Agronomic and physiological traits associated with genetic improvement of phosphorus use efficiency of wheat grown in a purple lithomorphic soil
Hongkun Yng, Renhu Chen, Yufeng Chen, Hn Li, Ting Wei, Wei Xie, Goqiong Fn,b,c,
a Crop Ecophysiology and Cultivation Key Laboratory of Sichuan Province, Sichuan Agricultural University, Chengdu 611130, Sichuan, China
b Key Laboratory of Crop Ecophysiology & Farming System in Southwest China, Ministry of Agriculture and Rural Affairs, Chengdu 611130, Sichuan, China
c State Key Laboratory of Crop Gene Exploration and Utilization in Southwest China, Ministry of Science and Technology, Chengdu 611130, Sichuan, China
Keywords:Grain yield Root P acquisition P remobilization and utilization Leaf photosynthesis Sucrose
A B S T R A C T Developing wheat that acquires and uses phosphorus (P) more efficiently is a promising and low-cost solution for increasing grain yield and reducing P-related environmental impacts.The present study identified agronomic and physiological traits that contribute to genetic variation in the P acquisition, remobilization, and utilization efficiency of 11 wheat cultivars from southwest China grown in P-deficient purple lithomorphic soil (Olsen P = 4.7) with balanced (75 kg P ha−1) and excess P (120 kg P ha−1) supplies. On average, soil P deficiency (-P) reduced root P uptake (17.0%-60.8%), P remobilization (33.9%-52.8%),dry mass yield(11.5%-39.2%),and grain yield(17.7%-54.4%).Balanced P(+P)increased grain yield via increased plant biomass rather than increased HI. -P increased phosphorus uptake efficiency (PUpE,4.5-fold), phosphorus utilization efficiency (PUtE, 1.25-fold), and phosphorus use efficiency (PUE, 5.4-fold) compared with those under +P, and PUtE explained most (58.1%-60.8%) of the genetic variation in PUE under both -P and +P. The high root P uptake of P-efficient cultivars under -P was regulated by root surface area and root length density in the 0-10 cm soil layer but not in the 10-20 and 20-40 cm soil layers, suggesting that a topsoil foraging strategy is a more economical approach than deeper root exploration for increasing P uptake. Root P uptake before anthesis and P remobilization after anthesis were critical for increasing the PUtE of wheat,given that P-efficient cultivars showed higher Pn(net photosynthetic rate) and sucrose levels than P-inefficient cultivars. Pn reduction by -P resulted from decreased Gs and Ci,and high evapotranspiration under+P increased shoot P%by increasing root P uptake.Genetic variation in the source-to-sink ratio was observed in consequence of a +P-induced allometric increase in sucrose in leaves and kernels. Owing to these beneficial effects, +P increased the kernel N and P yields of the 11 cultivars by 9.9%-52.4% and 12.3%-48.8%, respectively. The findings of this study could help improve wheat in future breeding efforts and P management by identifying desirable Pefficient phenotypes in P-deficient farming systems.
1. Introduction
Phosphorus (P) is a component of phospholipids, ATP, and nucleic acids and is therefore crucial for plant growth and development [1]. Plants often suffer from phosphorus starvation because root hairs can only acquire free phosphate (Pi) from soil, where it is characterized by low availability and mobility[2].Nearly 5.7 billion ha of farmland have insufficient root-available soil P, and an annual increase in P fertilizer of 2% is needed to maintain current grain yield. However, the price of P is projected to increase in the coming decade because natural rock phosphate reserves are continuously being depleted [3,4]. Genetic improvement of the phosphorus use efficiency (PUE) of crops so that grain yield is not affected by soil P deficiency is necessary and feasible for sustainable yield production in the coming decades [5].
Increasing PUE for crop growth requires increasing P uptake efficiency(PUpE)by roots from the soil and increasing P utilization efficiency (PUtE), leading to faster crop growth and higher grain yield in P-deficient soils [4,6]. The prerequisites for PUE improvement are that genetic diversity for PUE must be present,agronomic or physiological traits critical for increased PUE must be identified,and the environment for selection must have the potential to permit the expression of the desired traits[7].Genetic variation in PUE has been identified in maize[8],potato[4],wheat[7],pearl millet[9],rice[10],and sorghum[11].However,the contribution of PUpE and PUtE to PUE improvement varies among crops, environments,and soil P availabilities. PUpE was more critical for yield and PUE variation than PUtE in common bean [12], wheat [13], and maize[14], whereas PUtE was more critical for yield and PUE variation in maize [15] and potato [16]. PUpE was more critical for yield variation than PUtE under -P [9], whereas PUtE was more critical for yield variation under+P[17].The importance of PUpE and PUtE for PUE variation in wheat grown in P-deficient purple lithomorphic soil has not been investigated.
PUpE represents the capability of the root system to uptake inorganic Pi from the soil, and high P uptake requires a large amount of phosphate fertilizer because the diffusion of phosphate ions is slow (10-12-10-15m2s-1) [18]. For this reason, improvement of P acquisition by an ideal root system has become an economical approach for high- and low-input farming systems [19].Plants adapt to soil P deficiency by morphological, epigenetic,and metabolic modifications, and the most pronounced changes are in roots,given that root plasticity plays a crucial role in acquiring highly immobile soil P [20].The main mechanisms involved in PUpE improvement include elongation of the entire root system,reduced primary root growth, expanded lateral root surface area,shallower axial root growth,increased root thickness and root hair length, and increased root-to-shoot ratio [21,22]. P-efficient genotypes show a shallow-rooted architecture that increases P acquisition from topsoil in response to surface P fertilization[23,24].Root phenotype improvements in the vegetative growth period of crops are more effective than those in the reproductive growth period because grain yield can be fully ensured by remobilized P that is transferred from inactive senescing organs to kernels[23].Characterizing the genetic variability of critical P acquisition-associated traits in the root system is vital for increasing PUpE in crops.
PUtE represents the capability of crops to convert absorbed P into yield [4]. It is thus essential to ensure that plants maximize their use of the P that they take up from the soil, especially in low-P input systems. Aboveground plant parts are more affected by soil P deficits than are roots, and P deficiency inhibits plant growth directly by reducing sink activity and indirectly by inhibiting leaf photosynthesis [25,26]. Soil P deficiency reduces leaf photosynthesis, carbon metabolism, and biomass accumulation [27],mainly as a result of non-stomatal limitations [28,29], because of the critical role of Pi in regulating ribulose-1,5-bisphosphate(RUBP) regeneration, carboxylation activity, energy supply, stomatal size, and stomatal conductance [25]. P starvation inhibits leaf photosynthesis and crop growth and does not immediately lead to deficiency symptoms except under severe P deficit [30]. Sixty to ninety percent of root P is remobilized from senescing tissue to developing seeds in a P-deficient environment[31],and P remobilization is reduced under optimal P supply [26,32]. Although a non-stomatal limitation-inducedPnreduction mechanism under P deficient soils has been proposed [27-29], the significance of P remobilization and source-sink relations for PUtE improvement remains largely unknown.
Identifying source and sink relationships could reveal critical traits for genetic selection and yield improvement [33]. As the major product of photosynthesis, sucrose is a global regulator of plant response to P starvation [34]. InBrachiarialeaves [35] and soybeans [36], sucrose biosynthesis decreased with soil P deficit,as sucrose cleavage and sucrose transport were likely inhibited by P starvation.In other studies,soil P deficiency increased sucrose content because leaf photosynthesis and triose-P export from chloroplasts to the cytosol in exchange for Pi via the Pi translocator are likely restricted in leaves of rice[29],maize[37],common bean[38], andArabidopsis[39]. Thus, sucrose production in response to P starvation varies depending on plant species,organ,and environment. More evidence is needed to confirm this model in both leaves and developing kernels.
The complexity of P acquisition, remobilization, and utilization means that PUE improvement will most likely be achieved by combining several critical traits rather than using a single critical trait.Our previous results [40] suggested that genetic yield improvement in P-deficient soil was achieved through improvements in root nutrient uptake, light interception, solar energy conversion,and harvest index, indicating that indirect selection for PUE occurred.
The objectives of the present study were to(i)evaluate the contributions of P acquisition, remobilization, and utilization to grain yield and PUE variation under -P and +P, (ii) identify the critical agronomic and physiological traits that contribute to PUE and grain yield improvements of wheat in a P-deficient purple lithomorphic soil,and (iii)evaluate the response of the source and sink to soil P availabilities.The first hypothesis was that crop adaptation to soil P deficiency via a topsoil foraging strategy enables roots to acquire more P from soil. The second hypothesis was that crops maximize the utilization of acquired P by increasing P remobilization, leaf photosynthesis, and source-to-sink ratio.
2. Materials and methods
2.1. Experimental site and design
Field trials were conducted from 2018 to 2020 at the Renshou experimental station (29°51′N, 104°12′E) in southwestern China.The soil at the experimental site is classified as a purple lithomorphic soil under FAO taxonomy [41]. The root available soil P(Olsen-P) concentrations in the 0-20 and 20-40 cm soil layers were 4.7 mg kg-1and 2.8 mg kg-1, respectively. The soil had a pH of 7.13, 12.0% soil organic matter, and 0.68 g kg-1total P. The root available soil N, P, and K were 93.2, 4.7, and 110 mg kg-1,respectively. Soil organic matter was determined by the chromic acid digestion method, and available N, P, and K in the soil were measured by the Kjeldahl digestion method, Bray method, and flame emission spectrophotometry, respectively [42].
Experiments were laid out in a randomized complete block design with three replications in a split-plot arrangement of treatments where P level was the main plot and 11 cultivars with contrasting PUE values were subplots. Each plot was 20 m long and 20 m wide.Wheat seeds were sown on Oct 30 at an initial density of 2.2×106seedlings per hectare,and the row spacing was 20 cm.The experiment was conducted in P-deficient soil (Olsen-P = 4.7 mg kg-1) in two consecutive cropping seasons. Wheat was grown at three P levels:soil P deficiency(0 kg ha-1P,-P),balanced P supply(75 kg ha-1P,+P),and excess P (125 kg ha-1P), applied as calcium diphosphate before sowing. All plots were supplemented with 72 kg ha-1N as urea, 75 kg ha-1K2O as potassium chloride at sowing,and 48 kg ha-1N at the stem extension stage.In this dryland farming system,straw mulch-based no-tillage was applied to conserve the soil moisture available for plant growth [41].Commercial herbicides, pesticides, and fungicides were applied preventively at monthly intervals after tillering to avoid yield loss.
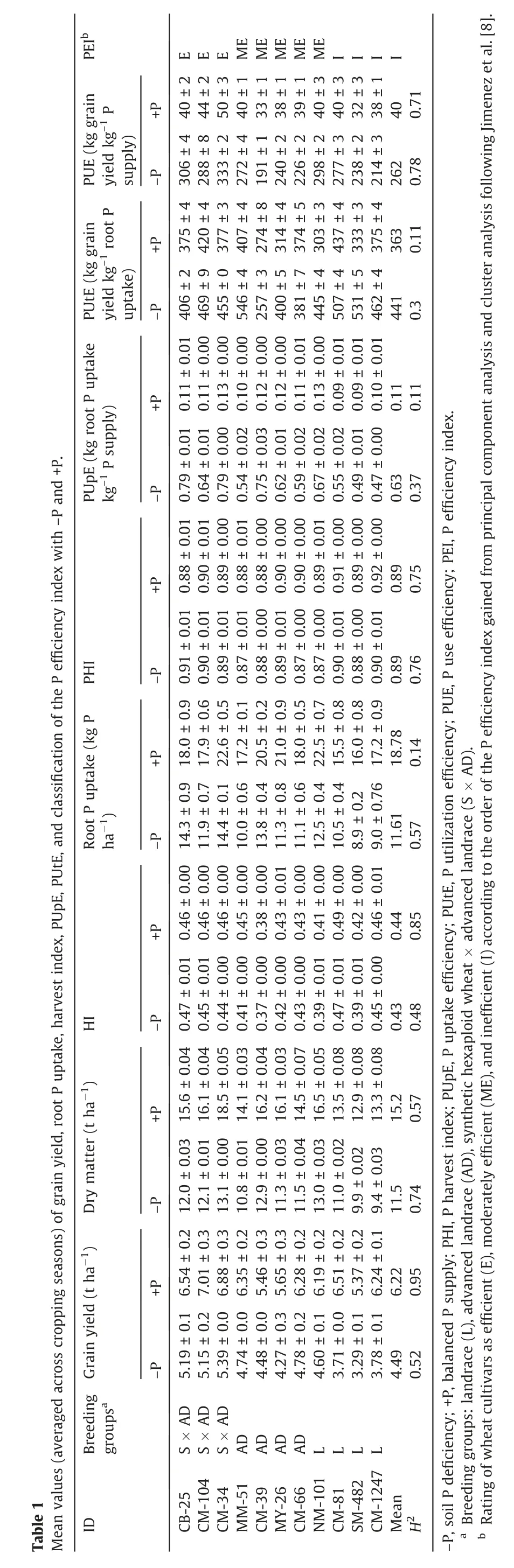
Based on our previous two-year field experiment [40,43], 11 typical cultivars, including landraces, advanced landraces, and modern cultivars, were chosen from 32 wheat (Triticum aestivumL.) cultivars released in southwest China between 1965 and 2017. All these cultivars are registered and widely grown (on >100,000 ha per year)in southwest China[40].Advanced landraces are advanced breeding lines of landraces that have been crossed with advanced-generation commercial hybrids (Table 1). Modern cultivars are synthetic hexaploid wheat-derived populations obtained by introducing multiple genomic regions of synthetic hexaploid wheat into landraces using an initial double top-cross strategy [44].
2.2. Plant measurements
2.2.1. Root morphology traits, PUE-associated variables, and grain yield
Root systems were assessed at the stem extension stage of wheat using the ‘‘shovelomics” technique [45]. Three wheat plants in each plot were randomly collected using the soil core method (φ = 200 mm). The whole root system of each plant with attached soil was excavated manually and placed in a mesh bag for laboratory measurements. The collected root samples were soaked in 1 mol L-1NaCl for 16 h to remove soil aggregates and then washed with a hydropneumatic washing machine. After removal of remaining mineral particles and organic debris, the root samples were rinsed and placed in distilled water until root scans could be performed. The root morphology traits in the 0-10 cm, 10-20 cm, and 20-40 cm soil layers were recorded digitally with a flatbed scanner (Epson, Tokyo, Japan) and quantified with WinRHIZO root analysis software (Regent Instruments,Ottawa, Canada) [46]. Root length density (RLD) was calculated as the total root length in the 0-10 cm, 10-20 cm, and 20-40 cm soil layers divided by the soil core volume. Root surface area density (RSAD) was calculated as the total root surface area in the 0-10 cm, 10-20 cm, and 20-40 cm soil layers divided by the soil core volume.
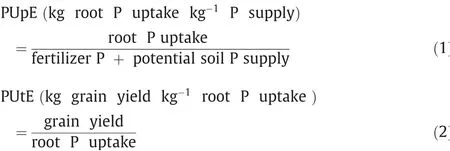
Aboveground plant dry mass was sampled in each plot from consecutive 30 wheat plants at the anthesis and maturation stages.The plant samples were separated into leaves, stems, chaff, and kernels; oven-dried at 105 °C for 30 min and 70 °C for 72 h; and weighed. P content was estimated from dried samples digested in H2SO4and H2O2and the molybdenum blue colorimetric method using a spectrophotometer [47]. P supply was calculated as the sum of potential soil P supply and fertilizer P [48]. Potential soil P supply at sowing was estimated using initial Olsen-P and bulk density [4]. Root P uptake was calculated as the product of dry matter and corresponding P concentrations. PUE is defined as the average grain yield produced per kg of P supply (Eq. (3)), and can be subdivided into PUpE and PUtE [4]. PUpE was calculated by dividing the root P uptake at harvest by P supply (Eq. (1)). PUtE was expressed as the kg grain yield per kg of root P uptake (Eq.(2)). Phosphorus harvest index (PHI) was determined as the ratio between accumulated P in kernels and total P accumulation at maturity (Eq. (4)).
?
Crop growth rate (CGR) was calculated as plant dry matter accumulated from anthesis to maturity divided by thermal time accumulated in the grain-filling stage. Post-anthesis dry matter accumulation was calculated as the difference between the dry matter at anthesis and that at maturity.Post-anthesis P accumulation was calculated as the difference between the P accumulations of the aboveground parts at anthesis and maturity [31]. P remobilization was calculated as aboveground root P uptake at anthesis minus straw,leaf,and chaff P at maturity(Eq.5).Dry matter remobilization (DMR) was calculated as the difference between dry matter at anthesis and that at physiological maturity, excluding grain weight (Eq. 6). P remobilization efficiency (PRE) was calculated as the P remobilization divided by the total aboveground P uptake at anthesis (Eq. 7). Dry matter remobilization efficiency(DMRE) was calculated as the percentage of remobilized dry matter relative to the dry matter of the whole plant at anthesis(Eq.8).The contribution of P remobilization to kernel P(CPRKP)was calculated as the amount of P remobilization divided by the accumulated P in kernels at maturity (Eq. 9). The contribution of DMR to grain yield (CDMRG) was calculated as the percentage of DMR to the dry matter of grains at maturity (Eq. 10). All values were adjusted to 0%moisture content.For estimates of DMR and P remobilization, it was assumed that all of the dry mass and P lost from vegetative organs were remobilized to developing organs.
2.2.2.Flag leaf photosynthesis,kernel sucrose content,and source-sink relationship
The flag leaves of the wheat plants (n= 4) in each plot were labeled at anthesis with a tag recording the date. Net photosynthetic rate (Pn), intercellular CO2concentration (Ci), and stomatal conductance(Gs)were recorded at 10,20,and 30 days after anthesis (DAA) using an LI-6800XT portable photosynthesis system (LICOR Biosciences, Lincoln, NE, USA). These values were recorded under 800 μmol m-2s-1light intensity,65%±5%relative humidity,and 380 μmol-1CO2L-1between 9:30 and 11:00 AM[49].All measurements were made on the middle portion of the flag leaf and were calculated as the mean of at least three measurements per plot.The sucrose content in both leaves and kernels was estimated using the KOH-resorcinol method [50]. Ethanol-soluble nonstructural carbohydrates were measured using the anthrone method[50].The methodology proposed previously[51]was used to calculate the relative change in source and sink ratio induced by +P compared with that under -P.
2.3. Statistical analysis
Statistical differences among treatments and cropping seasons were determined using the Generalized Linear Model Procedure in SAS 9.1.3 (SAS Institute Inc, Cary, NC, USA). In this procedure,cultivar and P level were set as fixed effects and cropping season was set as a random effect.A least significant difference(LSD)test was performed at 5% significance for mean comparisons. Linear regressions were fitted with GenStat version 16(VSN International,Hemel Hempstead, UK). Broad-sense heritability (H2) was

Grain yield was determined in a harvested area of 4 m2for each plot. Fertile spike number (FS) was counted in a harvest area of 4 m2,and kernel number per spike(KN)was counted for 30 spikes in each of the three trials.The kernels from each plot was air-dried,weighed, and held for moisture determination using a DMC-700 digital moisture tester (Seedburo, Chicago, IL, USA). The 1000-kernel weight (TKW) was measured at a kernel moisture content of 13.5%. The kernels from each plot was dried, ground into powder,and passed through a 0.15-mm screen.Kernel N for each sample was measured using a continuous flow analyzer (TRAACS model 2000 analyzer, Bran and Luebbe, Berlin, Germany). Harvest index (HI) was calculated as the ratio of kernel yield to total plant dry mass yield at maturity.
3. Results
3.1. Genetic variation of P efficiency variables under -P and +P
For root P uptake, remobilization, utilization, and grain yield, P level showed the largest effect, followed by environment (E) and genotype (G) (Table S1). In contrast, the root morphology traits,PHI, HI, PUE, and FS showed higher broad-sense heritability (H2)than traits related to dry matter, P uptake, and P remobilization.+P increased RLD and RSAD in the 0-10 cm soil layers,whereas RLD and RSAD in the 20-40 cm soil layers were decreased. -P reduced root P uptake,dry matter,grain yield,and HI,compared with those under +P. Dominance analysis (Table S2) showed that KN explained 51.5% of total yield variation, followed by FS (33.6%)and TKW (14.9%). -P reduced N and P yields in kernels by means of 26.2%and 43.4%,respectively, whereas the N:P ratios in kernels increased by 42.9% under -P compared with +P (Table S1). PUpE,PUtE, and PUE showed higherH2under -P than under +P. +P reduced PUpE, PUtE, and consequently PUE. The PUpE observed under -P was 4.5 times higher than that under +P, and the PUtE under-P was 1.25 times higher than that under+P.Consequently,the PUE under the P deficit soil was 5.4 times higher than that under+P.Thus,traits associated with P acquisition,remobilization,and utilization were more affected by soil P levels,while P harvest index and harvest index were more affected by genetic inheritance.
3.2. Root morphology traits, P acquisition, and dry mass yield
+P tended to increase RLD and RSAD in the 0-20 cm soil layer while reducing RLD (3.1%) and RSAD (23.7%), at the 20-40 cm soil depth(Table S1).Root P uptake was correlated with RLD and RSAD in the 0-10 cm soil layer but not in the 10-20 cm and 20-40 cm layers (Fig. 1). SM-482 and MY-26 showed the lowest RLD and RSAD under -P, whereas CM-34 and CM-104 showed the highest RLD and RSAD under +P.
Root P uptake under +P was positively related to root P uptake under-P,with a regression slope >1,and consistent in the anthesis and maturation stages (Fig. 2A). Root P uptake increased significantly with increasing P supply;a direct relationship between root P uptake and dry matter was observed at both soil P levels,and the steepest slope was observed under -P (slope = 0.35, Fig. 2B). A potential PUtE of 0.99 t dry matter kg-1root P uptake was estimated by linear regression,considering that the cultivars achieved the highest dry matter with a minimum root P uptake(Fig.2B).For the same levels of root P uptake,cultivars differed in plant dry matter and grain yield,and there was no(P>0.05)difference between root P uptake and grain yield when root P uptake from the soil exceeded 15.2 kg P ha-1(Fig. 2C).
The reduction in root P uptake was significantly related to dry matter and grain yield reductions, but the slope was less than 1,indicating that genetic improvement of low-P stress tolerance was achieved (Fig. 2D). The grain yield under -P was positively related to the grain yields under +P and ++P, with slopes of 0.87 and 0.72, respectively, suggesting that the genetic yield improvement under+P also reduced yield loss under-P(Fig.2E).The proportion of total plant P found in kernels(PHI) was higher than the proportion of dry mass found in kernels (HI) (Fig. 2F). Soil P levels showed no effect on PHI, and genetic yield improvement was significantly related to HI, but the slope of the relationship between the PHI and HI was less than 1,indicating that PHI did not increase to the same extent.The P-efficient cultivar CM-104 achieved a high grain yield at relatively low root P uptake in both soils with added P, suggesting its high capability in P utilization (Table 1). The Pefficient cultivars CB-25 and CM-34 achieved high kernel yields in both soils with added P, suggesting its high capability in P acquisition.
3.3. Internal P remobilization, kernel P yield, and the importance of PUpE and PUtE for PUE
P remobilization was positively related to aboveground plant dry matter (Fig. S1,R2= 0.67**), DMR (R2= 0.38*), FS (R2= 0.57**),and grain yield(R2=0.46**).Both P remobilization and DMR under-P were positively related to P remobilization and DMR under +P(Fig. 3A and B), and the slope of the regression line was above 1,indicating genetic P remobilization and DMR improvement. For the same level of P remobilization, cultivars differed greatly in DMR (Fig. 3C), suggesting significant variation in carbohydrates and P allocation.Averaging across P levels,the P-efficient cultivars CB-25 and CM-34 showed relatively high P remobilization and PHI values, whereas the P-inefficient cultivars SM-482 and CM-1247 had the lowest P remobilization under-P(Table 1).The increased grain yield under+P resulted from increased dry matter caused by increased CGR, and the steepest slope for the regression line was observed under +P (Fig. 3D). -P reduced the kernel P yield of 11 cultivars by 28.1%-59.9% and 28.8%-54.2% in the 2018-2019 and 2019-2020 cropping seasons,respectively,and grain yield was significantly related to kernel N and P yields per m2(Fig. 3 E and F).-P decreased the kernel N yield of the 11 cultivars by 9.9%-52.4%, whereas the N:P ratio increased by 12.3%-48.8% (Table S1).
Averaged across cropping seasons, PUE was related (R2= 0.60-0.87) to both PUpE and PUtE (Fig. 4A). -P tended to increase PUpE and PUtE in both cropping seasons. The ranges of PUpE of the 11 cultivars grown under -P were 0.55-0.97 and 0.35-0.37 kg root P uptake kg-1P supply in the 2018-2019 and 2019-2020 cropping seasons,respectively(Fig.4A;Table 1).PUtE was negatively related to shoot P level at both the anthesis and maturation stages(Fig. 4B), indicating that greater root P uptake reduced PUtE. The ranges of PUtE of the 11 cultivars grown under +P were 250-449 and 297-463 kg grain yield kg-1root P uptake in the 2018-2019 and 2019-2020 cropping seasons, respectively. As a product of PUpE and PUtE, PUE ranged between 31 and 343 kg grain yield kg-1P supply (Fig. 4A). Some cultivars, including CB-25, CM-104,and CM-34, showed the highest PUE (290-322 kg grain yield kg-1P supply)and PUpE(0.94-0.97 kg root P uptake kg-1P supply)under both -P and +P, whereas CM-1247 and SM-482 showed the lowest PUE and PUpE(Table 1,195-213 kg grain yield kg-1P supply). The dominant analysis model showed that NUpE explained 41.3% and 36.1% of the PUE variation, whereas NUtE explained 58.7%and 63.9%of the total PUE variation under-P and+P,respectively (Table S2). These results showed that high P remobilization causes wheat plants to have high PUtE.
3.4. Trait correlations and yield stability of cultivars under -P and +P
Under -P, the first principal component (PC1) accounted for 37.8% of variance and was dominated by variables describing P uptake,P remobilization,and PHI.PC2 accounted for 20.3%of variance and was dominated by CGR,dry matter production,and grain yield(Fig.5A).The relationships between these variables indicated that grain yield was positively correlated with KN, plant dry matter, the HI, and CGR. In contrast, HI was positively correlated with PHI,and these traits were positively correlated with PUtE and negatively correlated with shoot P levels, DMRE, and PUpE. Under +P,PC1 and PC2 accounted for respectively 33.7% and 23.6% of total variance (Fig. 5B). PC1 was dominated by variables describing P remobilization, shoot P, and PHI, whereas PC2 was dominated by root P uptake, PUpE, grain yield, and GN. PUE was correlated with CGR, plant dry matter, PHI, and GN, while HI and PHI were negatively correlated with root P uptake, shoot P, and DMR.
CB-25 and CM-104,and CM-34 were the three most stable cultivars with above-average yields (Fig. 5C and D). These cultivars were characterized by high productivity, P uptake, HI, PHI, PUpE,and PUE values. In contrast, SM-482 and CM-1247 showed the lowest yield stability under -P and +P. These cultivars showed lower P uptake,P remobilization,PUtE,and grain yield under both-P and+P(Fig.5).Thus,the 11 wheat cultivars can be classified as P-efficient, moderately P efficient, and P-inefficient cultivars according to their P efficiency index (Table 1) and yield stability(Fig. 5) under -P and +P.

Fig. 2. Relationship among root P uptake, dry matter, grain yield, and phosphorus harvest index of 11 cultivars under -P, +P, and ++P. Relationships between root P uptake under-P and+P(A),between root P uptake and dry matter(B)and grain yield(C),between root P uptake reduction by-P and dry matter reduction by-P(D),among grain yield under-P,+P and++P (E),and between HI and PHI (F).Dashed lines are 1:1 relationships. The dash-dot line(B)represents the maximal achievable PUtE under-P.The dotted line(C)represents the critical value of root P uptake(15.2 kg P ha-1).-P,soil P deficiency;+P,balanced P supply;++P,excess P supply;PHI,phosphorus harvest index;HI, harvest index. Values are expressed as mean ± standard error (n = 3). *, P <0.05; **, P <0.01.
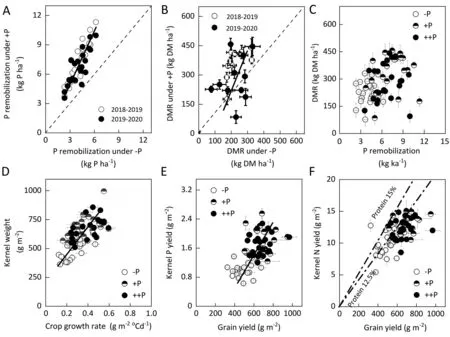
Fig. 3. Relationships among P remobilization, dry matter remobilization, kernel N and P yields of 11 wheat cultivars under -P, +P, and ++P. Relationships between P remobilization under -P and +P (A), between dry matter remobilization under -P and +P (B), between P remobilization and dry matter remobilization (C), between crop growth rate and kernel weight (D), and between grain yield and kernel P yield (E) and kernel N yield (F). Dashed lines (A, B) are 1:1 relationships. Dotted lines represent constant N concentrations of 2.3%and 5.6%for wheat kernels.-P,soil P deficiency;+P,balanced P supply;++P,excess P supply;DMR,dry matter remobilization.Values are expressed as mean ± standard error (n = 3).
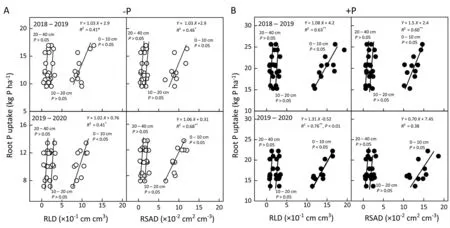
Fig.1. Relationships between root morphology traits and root P uptake in 11 cultivars under-P(A)and+P(B)in the 2018-2019 and 2019-2020 cropping seasons.RLD,root length density;RSAD,root surface area density;-P,soil P deficiency;+P,balanced P supply.All values were measured with three replications(n=3),*,P <0.05;**,P <0.01.
3.5. Leaf photosynthesis and source-sink relationships
-P tended to decrease flag leafPnfor both P-efficient and Pinefficient cultivars. However, the P-efficient cultivars (CB-25 and CM-34) always showed higherPnunder -P than the P-inefficient cultivar SM-482 (Fig. 6). Similarly, -P reducedgs,Ci, and E,whereas P-efficient cultivars always showed higherGs,Ci, andEunder -P. The dominance analysis showed that thePnreduction by-P was caused by bothGs(46.4%)andCi(53.9%),indicating nonstomatal limitation induced by-P.The P-efficient cultivars showed higherPnunder-P,resulting from reduced nonstomatal limitation.
Both the nonstructural carbohydrate and sucrose contents of flag leaves peaked at 20 days post-anthesis (DPA), while both of these values in kernels declined with time post-anthesis(Fig. 7A). Compared with that of the P-inefficient cultivar SM482,the leaf sucrose content of CM-34 and CB-25 increased by means of 15.7% and 6.7%, respectively, and kernel sucrose increased by means of 15.8 and 7.3%. Compared with those of the P-inefficient cultivar SM-482, leaf nonstructural carbohydrates of CM-34 and CB-25 increased by 7.4%and 2.5%,respectively,and kernel sucrose increased by 9.8% and 18.3%.
The slope of the regression line relating the sucrose levels in flag leaves and kernels peaked at 20 DPA (Fig. 7B). On average, -P reduced the sucrose ratio in leaves and kernels by 29.1% for the P-inefficient cultivar SM-482 and 24.9% and 22.2%, respectively,for the P-efficient cultivars CM-34 and CB-25. The relative change in the sucrose ratio between source and sink organs decreased with DPA (Fig. 7C); for the P-efficient cultivars CB-25 and CM-34,the decreases were 8.9% and 6.7%, respectively, from the value for the P-inefficient cultivar SM-482.
4. Discussion
4.1. The topsoil foraging strategy confers high P uptake efficiency on wheat plants
The differences among wheat cultivars with respect to root P uptake and PUpE could be attributed to genetic variation in RLD and RSAD. RLD and RSAD were positively related to P uptake,presumably through increased root surface area per unit soil volume, and increases in these variables increased the uptake of Pi from the soil [52,53]. Given that P transport to the root is mostly through diffusion, a larger root surface area provides better root-soil contact and is particularly important for P uptake in P-deficient soil [54]. Similar findings have been reported in wheat [32], chickpea [55], andBrassica napus[56],where soil P deficiency increased dry matter partitioning to roots by increasing root length, surface area, and volume[57,58]. Consequently, P-efficient cultivars (CB25) showed a higher P uptake than P-inefficient cultivars (SM482) in P-deficient soil (Fig. 8).
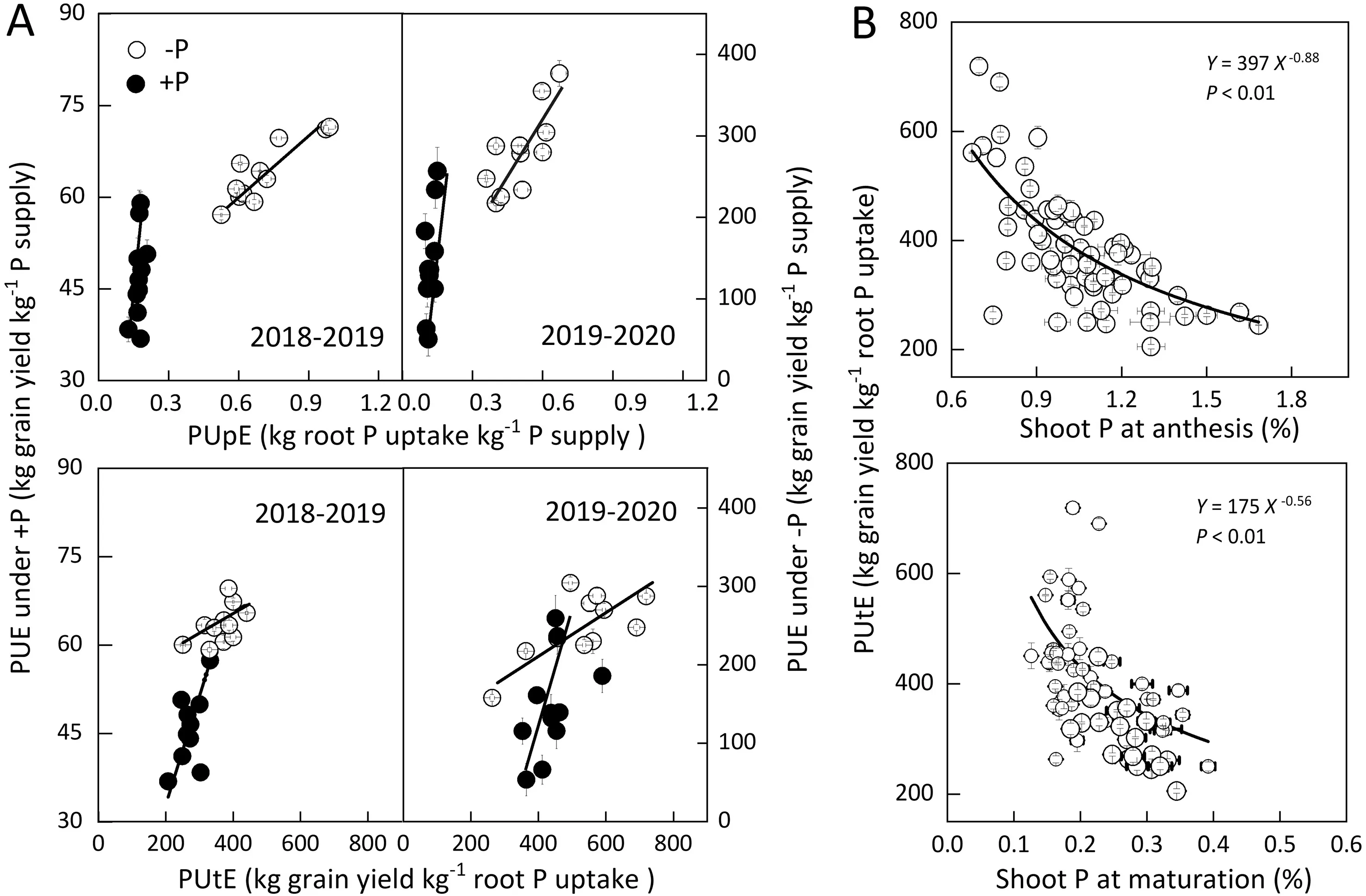
Fig.4. Relationships among shoot P level,PUpE,PUtE,and PUE in 11 cultivars under-P,and+P in the 2018-2019 and 2019-2020 cropping seasons.Relationship among PUpE,PUtE,and PUE(A),and between shoot P levels and PUtE(B).-P,soil P deficiency;+P,balanced P supply;PUE,phosphorus use efficiency;PUpE,phosphorus uptake efficiency.PUtE, phosphorus utilization efficiency. Values are expressed as mean ± standard error (n = 3).
Soil P deficiency also modified the distribution of roots in the soil (Fig. 8). In the present study, the RLD and RSAD in the topsoil layer(0-10 cm)contributed the most to the total variation in root P uptake,and RSAD was reduced in the 20-40 cm soil layers.These critical results are in line with findings observed inArabidopsisandBrassica napus[46], where soil P deficiency reduced primary roots and increased the number and length of lateral roots. P-efficient cultivars showed higher P uptake, dry matter, and grain yield in P-deficient soil via root foraging and P-mining strategies owing to the low mobility of P and relatively high P availability in surface soil layers[59].Shallow-rooted common bean[60]and maize[61]acquired more P than deeper-rooted genotypes in P-deficient soil.Deeper root exploration requires additional carbohydrate inputs[53,62].
P uptake was more affected by P supply than by genotype,while RLD and RSAD were more affected by genotype. P-efficient cultivars can show high plant dry matter in P-deficient soil[4],indicating that genotype selection for high RLD and RSAD increases the efficiency of P uptake from the soil. The heritabilities of RLD and RSAD at 0-10 cm and 20-40 cm were higher than those in the 10-20 cm soil layer(Table 1),suggesting that genetic modification of topsoil P acquisition is the most efficient approach for increasing P uptake under P-deficient soils(Fig.8).These findings support the first hypothesis,that crop adaptation to soil P deficiency via a topsoil foraging strategy enables roots to acquire more P from soil(Fig. 8).
The critical root P uptake, defined as the root P uptake above which crop yield does not respond to root P uptake, was 15.2 kg P ha-1. Wheat plants take up P from the soil up to a critical value(15.2 kg P ha-1) to maintain grain yield in P-limited soils. A series of results [63,64] showed that the critical value of soil Olsen-P is valuable for optimizing P fertilization management in P-deficient farming systems.However,soil Olsen-P differs across soil textures,environmental conditions, and cultivars [65,66]. Grain yield and soil Olsen-P relations are complex and determined by the soil P cycle,root P acquisition,internal P remobilization,and P utilization[65], explaining why the critical value of Olsen-P varies across environments. Considering both the Olsen-P in the soil and the critical P uptake of the wheat plant is, thus, more appropriate for wheat growers for optimizing P fertilization management than considering the critical soil Olsen-P alone.
4.2. P-efficient wheat cultivars with high P remobilization have high PUtE
PUtE(rather than PUpE)explained most of the variation in PUE under both-P(58.1%)and+P(60.8%).This finding is in agreement with widely accepted findings [4,9] suggesting that the contributions of PUpE and PUtE to PUE depend on soil type and P availability. In the present study, the relative contributions of PUpE and PUtE to PUE variation were estimated by dominance analysis rather than decomposition ofR2, thus generating more reliable results by which to rank the factors according to their relative importance in explaining PUE variation[67].This dominance analysis approach reduces distortion of the importance of predictor variables due to collinearity between PUpE and PUtE [4].
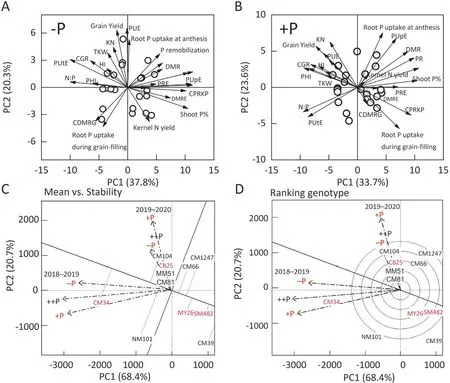
Fig.5. Productivity and yield stability performance of 11 wheat cultivars under-P,+P,and++P.Principal component analysis for 11 wheat cultivars under-P(A)and+P(B);productivity and yield stability of cultivars(C) under -P, +P,and ++P; and a GGE biplot(D) showing the ranking of cultivars in terms of both grain yield and stability across environments.The open symbols represent the 11 wheat cultivars,and the vectors represent the loading scores of variables for PC1 and PC2.-P,soil P deficiency;+P,balanced P supply; ++P, excess P supply; PUE, phosphorus use efficiency; KN, kernel weight; HI, harvest index; TKW, 1000-kernel weight; PHI, phosphorus harvest index; PA, root P uptake; PR, phosphorus remobilization; DM, dry matter; DMR, dry matter remobilization; DMRE, dry matter remobilization efficiency; CPRKP, the contribution of P remobilization to kernels P;PRE,phosphorus remobilization efficiency;PUtE,phosphorus utilization efficiency;PUpE, phosphorus uptake efficiency;CGR,crop growth rate.The cultivars shown in red represent P-efficient cultivars(CB25),moderately P-efficient cultivars(MY26),and P-inefficient cultivars(SM482)based on productivity and yield stability under -P, +P and ++P.
Greater shoot P resulting from increased root P uptake under+P led to a lower PUtE that was positively correlated with CGR. This finding indicates that using cultivars with lower shoot P% and higher CGR in P-deficient soil is the most efficient approach for increasing PUtE and grain yield, in agreement with findings in potato [4], pearl millet [9], and sorghum [68]. Maintaining high CGR in P-deficient soils and reducing the adverse effects of low shoot P on crop growth are challenging[69].Genetic improvement of the radiation utilization efficiency of wheat in P-deficient soils has been demonstrated to be a feasible approach to improve grain yield and PUE [70]. In the present study, the P-efficient cultivars showed high PUtE and low shoot P% in P-deficient soil, responses attributed to increased PRE. The ability of a plant to remobilize P from senescing organs to flag leaves or developing kernels represents the PRE, which is crucial for alleviating P starvation when P uptake is inhibited by low P supply [71]. P remobilization accounted for 60%-85% of root P uptake, and soil P deficiency decreased PRE.The range of PRE values(37.8%to 64.8%)was larger than the ranges observed in another study [30], a finding that might be attributed to the higher P stress levels in the present study.The P-efficient cultivars showed high P remobilization under-P (Tables 1 and 3), suggesting that increased P remobilization is an adaptive response in wheat cultivars under -P. However,greater P remobilization after anthesis leads to early senescence and reduced leaf photosynthesis [26], which might be attributed to the leaf P%being near the critical leaf P%(4 mg g-1DW)and consequently affectingPnassimilate production. Sufficient kernel P can be fully ensured by the internal transfer of P from leaves(35%), roots (28%), and stems (17%), even though the external P supply has been depleted [26]. All this evidence suggests that P uptake before anthesis is more critical than that in the grain filling stage in wheat because plants will first remobilize P accumulated in vacuolar phosphate [72]. In the present study, P remobilization and PRE increased with P supply,whereas P remobilized to kernels decreased with increasing P supply. These results might be attributed to the increased kernel number under+P than under-P.Similar results were observed in wheat [73], where P remobilization and DMR depended on the increased source-to-sink ratio. Thus,selection of cultivars with high dry matter and P remobilization can be critical for increasing grain yield. Recent studies have demonstrated that P remobilization is regulated by three purple acid phosphatases [74], and several critical transcription factors and genes are responsible for high P remobilization [75]. Further studies might reveal how these genes and transcription factors regulate P remobilization in a set of historical wheat cultivars.
The P-efficient cultivars presented a higher grain yield under Pdeficient soil as a consequence of increased KN (Fig. 8). These results were similar to a previous result [75], indicating that the effect of P supply on grain yield manifested before anthesis and that mainly FS and GN determined that grain yield. Since tiller development and tiller mortality are determined before anthesis and KN is determined primarily from stem extension to anthesis[76], a sufficient P supply before anthesis is more critical during the vegetative stage than during the grain filling stage. Direct evidence supporting this theory is that the post-anthesis P supply did not affect grain yield because kernel P nutrition can be fully ensured by P remobilization transfer from inactive sensing organs to kernels [26]. Thus, increasing P uptake before anthesis and P remobilization after anthesis could be the most efficient approach in crops for maximizing their yield potential in P-deficient soil.
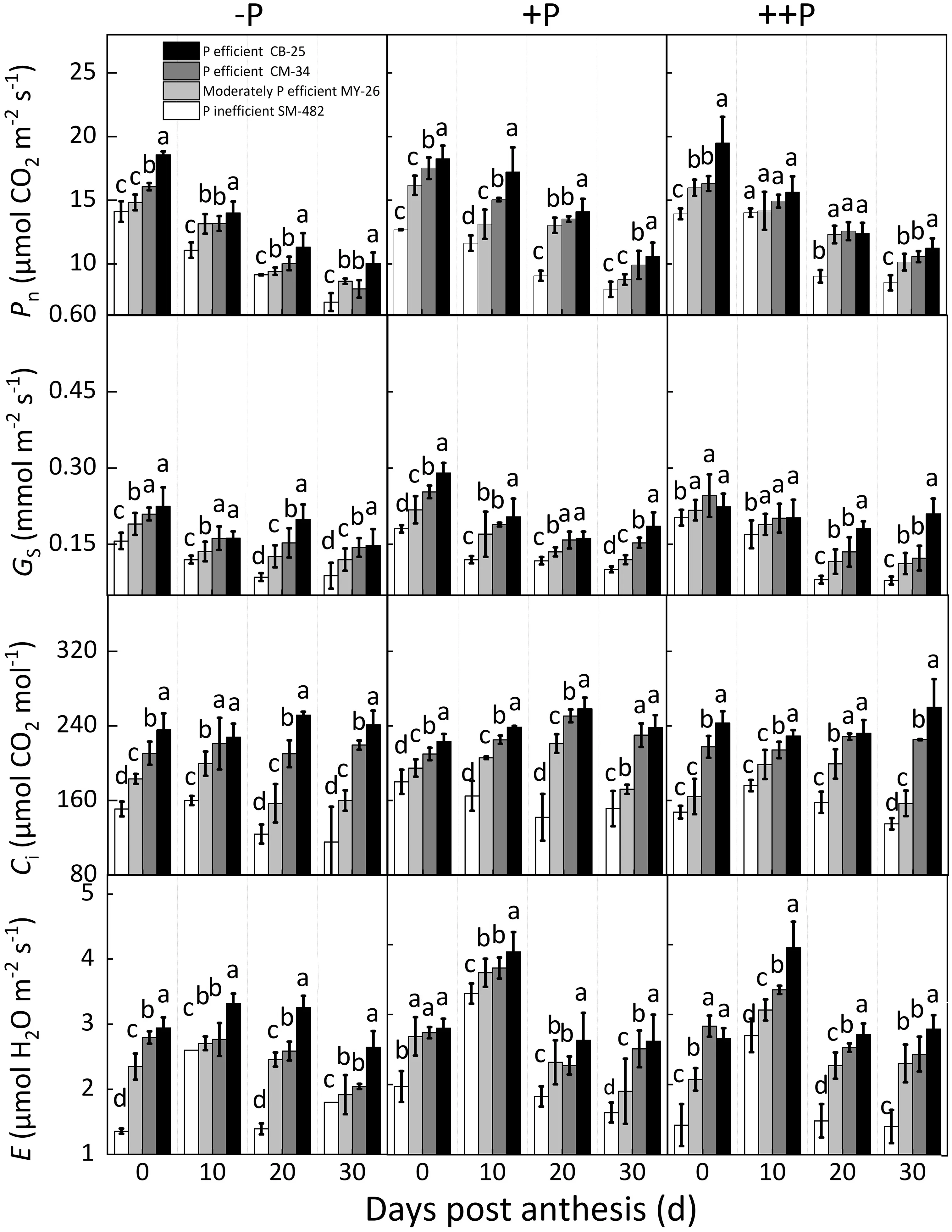
Fig.6. Dynamic changes in photosynthetic parameters of flag-leaves with days post-anthesis for P-efficient,moderately P-efficient, and P-inefficient cultivars under-P, +P,and++P.Pn,net photosynthetic rate;Gs,stomatal conductance;Ci,intercellular CO2 concentration;E,evaporation; -P,soil P deficiency;+P,balanced P supply;++P,excess P supply; Values are means ± standard errors. Different lowercase letters indicate significant difference at P <0.05.
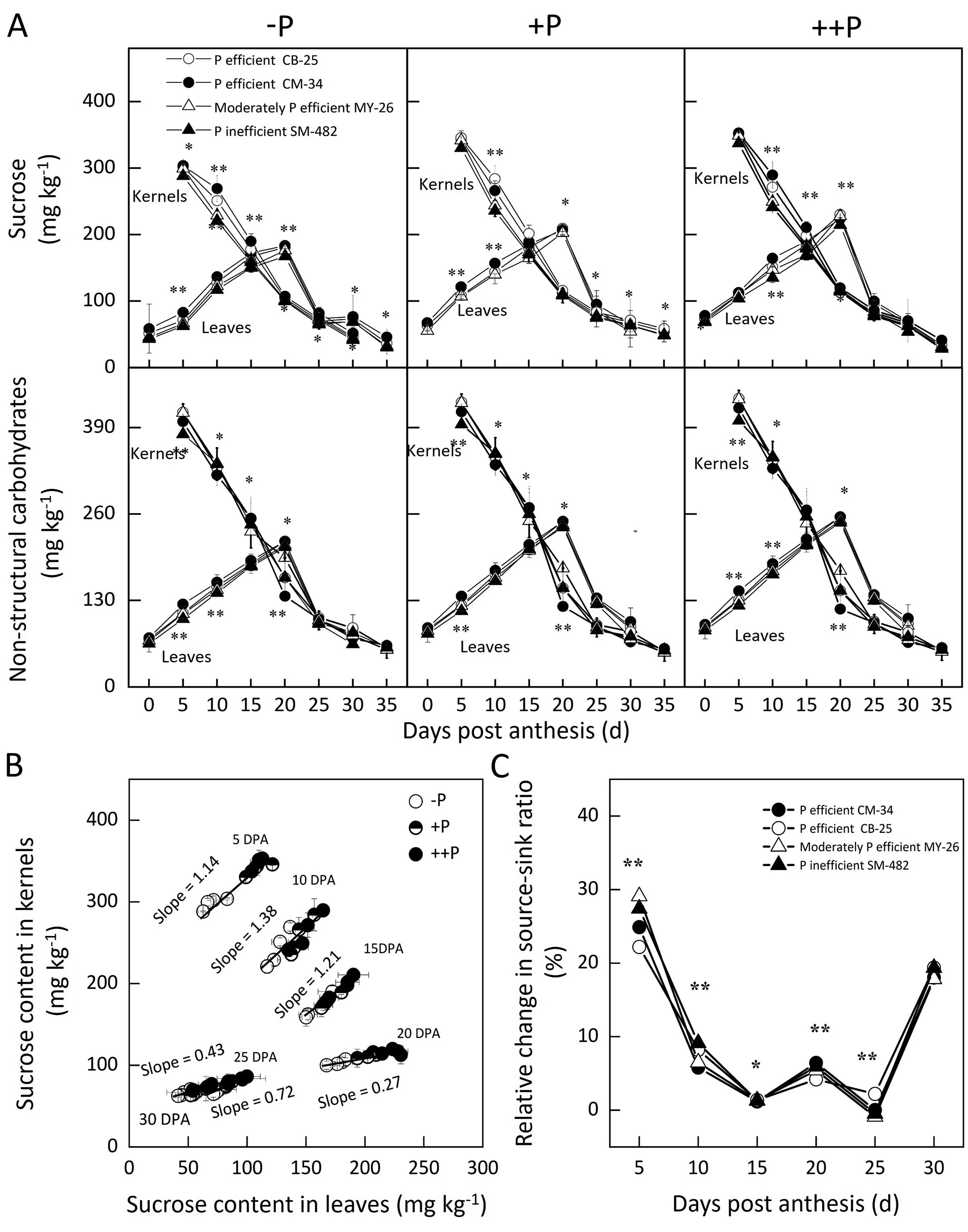
Fig. 7. Dynamic changes in source and sink relationships in P-efficient, moderately P-efficient, and P-inefficient cultivars under -P, +P, and ++P.Sucrose and non-structural carbohydrate contents in both leaves and kernels(A),the relationship between the sucrose content in leaves and kernels(B),and the relative change in the source-sink ratios(C) of P-efficient (CB-25 and CM-34), moderately P-efficient (MY-26), and P-inefficient cultivars (SM-482) in two wheat cropping seasons. The methodology proposed previously [51] was used to calculate the relative change in source and sink ratio induced by +P relative to that under -P. -P, soil P deficiency; +P, balanced P supply; ++P,excess P supply; Values are means ± standard errors. * and ** indicate significant differences at the 0.05 and 0.01 probability levels, respectively.
4.3. Changes in the source-to-sink relationship improved P utilization in P-deficient soil
Soil P deficiency reduced plant dry matter(11.5%-39.2%),a finding that can be attributed to the reducedPn(7.8%-12.2%,Fig.8),as confirmed by previous results [49]. However, aboveground dry mass is not always correlated withPnreduction[52],andPnreduction under soil P deficiency may be a strategy to adapt to P starvation. In the present study, soil P deficiency reducedPnresulting from stomatal limitations, given that bothGsandCiwere reduced by -P. However, in another study [29], -P reducedGsbut notCi,suggesting that the decrease inPnresulting from soil P deficiency was not caused by stomatal limitation. The prevailing consensus[77,78] is that soil P deficiency-induced nonstomatal limitation is more crucial than stomatal limitation forPnreduction because soil P deficiency reduces the generation of RUBP and triose phosphate export from the chloroplast stroma to the cytoplasm in exchange for Pi via Pi transporters.In the present study,the P-efficient cultivars CB-25 and CM-34 always showed higherPn,GsandCithan Pinefficient cultivars in P-deficient soil,suggesting that the efficient cultivars grown under-P with highPnhad P stress resistance.One of the critical findings from the present study is that P-efficient cultivars showed higher leaf evaporation in P-deficient soil. Rapid transpiration allowed more N and P acquisition through transpiration-driven mass flow, and close relations between leaf transpiration and P uptake have been observed in various plants[55]. However, the evidence for mass flow increasing P uptake[79]awaits further study in wheat.Our results support the hypothesis[49]that cultivars acquire high dry matter and photosynthesis rates confer P stress tolerance in P-deficient soil.

Fig. 8. Schematic representation of the possible mechanisms by which P-efficient cultivars adapt to P-deficient soil.
Soil P deficiency reduced sucrose levels in both kernels and flag leaves,a finding that might be attributed to P remobilization from senescing upper leaves to active sites such as flag leaves and kernels(Table 1).Thus,soil P deficiency reduced sucrose levels in both kernels and leaves. Soil P deficiency showed a significant effect on source and sink relations, indicating that sucrose availability in kernels was not reduced to the same extent as that in leaves because the sucrose stored in stems and leaves was remobilized from vegetative organs to kernels and roots during grain filling.The P-efficient cultivars always showed a lower source-to-sink ratio under soil P deficiency, a finding that might be attributed to their high DMR.These findings support the second hypothesis,that crops maximize the utilization of acquired P by increasing P remobilization, leaf photosynthesis, and source-to-sink relations.
4.4. Genetic PHI and PUE improvements coincidentally increased kernels N yield
The conserved nature of HI and PHI,as revealed by both ANOVA andH2, in response to soil P deficiency in a set of historical cultivars, is in line with findings in potato, maize, and wheat [4,80].Thus,the grain yield response to P supply can be attributed mainly to dry matter rather than to HI [81]. However, high genetic variation in HI and PHI was observed under different soil P levels, similar to the results observed in rice plants [82]. The P-efficient cultivars showed higher HI and PHI under both soil P levels than the P-efficient cultivars because of the introduction of semidwarf genes (Rht-8), which reduce plant height. Consequently, increased HI and PHI values were observed in P-efficient cultivars.However,HI did not increase to the same extent as PHI,indicating the importance of C and N economies, as +P also increased kernels N and P yields.
P harvested from grain is a major driver of the global P cycle,whereas a reduction in kernels P is beneficial for farmers,allowing them to reduce P fertilizer input and P-associated environmental pollution [83]. As expected, -P reduced root P uptake and kernels P yield. However, aboveground plant dry mass, kernels N yield,and N:P ratios unexpectedly increased under balanced P supply.A notable finding in the Glopnet [84] data set suggested that high N:P in leaves is associated with low P rather than greater N.In the present study,both kernels N and P yields were increased by+P,a finding that might be attributed to RLD and RSAD increasing both N and P uptake. The beneficial effect of increased kernels N and P yields under +P can improve the food processing quality of wheat flour and the establishment of plants in the following generation,owing to their role in the early stages of plant growth. One explanation for the increased kernels N yield and N:P under balanced P supply is that+P induced allometric increases in remobilized N and remobilized P. Another explanation from soybean and maize findings[73]is that+P enables plants to take up more N and P from the soil via increased leaf evaporation and N metabolism.P is stored as phytic acid and accumulates in the vacuoles of kernels.Phytic acid levels have been reported [85] to be closely associated with grain mineral nutrition because they interact with amino acids and chelated forms of minerals such as Fe3+,Zn2+,Mn2+and Ca2+;thus,the increase in PUE is accompanied by an increase in leaf mineral nutrition. Whether genetic improvements in PUE coincidentally lead to improved mineral nutrition awaits further study.
5. Conclusions
Genetic variation in PUE is ascribed mainly to PUtE (36.1%-41.3%) and less to PUpE (58.7%-63.9%) in both -P and +P soils.PUE with high PUpE was associated mainly with root P uptake being regulated by RLD and RSAD in the 0-10 cm but not the 10-20 and 20-40 cm soil layers,suggesting that topsoil acquisition rather than deeper root exploration is an economical approach for increasing P uptake under soil P deficiency. Root P uptake before anthesis and P remobilization after anthesis were critical for increasing the PUE of wheat, given that P-efficient cultivars showed highPnand sucrose levels under -P. Soil P deficiency reducedPnvia nonstomatal limitation, given that bothGsandCiwere reduced by -P, and leaf evapotranspiration increased shoot P% by increasing P uptake. +P induced allometric increases in sucrose content in leaves and kernels owing to the difference in DMR. Owing to these beneficial effects, +P increased the kernels N and P yields of the 11 cultivars by 9.9%-52.4% and 12.3%-48.8%, respectively. The findings of this study could help improve wheat in future breeding efforts and P management by identifying desired P efficiency phenotypes in P-deficient farming systems.The molecular mechanisms underlying P acquisition and internal P remobilization await further study.
CRediT authorship contribution statement
Hongkun Yang:Conceptualization, Methodology, Data curation, Writing.Renhua Chen:Data collection, Methodology, Conceptualization.Yufeng Chen:Data collection, Methodology.Han Li:Data collection.Ting Wei:Data collection.Wei Xie:Data collection.Gaoqiong Fan:Funding acquisition, Project administration.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We acknowledge our laboratory members for help with field management and data collection.We are grateful for financial support from the Sichuan Province Science and Technology Support Program (2021YJ0504, 2021YFYZ0002), National Key Research and Development Program of China (2016YFD0300406), Special Fund for Agro-scientific Research in the Public Interest(20150312705), and Crops Breeding Project in Sichuan Province(2016NYZ0051, 22ZDZX0018). We also thank the editor and three reviewers for their help in revising this manuscript.
Appendix A. Supplementary data
Supplementary data for this article can be found online at https://doi.org/10.1016/j.cj.2021.11.010.
- The Crop Journal的其它文章
- Research progress on the divergence and genetic basis of agronomic traits in xian and geng rice
- From model to alfalfa: Gene editing to obtain semidwarf and prostrate growth habits
- The chloroplast-localized protein LTA1 regulates tiller angle and yield of rice
- Genome-wide association study and transcriptome analysis reveal new QTL and candidate genes for nitrogen-deficiency tolerance in rice
- Advances in the functional study of glutamine synthetase in plant abiotic stress tolerance response
- Identification of microRNAs regulating grain filling of rice inferior spikelets in response to moderate soil drying post-anthesis

