultivars and cultivation methods [51]. The new cultivars were shorter,showed increased grain yield,and were more resistant to damage by wind and rain [52]. Semidwarfism results from the disruption of the GA signaling pathway or blockage of the GA synthesis pathway. However, this phenotype has not been reported in cultivated alfalfa.MsGA3ox1is the first gene found in this study to play key roles in GA-regulated plant height in cultivated alfalfa.
In this article, our results showed that the expression patterns ofMtGA3ox1andMtGA3ox2differed.MtGA3ox1showed relatively high expression in stems and leaves,and is a major gene regulating plant architecture.Recently,MtGA3ox1 has been reported[53-54]to be involved in plant growth,axillary bud outgrowth and the formation of leaf margins,but there is a lack of phenotypic studies onMtGA3ox2mutants. We found that althoughMtGA3ox2was expressed in roots, stems, leaves and nodules, its mutation had no visible effect on plant growth and development. We observed thatMtGA3ox2was clearly expressed in stomata. Its function in stomata awaits further study. We also found that bothMtGA3ox1andMtGA3ox2were expressed in nodules,but the mutation of neither of these genes affected nitrogenase activity. InArabidopsis,fourGA3oxgenes have been identified (AtGA3ox1-AtGA3ox4)[31,55-57]. According to their expression patterns, GA3ox1 and GA3ox2 are required for vegetative growth and development,and GA3ox1, GA3ox3, and GA3ox4 function in the development of reproductive organs [58]. To identify the roles of AtGA3ox1 and AtGA3ox2 in plant development, mutants with defects inGA3ox1andGA3ox2were isolated. Thega3ox1mutant showed a semidwarf phenotype, whereas the homozygousga3ox2-1mutant showed no aberrant phenotype [58]. Our findings were similar to these. The phenotypes ofga3ox1/ga3ox2double mutants inArabidopsisshowed that GA3ox1 and GA3ox2 are functionally redundant. We failed to obtainMtGA3ox1/MtGA3ox2double mutants, which would shed light on the functional differentiation and cooperation between MtGA3ox1 and MtGA3ox2.
Genome editing could accelerate crop breeding.The semidwarf and prostrate alfalfa obtained by gene editing ofMsGA3ox1has the advantages of multiple branches, a high leaf/stem ratio, and high crude protein. Alfalfa with these characteristics may offer the advantages of increased ground coverage,weed control,and moisture retention. When alfalfa was included in a crop intercropping scheme, the architecture of semidwarf alfalfa did not affect the light utilization of other crops. Alfalfa with a prostrate growth habit can be used as a mulch in orchards without affecting normal farming, and can also provide a high-quality protein source for poultry.
CRediT authorship contribution statement
Lihua Zheng:Investigation, Data curation, Writing - original draft, Writing - review&editing.Jiangqi Wen:Investigation, Formal analysis.Jinling Liu:Investigation, Data curation.Xiangzhao Meng:Investigation, Data curation.Peng Liu:Investigation, Data curation.Na Cao:Investigation, Data curation.Jiangli Dong:Supervision, Funding acquisition, Project administration, Writing- review & editing.Tao Wang:Supervision, Funding acquisition,Project administration, Writing - review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the Agricultural Variety Improvement Project of Shandong Province (2019LZGC010) and the National Key Research and Development Program of China(2019YFD1002701).
Appendix A. Supplementary data
Supplementary data for this article can be found online at https://doi.org/10.1016/j.cj.2021.11.008.
- The Crop Journal的其它文章
- Research progress on the divergence and genetic basis of agronomic traits in xian and geng rice
- The chloroplast-localized protein LTA1 regulates tiller angle and yield of rice
- Genome-wide association study and transcriptome analysis reveal new QTL and candidate genes for nitrogen-deficiency tolerance in rice
- Advances in the functional study of glutamine synthetase in plant abiotic stress tolerance response
- Identification of microRNAs regulating grain filling of rice inferior spikelets in response to moderate soil drying post-anthesis
- TaGW2L, a GW2-like RING finger E3 ligase, positively regulates heading date in common wheat (Triticum aestivum L.)

