Trends in medication use and treatment patterns in Chinese patients with inflammatory bowel disease
Ling-Ya Yao, Bu-Le Shao, Feng Tian, Mei Ye, Yu-Qin Li, Xiao-Lei Wang, Lin Wang, Shao-Qi Yang, Xiao-PingLv, Yan Jia, Xue-Hong Wang, Xiao-Qi Zhang, Yan-Ling Wei, Qian Cao
Abstract
Key Words: Inflammatory bowel disease; Crohn's disease; Ulcerative colitis; Medication trends; Treatment pattern; Infliximab
lNTRODUCTlON
Inflammatory bowel diseases (IBD), consisting of Crohn’s disease (CD) and ulcerative colitis (UC), are chronic debilitating disorders characterized by alternating periods of relapse and remission[1,2]. With a growing incidence worldwide, IBD cases in China are expected to exceed 1.5 million by 2025[3]. In light of the need for lifelong medical interventions, IBD poses a global public health challenge.
Owing to the rapid development of new drugs, IBD medications have launched in succession and changed in the past few decades, especially following the discovery of infliximab (IFX), the first antitumor necrosis factor agent, which has revolutionized the field[4,5]. Several attempts have been made by Western and other Asian countries to provide rough medication changes. Specifically, increasing proportions of immunomodulators and biological agents have been observed, accompanied by decreasing prescription rates of 5-aminosalicylates (5-ASA) and corticosteroids (CS)[6-8]. A few studies have been carried out on annual changes in IBD medications, including 5-ASA and CS[9,10]. However,no long-term analysis of medication tendency has been conducted in a large population of Chinese patients with IBD.
Drug diversification enables treatment combinations for better therapeutic effects[11]. However,combination treatment also correlates with increasing risks of side effects[12,13]. Therefore, patients might switch treatment strategies to acquire optimal effects from IBD medications under different circumstances. Currently, minimal attention has been paid to periodic changes in IBD treatment strategies. Additionally, initial treatment strategies affect follow-up therapeutic patterns to some extent;thus, investigating the possible predictors for initial treatment strategies may help to better understand periodic changes in therapeutic patterns in patients with IBD.
Therefore, this study aimed to provide fresh insights into temporal trends in medication prescriptions among the Chinese patients with IBD for over 20 years. The study also aimed to investigate long-term periodic changes in treatment paradigms and identify the possible factors that influence initial drug strategies.
MATERlALS AND METHODS
Study design
This multicenter retrospective cohort study included 12 IBD referral centers across all seven administrative regions in China with diverse socioeconomic backgrounds. The study protocol was reviewed and approved by Sir Run Run Shaw Hospital, College of Medicine Zhejiang University Institutional Review Board (Approval No. 20210714-31) with approval for all hospitals involved in the study. The requirement for patient informed consent was waived due to the retrospective nature of this study.
Study population
Incident adult patients with a definite diagnosis of CD or UC according to the Chinese consensus on IBD diagnosis, which was similar to that of the European Crohn’s and Colitis Organization (ECCO)consensus, between January 1, 1999 and December 31, 2019 were included[14,15]. Patients were excluded from analyses for medication trends if the cessation date of medications was unclear.Additionally, patients were further excluded from analyses for treatment patterns if: (1) They were followed up for < 3 years since diagnosis; or (2) No prescription of either 5-ASA, CS, immunosuppressants (IMS), or IFX was administered throughout follow-up. Cases were followed from diagnosis until loss to follow-up, or December 31, 2020, whichever came first.
Data collection and definition
Patients were identified using International Classification of Diseases 8thand 10threvision codes for CD(563.00-563.09 and K50) and UC (563.19, 569.04 and K51), respectively. Data regarding baseline characteristics and prescriptions since diagnosis were collected by reviewing medical records and digitized into the Epidata 3.0. Data quality was ensured using standardized case report forms and careful manual scrutinization.
Demographic parameters including sex, date of first IBD-related symptoms, date of initial diagnosis,smoking status, region of urbanization, occupation, and clinical parameters including disease location,disease activity, extraintestinal manifestations (EIMs), IBD family history, gastrointestinal surgical history, and medication use within six months before diagnosis were collected from patients with IBD.Additionally, disease behavior, perianal involvement, and perianal surgical history were also collected from patients with CD. Other relevant variables were calculated, including median age of onset and diagnosis, median interval from onset of symptoms to diagnosis, median follow-up duration, and body mass index (BMI) at diagnosis.
Smoking status was defined by smoking habits at diagnosis. EIMs classification originated from the 2016 ECCO consensus[9]. Disease location for IBD and CD behavior were defined according to the Montreal classification[10]. Disease activity was acquired from medical records using the Crohn’s Disease Activity Index (CDAI) and Mayo score for CD and UC, respectively, and was assessed and recorded by IBD nurses when patients were first diagnosed[16,17].
Statistical analysis
Continuous variables are presented as medians with interquartile ranges (IQRs), and categorical variables as numbers with percentages. A T-test or Mann-Whitney U test was used to compare continuous variables, and Chi-square test or Fisher’s exact test was used to compare categorical variables.
The initiation date of medications (including 5-ASA, CS, IMS, and IFX) since diagnosis was set as the first prescription date, and the last date was calculated as the first prescription date plus the number of prescription days. Annual percentage of each drug used among all available patients was plotted against every calendar year to demonstrate medication trends from 1999 to 2020. To conduct subgroup analysis for periodic changes in treatment paradigms, the study period was divided according to the following time points since diagnosis: 1, 3, 6, 12, 24, and 36 mo. The frequency and percentage of different drug combination strategies were plotted against different periods to illustrate periodic changes in drug patterns with a 3-year follow-up. To investigate possible factors for initial treatment strategies, we incorporated drugs into the following combination strategies. For CD, initial medications were classified as: (1) CS (CS monotherapy or combined with 5-ASA); (2) CS + IMS (CS combined with IMS); and (3) IFX [IFX monotherapy or combined with conventional drugs (5-ASA, CS, or IMS)]. For UC, initial medications were classified as: (1) 5-ASA (5-ASA monotherapy); (2) CS (CS monotherapy or combined with 5-ASA); and (3) IFX/IMS (IFX monotherapy, IMS monotherapy, or IFX combined with IMS). Predictors for initial drug strategies were identified using logistic regression analysis and presented as odds ratios and 95% confidence intervals.
Pvalue < 0.05 were considered a statistically significant difference. Statistical analyses were conducted using SAS 9.4 (SAS Institute, Cary NC, United States), and graphs were plotted using R software (version 4.1) with the “ggplot2” and “ggalluvial” packages[18,19].
RESULTS
Baseline characteristics
Altogether, 3610 patients were included in the analysis for temporal changes in medication use (2208 and 1402 patients with CD and UC, respectively). Furthermore, 957 patients were included in the analysis for periodic changes in treatment patterns (686 and 271 patients with CD and UC, respectively),after excluding 2452 patients who were followed up < 3 years since diagnosis, and 201 patients with no prescription of either 5-ASA, CS, IMS, or IFX throughout follow-up (Figure 1).
Patient baseline characteristics are summarized in Table 1. The median (IQR) follow-up duration was 1.6 (0.3-3.8) years, the median (IQR) age at diagnosis was 33.0 (25.0-48.0) years, and male accounted for 46.2% of patients. Similar patterns were found in the region of urbanization and IBD family history between CD and UC, except for male sex (P< 0.0001), median age of onset (P< 0.0001), median age at diagnosis (P< 0.0001), median interval from onset to diagnosis (P< 0.0001), median follow-up duration(P< 0.0001), smoking status (P< 0.0001), occupation (P< 0.0001), BMI category (P< 0.0001), EIM classification (P< 0.0001), gastrointestinal surgical history (P< 0.0001), and drug history (medication use within six months before diagnosis;P< 0.0001).
The differences in baseline characteristics between included and excluded patients in the analysis for periodic changes in treatment patterns are shown in Table 2. Briefly, patients who were included in further analysis were prone to be younger at the age of onset and diagnosis (P< 0.001), accompanied with EIMs (P= 0.0096), and underwent perianal surgeries before diagnosis (P< 0.0001). Moreover,patients with UC had a higher risk of proctitis and left-sided involvement (P= 0.0005), and patients with CD had higher proportion of moderate severity (P= 0.0002).
Temporal trends in medication use
Proportions of different prescriptions during the 20-year follow-up of patients with CD are depicted in Figure 2A and Supplementary Table 1. The prescription rate of 5-ASA showed a steady decline from 50.0% in 2002 to 5.5% in 2020, and a similar trend was observed for CS. Meanwhile, the prescription rate of IMS increased prominently from 2005 onwards, but decreased slightly between 2018 and 2020. The prescription rate of IFX has gradually increased since 2008, reaching 60.3% in 2020.
We also reported the proportions of different prescriptions among patients with UC (Figure 2B and Supplementary Table 2). 5-ASA and CS were steadily prescribed between 1999 and 2020. IMS was first prescribed in 2007, increasing slightly to 8.5% in 2020. Parallel to IMS, a gentle increase was observed in the proportion of IFX prescriptions between 2009 and 2020.
Periodic changes in treatment patterns
Therapeutic regimens were evaluated for three consecutive years after diagnosis. As IFX was first prescribed between 2008 and 2009 in China, we further divided the total cohort into Cohorts I(1999-2008) and II (2009-2020), to evaluate the potential impact of IFX availability on therapeutic strategies.
IFX (22.3%), IMS (7.1%), and IMS combined with IFX (12.0%) accounted for nearly half of initial(within the first month after diagnosis) treatment strategies for patients with CD (Figure 3A and Supplementary Table 3). Interestingly, 51.6% of patients ceased medical treatment within 1-3 mo, but 41.7% re-prescribed in the following period. Specifically, a prominent increase in monotherapy orcombination therapy containing IMS and IFX was observed during the subsequent 6-36 mo. Meanwhile,conventional therapies including 5-ASA, CS, and CS combined with IMS were steadily used. When comparing the two cohorts, we found that all initial prescriptions originated from conventional medications in Cohort I, while therapies comprising of IFX or IMS accounted for 45.0% of all initial prescriptions in Cohort II (Supplementary Figures 1 and 2).

Disease behavior, n (%)B1-inflammatory disease -1175 (53.3)-B2-stricturing disease 690 (31.3)B3-penetrating disease 338 (15.4)Perianal involvement, n (%)Fistula -772 (35.0)-Abscess 392 (17.8)Fissure 38 (1.7)Extraintestinal manifestations, n (%)464 (12.9)357 (16.2)107 (7.6)< 0.0001 Family history of IBD, n (%)27 (0.7)20 (0.9)7 (0.5)0.1671 History of gastrointestinal surgery, n (%)594 (16.5)524 (23.7)70 (5.0)< 0.0001 History of perianal surgery, n (%)471 (13.0)471 (21.3)0 (0.0)< 0.0001 Medication use before diagnosis (≤ 6 mo), n(%)908 (25.2)440 (19.9)468 (33.4)< 0.0001 The classification of body mass index: Underweight: < 18.5; Normal: 18.5-23.9; Overweight: 24-28; Obese: > 28. Disease behavior was not available in 5 patients. IBD: Inflammatory bowel disease; CD: Crohn’s disease; UC: Ulcerative colitis; IQR: Interquartile ranges; BMI: Body mass index; CDAI: Crohn’s Disease Activity Index.

Figure 1 Flowchart of inflammatory bowel disease selection and analysis. CD: Crohn’s disease; UC: Ulcerative colitis; ASA: Aminosalicylates; CS:Corticosteroids; IMS: Immunosuppressants; IFX: Infliximab.
Data on UC are presented in Figure 3B and Supplementary Table 4. 5-ASA (56.1%) was the most frequently prescribed medication in initial strategies, followed by CS (20.7%). Similar to CD, 60.1% of patients stopped medications within 1-3 mo, but 38.7% were put back on the same or other drugs for treatment progression, resulting in a steady increase of 5-ASA, CS, and IFX/IMS prescriptions in the following period. Compared with Cohort I in initial strategies, we still found 5-ASA and CS comprised a majority of prescriptions in Cohort II (Supplementary Figures 3 and 4).
Factors associated with initial drug strategies
Since initial therapeutic regimens may affect follow-up strategies to some extent, we further invest-igated potential factors impacting initial drug strategies.
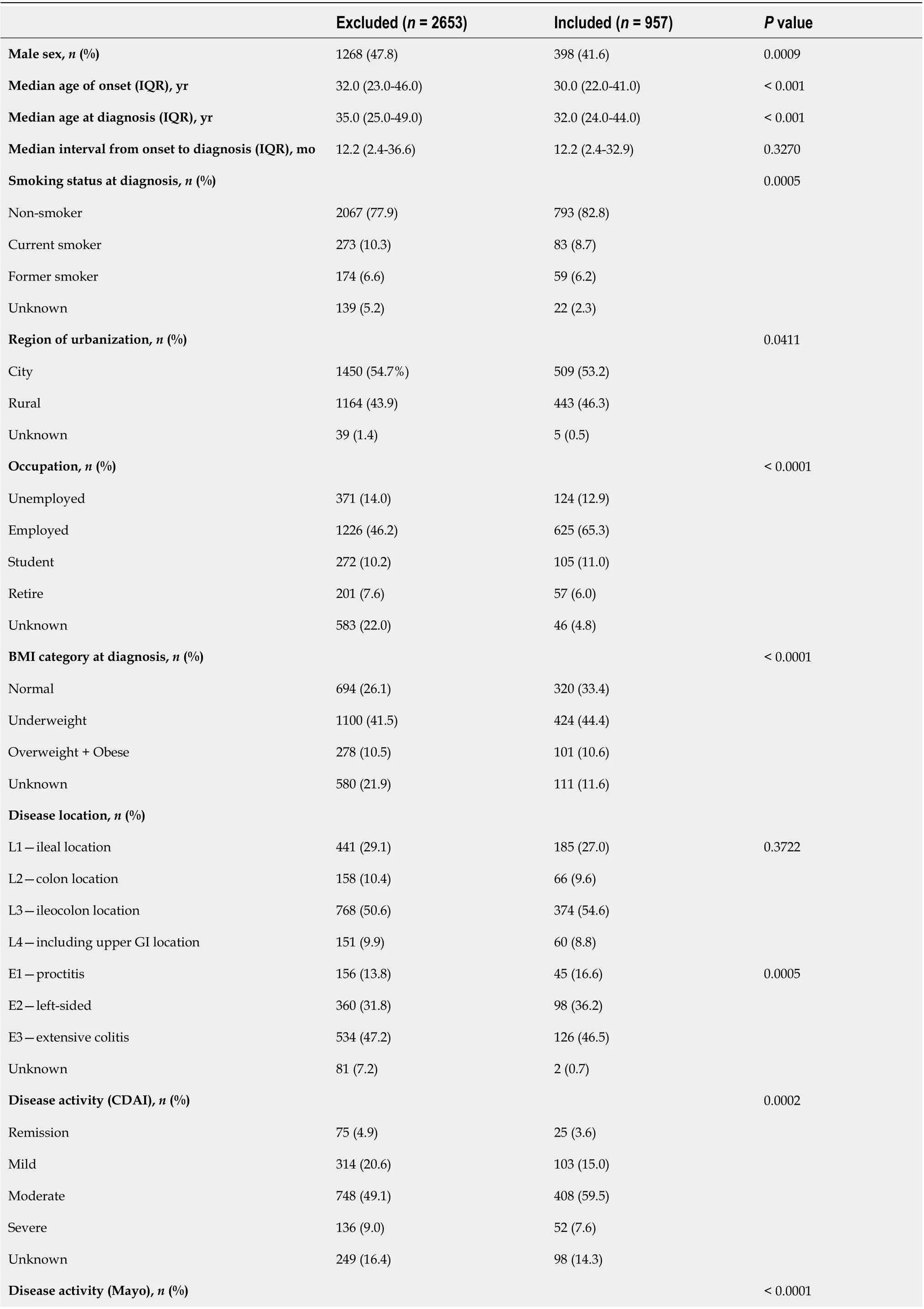
Table 2 Comparison of demographic and clinical characteristics of patients with inflammatory bowel disease included and excluded from analysis in 1999-2020

Remission 18 (1.6)4 (1.5)Mild 231 (20.4)71 (26.2)Moderate 406 (35.9)121 (44.7)Severe 192 (17.0)60 (22.1)Unknown 284 (25.1)15 (5.5)Disease behavior (CD), n (%)0.4273 B1-inflammatory disease 797 (52.5)378 (55.2)B2-stricturing disease 488 (32.1)202 (29.5)B3-penetrating disease 233 (15.4)105 (15.3)Perianal involvement (CD), n (%)0.0800 Fistula 524 (34.4)248 (36.2)Abscess 262 (17.2)130 (19.0)Fissure 24 (1.6)14 (2.0)Extraintestinal manifestations, n (%)318 (12.0)146 (15.3)0.0096 Family history, n (%)19 (0.7)8 (0.8)0.7124 History of gastrointestinal surgery, n (%)423 (15.9)171 (17.9)0.1687 History of perianal surgery, n (%)263 (9.9)208 (21.7)< 0.0001 Medication use within 6 mo before diagnosis, n (%)646 (24.3)262 (27.4)0.0643 The classification of body mass index: Underweight: < 18.5; Normal: 18.5-23.9; Overweight: 24-28; Obese > 28. Disease behavior was not available in 1407 patients. IQR: Interquartile ranges; BMI: Body mass index; CDAI: Crohn’s disease Activity Index; CD: Crohn’s Disease.
Table 3 presents factors for commencing different medications compared with a no prescription group among patients with CD. Specifically, variables including urban living, L3, B3, gastrointestinal surgical history, and diagnosis after 2009 yielded the most significant predictors of patients not commencing CS for initial treatment strategies. However, patients with a drug history had a higher probability of commencing CS. When compared with the no prescription group, patients of male sex,urban living, BMI ≥ 24.0 kg/m2, B3, and gastrointestinal surgical history were less likely to be prescribed CS combined with IMS as initial treatment. In contrast, patients with L4, EIMs, drug history, and perianal surgical history were more likely to be prescribed CS combined with IMS strategy. In terms of comparison between the IFX and no prescription groups, patients who were smokers, had a BMI ≥ 24.0 kg/m2, B2 or B3, and had a gastrointestinal surgical history had a fewer possibility to be prescribed IFX.Contrarily, patients who were students, L4, had moderate severity, and had a perianal surgical history were more likely to be prescribed IFX.
Table 4 presents the variables that determined initial therapeutic regimens in patients with UC.Particularly, patients with a drug history tended to maintain treatments after diagnosis, regardless of type (5-ASA, CS, IMS, or IFX). Variables including current smoker, severe disease activity, and diagnosis after 2009 were the most significant factors for not commencing 5-ASA, whereas variables including employed or retired and E3 were related to a higher probability of commencing 5-ASA.Likewise, patients with a BMI ≥ 24.0 kg/m2and diagnosed after 2009 were less likely to be prescribed CS, and those with E3 and EIMs involvement were likely to be prescribed CS. Moreover, patients with a drug history were more likely to be prescribed IFX/IMS for initial treatment.
DlSCUSSlON
To the best of our knowledge, no multicenter cohort study has been conducted to investigate temporal trends in long-term medication use and periodic changes in treatment paradigms in Chinese IBD patients. Our findings showed that prescriptions of IMS and IFX gradually increased in IBD over the past 20 years, paralleled by decreasing prescriptions of 5-ASA and CS for CD but not for UC.Additionally, periodic changes in treatment patterns revealed a switching profile from conventional medications to IFX in CD, while 5-ASA and CS still took irreplaceable positions in UC.

Table 3 lnfluencing factors of initial drug strategies in patients with Crohn’s disease

Extraintestinal manifestations No Ref -Ref -Ref -Yes 1.10 (0.65-1.87)0.71051.88 (1.23-2.87)0.00370.93 (0.67-1.28)0.6605 Family history of IBD No Ref -Ref -Ref -Yes 1.46 (0.14-15.30)0.75141.70 (0.22-13.10)0.61041.67 (0.37-7.62)0.5082 History of gastrointestinal surgery No Ref -Ref -Ref -Yes 0.35 (0.20-0.60)0.00020.35 (0.20-0.59)< 0.00010.55 (0.41-0.73)< 0.0001 History of perianal surgery No Ref -Ref -Ref -Yes 1.36 ( 0.77-2.40)0.29481.86 (1.20-2.87)0.00522.68 (1.92-3.73)< 0.0001 Medication use before diagnosis (≤ 6 mo)No Ref -Ref -Ref -Yes 1.94 (1.16-3.23)0.01113.06 (2.04-4.58)< 0.00011.33 (0.95-1.85)0.0934 Calendar year of diagnosis< 2009 Ref -Ref -Ref -≥ 20090.19 (0.09-0.40)< 0.00010.77 (0.25-2.33)0.6393 NS NS CS: Corticosteroids; IMS: Immunosuppressants; IFX: Infliximab; OR: Odd ratio; CI: Confidence interval; BMI: Body mass index; CDAI: Crohn’s Disease Activity Index; IBD: Inflammatory bowel disease; NS: Non-significant.
In China, more than 1.5 million people are expected to suffer from IBD by 2025[3]. Therefore, the study population accounted for merely 0.2% of the total estimated Chinese patients with IBD. However,because there is lack of national registries covering all IBD patients, it is difficult to acquire complete data on the diagnosis, treatment, and prognosis of all Chinese patients with IBD. A well-organized national IBD registry in the near future will help facilitate larger clinical studies in China. Besides, IBD referral centers in our study are distributed across all seven administrative regions in China with diverse socioeconomic backgrounds. To some extent, the study population represents in-patients from Chinese referral centers. Moreover, we found similar patterns in demographic and clinical characteristics by further comparing IBD patients in our study with those in other large-sample Chinese studies,which also reflects the representativeness of our study to a certain extent[20,21].
In this study, the median (IQR) age of onset and diagnosis was 31.0 (23.0-45.0) years and 33.0(25.0-48.0) years, respectively, similar to other Asian and Western populations[22,23]. The total median duration of follow-up was short. There are two possible explanations for this. On one hand, the study population mainly stems from IBD referral centers and therefore many patients will go back to grassroots hospitals for following treatment after acquiring definite IBD diagnosis and initial treatment strategies, which may result in loss to follow-up. On the other hand, due to the observational design of this study, most information originated from medical records or databases. It would be a huge cost to follow such a large number of patients. Despite the short follow-up period, which may affect medication trend analysis, there were still more than 1000 patients who were followed for more than three years,and our analysis towards long-term changes in treatment patterns provided a credible result, which can assist in clinical drug management. We also compared baseline characteristics between CD and UC patients. Regarding demographic differences, CD patients seemed more likely to be younger at onset or diagnosis and have longer intervals from onset to diagnosis and follow-up duration. This is possibly due to more sophisticated processes in CD diagnosis and treatment. However, UC patients were more likely to be overweight compared to those with CD. UC patients who seldom implicate small intestine that would cause malabsorption of nutrients and following weight loss might explain BMI differences.In regard to clinical discrepancies, more CD patients had EIMs involvement, family history, and gastrointestinal and perianal surgical histories than UC. Patients with CD often undergo intestinal resection for perforation, ileocecal junction inflammation, or perianal surgeries before diagnosis.Contrarily, more medications were likely prescribed for UC before diagnosis, which was probably due to the fact that 5-ASA was also prescribed for patients with undetermined intestinal ulcers before definite diagnosis. Furthermore, L3 was the most frequent disease location for CD, while B1 and B2 predominated disease behaviors, sharing similar clinical patterns with other Asian cohorts[22,24].

Table 4 lnfluencing factors of initial drug strategy in patients with ulcerative colitis

No Ref -Ref -Ref -Yes 0.68 (0.09-4.94)0.70410.87 (0.08-10.00)0.9096 NS NS History of gastrointestinal surgery No Ref -Ref -Ref -Yes 0.68 (0.36-1.26)0.21630.57 (0.25-1.31)0.18240.88 (0.18-4.23)0.8683 Medication use before diagnosis (≤ 6 mo)No Ref -Ref -Ref -Yes 10.2 (7.19-14.5)< 0.00017.23 (4.71-11.10)< 0.00019.88 (4.77-20.50)< 0.0001 Calendar year of diagnosis< 2009 Ref -Ref -Ref -≥ 20090.31 (0.22-0.45)< 0.00010.51 (0.30-0.85)0.00973.12 (0.41-23.90)0.27485-ASA: 5-aminosalicylates; CS: Corticosteroids; IMS: Immunosuppressants; IFX: Infliximab; OR: Odd ratio; CI: Confidence interval; BMI: Body mass index;NS: Non-significant; IBD: Inflammatory bowel disease.

Figure 2 Temporal trends in medication use in patients with inflammatory bowel disease. A: Trends in medication use in patients with Crohn’s disease; B: Trends in medication use in patients with ulcerative colitis. ASA: Aminosalicylates; CS: Corticosteroids; IMS: Immunosuppressants; IFX: Infliximab.

Figure 3 Periodic changes in treatment patterns in patients with inflammatory bowel disease. A: Crohn’s disease; B: Ulcerative colitis. ASA:Aminosalicylates; CS: Corticosteroids; IMS: Immunosuppressants; IFX: Infliximab.
Currently, IBD medications mainly include 5-ASA, CS, IMS, and biological agents. In our study, trend analysis for CD found a prominent decline in prescriptions of 5-ASA and CS in parallel to a significant increase in prescriptions of IMS and IFX from 1999 to 2020. A previous national study from the American database reported that 37.3% of patients with CD were prescribed 5-ASA between 2009 and 2014, despite the debatable effectiveness of 5-ASA[25]. Nevertheless, the latest ECCO guideline no longer recommends 5-ASA in patients with mild-to-moderate CD[26]. In our analysis, 5-ASA usage significantly decreased from 39.6% in 2009 to 5.5% in 2020, reflecting a positive trend in narrowing the knowledge gap between Chinese providers and IBD consensus. We also observed an apparent reduction in CS prescription onwards, which was probably due to improved awareness of steroidsparing, earlier treatment after diagnosis, closer surveillance for disease relapse, or more attention on adverse effect of CS[27]. Moreover, IMS emerged in 2005, and prescription rates increased strikingly afterwards, except for a slight decrease between 2018 and 2020. The effect of maintenance treatment with IMS administered to patients with CD who are steroid-dependent has been testified[28]. IFX prominently increased between 2008 and 2020. These may reflect deeper market infiltration of IFX and better treatment adherence. It is worth noting that insurance coverage of IFX was not achieved until November 28, 2019, the day IFX entered the national medical insurance. This policy will influence the decision making of Chinese IBD patients on whether to choose IFX or other IMS and may explain treatment discrepancies between Chinese patients and those from other countries. Regarding trend analysis in UC patients, 5-ASA and CS were steadily prescribed, accompanied by a modest increase in IMS and IFX, demonstrating 5-ASA as a milestone during therapeutic armamentarium extension. The American Gastroenterological Association recommends treating patients with mild-to-moderate left sided UC with mesalamine or diazo-bonded 5-ASA compounds[29]. For patients with extensive mildto-moderate UC, ECCO recommends the addition of rectal 5-ASA to oral therapy, as combined oral and rectal therapy delivers a greater effective dose to the affected areas of colon and leads to higher rates of remission[30]. In our study, 51.6% of CD patients and 60.1% of UC patients ceased medical treatment within 1-3 mo. We realized the contradicting results from our study with the current strategy applied worldwide. Our treatment data are mainly derived from IBD referral centers where patients may cease treatment after returning home with only one-month prescriptions. This may be explained by the following reasons which reflect the specific situation of Chinese IBD management: (1) Lack of communication between referral centers and grass-roots hospitals during the early period of IBD treatment; (2) Poor medication adherence at the patient level[31]; and (3) Knowledge gap between doctors and guidelines worldwide. Overall, we are the first to depict temporal changes in medications among a large Chinese IBD population, which has not been well elucidated before.
To further investigate long-term changes in treatment patterns, we included patients who were followed for at least three consecutive years. Our analysis showed that monotherapy or combination therapy with IFX and IMS accounted for nearly half of initial treatment strategies for CD. In fact, 12.7%were prescribed 5-ASA as the initial drug, among which nearly two-thirds ceased treatment within 1-3 mo. However, most patients switched to up-level drugs afterwards, probably reflecting the limitations of 5-ASA for disease maintenance. According to a previous study, treatment strategies for patients with moderate-to-severe CD could be listed as: (1) Conventional step-care therapy (CS and IMS are prescribed sequentially); (2) Accelerated step-care therapy (a tapering process of CS together with IMS);and (3) Top-down therapy (early combination of IMS and IFX). In our study, 7.2% of cases were prescribed CS as the initial drug, most of whom received conventional step-care therapy three months after diagnosis[32]. Another 13.1% were prescribed CS combined with IMS as the initial strategy, most of whom followed an accelerated step-care regimen. Moreover, top-down therapy was the first choice for almost half of the population. Despite short-term interruption, most patients recovered with IMS,IFX, or a minority of CS or 5-ASA. We noted that conventional medications were the main initial strategies in Cohort I, comprising mostly of two step-care therapies. By contrast, the initial approach in Cohort II was predominated by top-down therapies. Regarding therapeutic patterns in UC patients,initial treatments mainly included 5-ASA and CS. As the principal therapy in initial strategies, 5-ASA was highly effective for UC[33]. Similar to CD patterns, patients with UC who stopped treatment within 1-3 mo were re-prescribed different levels of medications afterwards, reflecting better adherence and more standardized treatment. Additionally, 5-ASA and CS were only initial strategies in Cohort I,complying with clinical settings in the early period of IBD management. In general, our analysis provides a unique insight into long-term therapeutic paradigms in Chinese population.
Since initial treatments were crucial for long-term therapeutic paradigms, we next investigated potential predictors for initial drugs. In CD patients, L3 congruously reduced the possibility of commencing CS, whereas L4 increased the possibility of commencing CS combined with IMS or commencing IFX. This is likely due to the fact that patients with upper gastrointestinal involvement are prone to behave in a more sophisticated course, requiring IMS or IFX for maintenance of remission.Intriguingly, patients with penetrating behavior and gastrointestinal surgical history appeared to have a lower likelihood of being prescribed either drug, given the intricate and drug-refractory nature, calling for short-term surgical intervention after diagnosis. We also observed an increase in CS combined with IMS or IFX in patients who underwent perianal surgeries before diagnosis. A possible explanation is that poor healing after perianal surgeries provide useful evidence for making detailed diagnosis and more appropriate selection of therapeutic strategies. Regarding factors in UC, we found an appreciable correlation between all strategies and medication history. This finding should be carefully generalized to other UC populations. Thus, future investigation elucidating detailed association between treatments before diagnosis and initial therapeutic paradigms in larger population is warranted. Interestingly,patients with E3 reflected a higher risk of 5-ASA and CS medications, possibly explained by the fact that patients with extensive colitis naturally tended to adopt medications that might relieve symptoms.Moreover, our observations indicated that patients diagnosed after 2009 were less likely to be prescribed 5-ASA or CS as initial therapies, which is mainly due to IFX insurance coverage or improved awareness of steroid-sparing medications. Generally, these results suggest that baseline characteristics probably affect initial treatment strategies to a certain extent.
There are several strengths in this study. First, patients were from IBD referral centers, which covered all the administrative regions in China. The population represented a broad spectrum of Chinese patients with IBD for over 20 years, facilitating temporal trends evaluation in medication use and drug strategies. Second, detailed baseline characteristics and prescriptions through follow-up were retrieved from consecutive medical records, which may reflect actual drug intake to some extent. Lastly, no study has analyzed periodic changes in treatment paradigms for three consecutive years and associated factors with initial drug strategies in Chinese IBD patients.
The study also has limitations. First, extrapolation of medication trends to the entire country and other Asian countries remains unproven due to lack of the national registry in China and potential differences in racial phenotypes and treatment strategies worldwide. A wholesome IBD registry system and multicenter studies among Asian populations are needed in the near future. Second, potential information and confounding bias exist due to observational design. Therefore, we assessed baseline factors thoroughly and employed multivariate logistic regression analysis to minimize bias. Moreover,outcomes, including hospitalization, surgeries, or phenotype progression were not collected, limiting further investigation in the correlation between different drug strategies and long-term outcomes.
CONCLUSlON
In conclusion, this study provides insights into temporal trends in long-term medication use and periodic changes in treatment patterns for Chinese patients with IBD. The findings suggest that prescriptions of IMS and IFX increased in parallel with steady or decreasing prescriptions of 5-ASA and CS in real-world settings. The study also shed light on periodic changes in treatment patterns, reflecting a switching profile from conventional drugs to IFX in CD. Further research into the full breadth of trends in medication use and treatment patterns should be conducted for additional compliance with guidelines for IBD management.
ARTlCLE HlGHLlGHTS

ACKNOWLEDGEMENTS
We want to thank Professor Yun-Xian Yu from Zhejiang University to help with reviewing the statistical methods and proofreading the manuscript of this study.
FOOTNOTES
Author contributions:Cao Q formulated the research idea; Yao LY developed the study protocol; Yao LY, Tian F, Ye M, Li YQ, Wang XL, Wang L, Yang SQ, Lv XP, Jia Y, Wang XH, Zhang XQ and Wei YL conducted the patient identification and data collection; Shao BL and Yao LY conducted the data analysis; Yao LY, Shao BL and Cao Q developed the manuscript; and all authors have read and approve the final manuscript.
lnstitutional review board statement:The study protocol was reviewed and approved by Sir Run Run Shaw Hospital,College of Medicine Zhejiang University Institutional Review Board with approval for all hospitals involved in the study, No. 20210714-31.
lnformed consent statement:The requirement for patient informed consent was waived due to the retrospective nature of this study.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Data sharing statement:Technical appendix, statistical code, and dataset available from the corresponding author at caoq@zju.edu.cn.
STROBE statement:The authors have read the STROBE Statement-checklist of items, and the manuscript was prepared and revised according to the STROBE Statement-checklist of items.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORClD number:Qian Cao 0000-0001-7938-7532.
S-Editor:Fan JR
L-Editor:A
P-Editor:Fan JR
 World Journal of Gastroenterology2022年30期
World Journal of Gastroenterology2022年30期
- World Journal of Gastroenterology的其它文章
- Role of one-step nucleic acid amplification in colorectal cancer lymph node metastases detection
- Current perspectives on the role of liver transplantation for Langerhans cell histiocytosis: A narrative review
- Gut microbiota, inflammatory bowel disease and colorectal cancer
- Thrombocytopenia in chronic liver disease: Physiopathology and new therapeutic strategies before invasive procedures
- P2X7 receptor blockade decreases inflammation, apoptosis, and enteric neuron loss during Clostridioides difficile toxin A-induced ileitis in mice
- Serological profiling of Crohn’s disease and ulcerative colitis patients reveals anti-microbial antibody signatures
