Role of one-step nucleic acid amplification in colorectal cancer lymph node metastases detection
Francesco Crafa, Serafino Vanella, Onofrio A Catalano, Kelsey L Pomykala,Mario Baiamonte
Abstract Current histopathological staging procedures in colorectal cancer (CRC) depend on midline division of the lymph nodes (LNs) with one section of hematoxylin and eosin staining. Cancer cells outside this transection line may be missed, which could lead to understaging of Union for International Cancer Control Stage II high-risk patients. The one-step nucleic acid amplification (OSNA) assay has emerged as a rapid molecular diagnostic tool for LN metastases detection. It is a molecular technique that can analyze the entire LN tissue using a reversetranscriptase loop-mediated isothermal amplification reaction to detect tumorspecific cytokeratin 19 mRNA. Our findings suggest that the OSNA assay has a high diagnostic accuracy in detecting metastatic LNs in CRC and a high negative predictive value. OSNA is a standardized, observer-independent technique,which may lead to more accurate staging. It has been suggested that in stage II CRC, the upstaging can reach 25% and these patients can access postoperative adjuvant chemotherapy. Moreover, intraoperative OSNA sentinel node evaluation may allow early CRC to be treated with organ-preserving surgery, while in more advanced-stage disease, a tailored lymphadenectomy can be performed considering the presence of aberrant lymphatic drainage and skip metastases.
Key Words: Colorectal malignancies; One-step nucleic acid amplification; Diagnostic accuracy; Negative predictive value; Upstaging; Organ-sparing surgery; Tailored lymphadenectomy
lNTRODUCTlON
Of the gastrointestinal cancers, the colorectal cancer (CRC) is the most represented. Among the indication criteria for chemotherapy, lymph node (LN) positivity (stage III) is the most important[1].The histopathological study of the LNs is performed on one or at most two sections of each LN with hematoxylin and eosin (HE). Therefore the conventional study presents the possibility of not detecting micro-metastases (MMs) or macro-metastases leading to an "understaging". The high relapse rates(20%-25%) in patients with negative LNs could be due to this "understaging"[2]. Multilevel LN sectioning combined with immunohistochemistry (IHC) can improve the detection rate of small nodal tumor infiltrates [i.e.isolated tumor cells (ITCs) and MMs], although it is a costly and protracted process[3-6].
Tsujimotoet al[7] were the first to describe the one-step nucleic acid amplification (OSNA) assay for detecting LN metastases (LNMs) in patients with breast cancer (BC). Numerous studies have followed which have confirmed the high sensitivity of OSNA in detecting LNMs of breast, gastric and CRCs[8-15]. Other studies[16-18] have underlined the usefulness of the OSNA assay as a complementary tool for diagnosing LNMs and upstaging in histologically node-negative stage II CRC.
The sentinel LN (SLN) is gaining more and more consensus because it allows to perform a more conservative surgery with considerable advantages, when applicable for the patient and for the operating times. Obviously in the early stages of CRCs this could play an important role, allowing to realize, in case of absence of lymph node metastases on SLNs, an organ preserving surgery.
In this review, we analyzed the use of OSNA in detecting LNMs in CRC.
LlTERATURE SEARCH
Search strategy
After developing and piloting search terms, MEDLINE, SCOPUS, ClinicalTrials.gov, and Cochrane Database were used to conduct a comprehensive computerized literature search for articles pertaining to OSNA use in detecting LNMs in CRC. Medical subject headings terms and keywords were combined:colorectal malignant, cancer, colorectal tumor, colorectal neoplasm, carcinoma, lymph node metastasis,SLN, one-step nucleic acid amplification, OSNA, cytokeratin 19, CK-19, predictive value, upstaging,organ-sparing surgery, and tailored lymphadenectomy. The electronic search was supplemented by reviewing reference lists of included studies and previous systematic reviews. No time limitation was stipulated for the search, which was last updated December 20, 2021. In addition, we retrieved and cited high-quality references using the Reference Citation Analysis database (https://www.referencecitationanalysis.com/).
Study selection, data extraction, and quality assessment
The retrieval of articles was completed in three consecutive stages. Following reduplication of the sum of collected articles, their titles and abstracts underwent further screening and those deemed ineligible were removed. For duplicates, the most recent or complete publication was chosen. The remaining papers were evaluated in full text. Two reviewers (MB, SV) extracted data in duplicate using a standardized data extraction sheet.
LYMPHATlC DRAlNAGE lN CRC
The American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC)staging score divides the stages according to how many metastatic lymph nodes are present. Based on the location of the primary tumor, those with a course adjacent to the main vascular branches near the affected colon are considered regional lymph nodes. In particular, starting from the rectum up to the right colon, in addition to the peri-colic lymph nodes, regional lymph nodes are considered, those adjacent to the rectal arteries, the sigmoid arteries, the left colonic artery, the inferior mesenteric artery,the middle colic artery, the right colic artery, the ileocolic artery[19].
In AJCC/UICC tumor-node-metastasis (TNM) staging system[20-22], patients with no metastatic LNs are N0, cases with one to three metastatic LNs are N1, and cases with more than three positive LNs are N2. Moreover, the N1 category is subdivided into N1a (1 metastatic LN), N1b (2-3 metastatic LNs), and N1c (no regional LNs are positive but there are tumor deposits in the subserosa, mesentery or nonperitonealized pericolic or perirectal/mesorectal tissues), whereas the N2 category is subdivided into N2a (4-6 metastatic LNs) and N2b (7 or more metastatic LNs). The minimum number of examined LNs needed for adequate staging should not be less than 12 to minimize the possibility of stage migration[19,23-27].
The Japanese Society for Cancer of the Colon and Rectum (JSCCR) staging score classifies the involved LNs based on location and number. This system divides the regional LNs into three groups:main, intermediate and peri-colic. Regional LNs depend on their adjacency to the blood vessels following the primary tumor site. LNs adjacent to the marginal arcade are pericolic nodes, the LNs along the course of the main vessels of the colon are intermediate nodes (sigmoid arteries, the left colonic artery, the inferior mesenteric artery, the right and left middle colic artery, the right colic artery,the ileocolic artery). Lymph nodes located proximal to the origin of the main colonic vascular branches of the inferior and superior mesenteric artery are the main nodes. LNMs are classified as N1 if up to 3 peri-colic or intermediate LNs are involved, N2 if they are ≥ 4, N3 when the main LNs are involved[28,29].
ITCs and MMs
When single or few tumor cells smaller than 0.2 mm are found, these are called ITCs, if instead the deposits have a diameter between 0.2 and 2.0 mm these are called MMs. When ITCs or MMs with HE or IHC are found, they are classified as pN0 (i +), if instead the deposits are diagnosed only by reverse transcriptase polymerase chain reaction (RT-PCR), they are classified as pN0 (mol +)[19,20]. MMs, ITCs,and occult metastasis have been reported in 4.2%, 19.3%, and 5% of patients with stage I and II CRC,respectively, and attracted interest as prognostic factors[30-33].
Tumor deposits
In the literature, tumor deposits (TDs) are defined as foci of tumor separated from the main neoplasm and found in peri-rectal or peri-colonic adipose tissue or in mesocolon in the lymphatic drainage area, in the absence of identifiable LN tissue.
It is postulated that they are produced either by discontinuous dissemination of the tumor or by vascular/perineural dissemination. TDs can be found in 10.2%-22% of CRC cases and it has been suggested that TDs may represent a LN, a vascular structure, or a nerve completely replaced by carcinoma[34].
Several studies[35,36] have shown decreased disease-specific survival and overall survival (OS) in the presence of TDs. Moreover, the survival outcomes worsen when TDs occur concomitantly with LNMs.Other studies confirmed this evidence in CRC. It has been suggested that TDs have negative prognostic value but are not sufficiently categorized in the current TNM staging and the number and/or presence of the TD should be added to the number of LNMs to define the final N stage creating a specific category for TDs with LNM, which could be called category N2c or N3[37-41].
CLlNlCAL STAGE OF NODAL METASTASES
Diagnostic imaging
Diagnostic imaging assessment of lymphadenopathy in CRCs is challenging. Individual imaging modalities face specific intrinsic limitations, for example, transrectal ultrasound is operator-dependent,detrimentally affected by a small field of view, and cannot be employed in stenosing or rectal cancers.Computed tomography (CT) is hampered by its low soft tissue contrast resolution which, besides negatively impacting detection, precludes evaluation of fine LN details and therefore must rely only on LN size for assessing lymphadenopathy. Magnetic resonance imaging (MRI) requires long acquisition times and is prone to artifacts in the case of poor patient cooperation. Fluorodeoxyglucose (FDG)-positron emission tomography (PET)/CT and PET/MRI necessitate exposure to radiation, and its yields are influenced by the amount of metabolically avid cells in the affected LNs.
Size
Lymphadenopathy is also intrinsically challenging. Despite malignant LNs tending to be larger than their benign counterparts, there is wide size superimposition between malignant and benign LNs. In one study that evaluated only LNs ≥ 5 mm, short axis range was 6-12 mm for malignant LNs and 5-13 mm for benign LNs[42,43]. Moreover, the size of metastatic LNs is often at the lower limits of imaging spatial resolution. In a study, median LN short axis was 3.2 mm for malignant LNs and 2.8 mm for benign LNs[44]. Additionally, 30%-94% of metastatic LNs from rectal cancer are < 5 mm in short axis[45-48]. To make things even more challenging, some benign etiologies, especially inflammation, might increase LNs size.
MRI
Due its superior anatomic layout and high soft tissue contrast resolution, MRI can explore LN nature trough size and morphologic criteria. LN size criteria have yielded different results in different studies.However, across studies, the bigger the short axis threshold, the higher the specificity and lower the sensitivity; a 3 mm short axis has been associated with 92% sensitivity, 3% specificity, and 40% accuracy;a short axis threshold of 9 mm has been associated with the opposite trend, giving 8% sensitivity, 100%specificity, and 62% accuracy[42]. Short axis thresholds of 7.2-7.5 mm have reached 32%-87% sensitivity and 70%-94% specificity, with 68% of accuracy[42,43]. However, the most accepted short axis cut off for lymphadenopathy in rectal cancer is 5 mm, yielding sensitivities of 50%-72%, specificities of 46%-60%,and an accuracy of 57%[42,46-50].
Beside size, several other MRI criteria can be used, with diffusion weighted imaging (DWI) believed to be one of the most promising tools. However, DWI has proven inadequate for this purpose so far.High b-value DWI is a powerful tool for LN detection. However, DWI and even apparent diffusion coefficient values[43,51,52] are unable to discriminate benign from malignant LNs (accuracy 40%).Therefore, in our practice, we use DWI just to detect all LNs, relegating the differentiation of benign from malignant ones to morphologic, size and/or metabolic criteria.
Chemical shift effect (CSE) refers to a black or bright border outlining organ contours, including LNs.In the case of neoplastic growth in the subcapsular sinus, the resonance frequencies of hydrogen protons in the subcapsular sinus and in the adjacent fat are similar, resulting in the loss of CSE.
Four patterns of LN CSE have been described: continuous and smooth, continuous, and irregular,discontinuous, and irregular, or absent[44]. Once neoplastic cells have colonized the subcapsular sinus,they easily spread outside of the LN, likely more rapidly than toward the medulla. This results in irregular or obscure LN contours, reported in 60%-65% of malignant LNs and in 16%-20% of benign LNs, leading to sensitivity of 88%, specificity of 23%, and accuracy of 50% in one study. On the other hand, a smooth external contour has been described in 80%-84% of benign LNs and in 34%-40% of malignant LNs[42-44].
The internal structure of LNs may be heterogenous in the settings of metastases; this has been reported in 26%-52% of benign and 54%-91% of malignant LNs, with a sensitivity of 84%, specificity of 31%, and accuracy of 53%; on the other hand, homogeneous internal structure, has been observed in 48%-73% of benign and 8%-46% of malignant LNs[42-44]. The combination of inhomogeneous signal intensity and indistinct/irregular borders has been shown to yield sensitivity, specificity, and accuracy of 56%, 91%, and 77%, respectively[42].
Currently, LN size is the most used criterion to discriminate between malignant and benign LNs on MRI, but given the previously discussed inherent limitations, it is often integrated with the morphologic criteria described above. According to the European Society of Abdominal and Gastrointestinal Radiology (ESGAR) guidelines, a LN is considered metastatic in the case of[53]: Short axis diameter ≥ 9 mm, short axis 5-8 mm plus ≥ 2 morphologic criteria, short axis < 5 mm plus 3 morphologic criteria, or mucinous LN regardless of the size. Morphologic criteria chosen by ESGAR are round shape, irregular borders, and heterogenous signal.
However, despite all of the above efforts, even MRI, the most promising imaging modality for LN evaluation is still inadequate for the scope. A recent study that explored the staging performance of MRI in rectal cancer, using surgical pathology as a standard of reference, showed that MRI LN status was correctly assigned in 68% of cases, overstaged in 28%, and understaged in 4%. Moreover, only 40% of MRI-positive LN cases were pathologically confirmed[54]. These results are in line with a FDGPET/MRI study where N status was overstaged by MRI in 22.6% of patients and by PET/MRI in 8% of cases; correct N status was assigned by MRI in 58% of patients and by PET/MRI in 79% of patients[55].
PET
FDG-PET, in addition to structural information, includes the advantage of assessing the metabolic activity of colorectal patient LNs. However, even with metabolic information, sensitivity is limited. A recent meta-analysis including 13 studies published between 2007 and 2019 evaluated the pretreatment ability of 18F-FDG PET/CT as a staging modality to detect metastatic LNs in CRC[15]. The pooled sensitivity, specificity, positive and negative likelihood ratios were 65%, 75%, 4.57, and 0.37,respectively. Prospective studies have demonstrated higher sensitivity and specificity compared to retrospective studies, and studies with sample sizes greater than 100 and that used a cut off value of maximum standardized uptake value (SUV) ≤ 2.5 revealed better accuracy. An older meta-analysis of CRC patients found an even lower pooled sensitivity of 42.9% for detecting LN metastasis, but a higher specificity of 87.9%[56,57]. Differences in meta-analysis outcomes are thought to be due to the heterogeneity of baseline patient characteristics and included article methodologies. Regardless of the variances between the two meta-analyses, FDG-PET has limitations in sensitivity[58-61], likely due to a partial volume effect when assessing the SUV of small LNs (< 10 mm), as well as limitations in spatial resolution when differentiating between extension of primary tumor and adjacent positive LNs[62-64].Specificity on the other hand is limited by false positives seen most often in reactive LNs.
Innovations
Advancements in the imaging evaluation of LNs in CRC are going to happen in the very near future due to innovative scanning technologies such as PET/MRI, which can investigate tumor biology,phenotypes, improve diagnosis, and impact the management of several solid organ malignancies including CRC[55,65-72], innovative radiopharmaceuticals such as fibroblast activation protein inhibitor(FAPI), which is already outperforming FDG in several settings[73,74], and due to the endless possibilities opened by artificial intelligence. Regarding FAPI, a study[74] comparing 68Ga-FAPI and 18F-FDG uptake in 35 patients with gastric, duodenal, and CRCs, showed a significantly higher sensitivity with 68Ga-FAPI PET/CT compared to 18F-FDG PET/CT (79%vs54%) but an equivalent specificity (82%vs89%). Artificial intelligence is going to play a major role in diagnostic imaging evaluation of LNs. A recently published metanalysis, which focused on LN staging in CRC, showed that deep learning and radiomics outperform radiologists, with deep learning also being superior to radiomics. In rectal cancer,on a per patient basis, pooled area under receiver operator characteristic curve was 0.017 for deep learning, 0.808 for radiomics, and 0.727 for radiologists; and sensitivity and specificity were 89% and 94% for deep learning, 78% and 73% for radiomics, and 68% and 70% for radiologists respectively[75].
CONTROVERSlES lN LN DlSSECTlON lN CRC
Several studies have shown that in more than 80% of cases, the first metastatic LN in CRC is a paracolic LN located 5 cm or less from the tumor[76-81]. Besides this classic lymphatic drainage, aberrant drainage within the regional LNs can exist. Such drainage leads directly to main LN stations near the superior and inferior mesenteric vessels or to colic and paracolic LNs located a significant distance from the tumor. The prevalence of aberrant lymphatic drainage is reportedly up to 20%[82,83]. Drainage of this nature influences the scope of lymphadenectomy since “aberrant” LNs are potential locations for“skip metastases”[76,77,84-86].
In some individual studies, a higher rate of aberrant lymphatic drainage reaching up to 29% has been observed in patients undergoing lymphatic mapping[87]. There are some different points of view on the resection type between East and West. The Japanese concept is partial resection of the bowel according feeding artery (short bowel specimen, long lymph vascular pedicle), and the opposite European concept is wide resection of the bowel such as hemicolectomy or extended hemicolectomy.
European Society of Medical Oncology (ESMO) recommends that local excision could be considered in the early colon cancer (CC) Stage 0 (Tis) and in selected T1N0M0 (G1-2, N0). ESMO and National Comprehensive Cancer Network (NCCN) recommend wide surgical resection with a safe margin(ESMO suggests at least 5 cm from the tumor), and en bloc removal of LNs with the feeding arterial arcade (regional nodes). NCCN suggests removing only suspicious LNs that are not contained in the arcade[25,26,88,89].
The best results in terms of prognosis after the introduction of the TME concept in the treatment of rectal cancer led to the Hohenbergeret al[90] hypothesis in which surgical dissection according to embryological planes could lead to a similar improvement also in surgery of the. On this hypothesis is based the complete mesocolic excision (CME) associated with the concept of central vascular ligation(CVL) in which the main blood vessels are tied at the origin after a dissection according to the embryological planes (i.e.removing the surgical trunk of Gillot in right-sided CC)[90].
JSCCR provides a detailed description of the extent of surgical lymphadenectomy, based on tumor stage. In brief, JSCCR advocates central (D3) lymphadenectomy in selected T2 and in all T3-T4 cancers,as well as in all N-positive patients.
In the study by Westet al[91] the CME with CVL does not show significant differences with the Japanese D3 as regards the quality of the mesocolon surgical plan and the free margins. In the Japanese school, as indicated in the JSCCR guidelines, the longitudinal extension is less important; consequently the number of lymph nodes and the mesenteric surface were lower. Even if the operative pieces in western countries have a greater longitudinal extension than in the Asian ones, the TNM system does not include in its nomenclature the localization of regional lymph nodes. The indications to D3 Lymphadenectomy with CVL in the Western countries are still a subject of debate.
CONTROVERSlES lN LN DlSSECTlON lN RECTAL CANCER
Rectal cancer surgery is based on[92-94] TME or tumor-specific mesorectal excision (TSME). TME is a procedure that resects all of the mesorectum just above the anal canal[92]. TSME is a procedure for partially resecting the mesorectum according to the location of the tumor[94]. In Western countries, in the rectal cancer, lateral LNM is generally considered a metastatic disease and neoadjuvant chemoradiotherapy combined with TME is widely used. By contrast, the Japanese Classification of Colorectal Carcinoma[95-98] defines lateral LNs as regional LNs in the internal iliac, obturator and common iliac subregions. The JSCCR Guidelines for the treatment of CRC[28,29] consider mesorectal excision as D1 resection and recommend a D3 procedure (TME with lateral LN dissection) as standard treatment for T3 or more middle and lower located rectal cancer.
Some studies have shown that extensive lymphadenectomy is associated with improved prognosis in patients with more advanced stage CRC, even though numerous postoperative complications related to this extensive surgery are described.
Among colorectal surgeons, it is now accepted that "one size does not fit all", and there is increasing agreement regarding the need for a more targeted surgery and a tailored lymphadenectomy[98-100].The real challenge is careful patient selection.
HYSTOPATHOLOGlCAL DlAGNOSlS OF LNMs lN CRC
A relevant clinical finding is the fact that up to 30% of patients with CRC diagnosed as pN0 following surgery will die within 5 years due to regional recurrence or distant metastases[87,101-104].
A discussion to establish criteria for defining high-risk stage II patients who could benefit from adjuvant therapy was undertaken by the Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer and National Surgical Adjuvant Breast and Bowel Project studies. Presently, the high-risk group, according to ESMO and NCCN treatment standards, comprises patients with T4 tumors (especially T4b), a high grade of histological malignancy, infiltration of vessels and perineural tissue, tumor budding (TB), a small number of removed LNs (< 12), and emergency surgery[101,105,106]. Several studies have identified prognostic genes that may select high-risk patients for adjuvant treatment[107-112], but only a few are routinely used in clinical practice.
Mismatch repair (MMR) genes act in DNA repair pathways. MMR deficiency results from the loss of function of their products (MMR-D), leading to microsatellite instability (MSI). MSI increases CRC risk by increasing tumor mutational burden and the number of tumor-infiltrating lymphocytes (TILs). There are two categories of CRC with MSI: MSI-high (MSI-H) and MSI-low (MSI-L). Instability in more than 30% of the markers as detected by PCR is defined as MSI-H, and alteration in 10%-30% of the markers is considered MSI-L. The MSI-H is associated with a high mutational burden in DNA.
Frameshift mutations can create antigenic epitopes that make MSI-H/MMR-D tumors more immunogenic compared with microsatellite-stable tumors. MSI-induced frameshift mutations produce a significant number of neoantigens. Accordingly, MSI-H/MMR-D tumors manifest a great number of TILs, many of which can be directed against tumor-related neoantigens[107].
Despite this, the most important risk factor is the presence of unidentified LN MMs and macrometastases. Rahbariet al[113] concluded that the presence of LN MMs is associated with poor OS and shorter disease-free survival (DFS) in stage II CRC patients. Therefore, the problem is to identify diagnostic methods that can improve selection based on this criterion in terms of both cost and effectiveness[101,114,115]. The relevant literature shows that examination of only one LN slide using HE staining leaves up to 33% of metastases unidentified. A single slide with HE staining through the center of a node 1 cm in diameter provides information on < 1% of its volume[114-118].
Additional HE histopathologic analyses of serial sections allows for the identification of micrometastatic disease in up to 20% of LNs determined to be negative by standard HE methods[119]. However,performing HE histopathologic analyses of sections can be technically challenging and time consuming,as well as entailing significantly greater cost. Other histopathologic methods utilized for more accurate assessment of the status of the regional LNs, such as IHC using antibodies against human cytokeratin(CK) or RT-PCR, require even more time and incur an even higher cost.
lDENTlFlCATlON OF AN OPTlMAL mRNA MARKER FOR THE OSNA ASSAY lN CRC METASTATlC NODES
Yamamotoet al[13] reported the background for the identification of CK19 mRNA as an optimal marker for the OSNA assay in CRC. Yamamotoet al[13] examined 98 candidate mRNA genetic markers, which were from a genome-wide database, by comparing an expression frequency in CC. After four sequencing phases, CK19, carcinoembryonic antigen (CEA), and CK20 mRNAs were evaluated using the OSNA assay. The expression of CK19 mRNA was observed in all pathologically positive LNs;however, CEA and CK20 mRNAs were not found in metastatic nodes.
DlAGNOSTlC PERFORMANCE OF THE OSNA ASSAY lN CRC
A novel technique for pathological examination, OSNA, uses the reverse transcription loop-mediated isothermal amplification method to amplify CK19 mRNA. In contrast to the current routine histopathological examination, it can examine whole LNs and detect metastases in a sufficiently short time(Table 1). A standard curve previously determined with three calibrators containing different CK19 mRNA copy numbers was used to calculate the amount of CK19 mRNA. Positive and negative control samples were used to ensure the quality of the assay.
A limit value of 250 copies/mL of CK19 mRNA copy had been choosed. A value less than 250 copies/mL was considered negative for metastasis, on the contrary, a value ≥ 250 copies/mL was considered positive. Previous studies defined this by the logarithmic midpoint between the maximum value of the CK19 mRNA copy number in non-metastatic patients and minus 2 or 3 standard deviations(SDs) from the average of CK19 mRNA copy number in node-positive patients. These studies also defined the MM threshold between 250 and 4999 CK19 mRNA copies/mL. LNs with 5000 or more mRNA copies/mL were considered macrometastases[7,120,121]. The utility of conventional OSNA as a molecular staging method has been demonstrated for various cancers[9,17,122-124].
Although most of the studies evaluated in this review were prospective in design, none was a randomized controlled trial (Table 1). The studies comparing the diagnostic performance between OSNA and pathological examination for the detection of LNMs in CRC are shown in Tables 2 and 3.Our review on OSNA and CRC shows high sensitivity, few false negatives results, and a concordance rate with pathological findings ranging from 61.8% to 98.7% (Table 2). Moreover, studies have shown that OSNA results in upstaging in about 25% of initially nodal-negative CRC patients after conventional HE analysis (Table 3). With the OSNA approach, the lymph node is homogenized without the need for other preparations and the results are ready in less than 40 min for 3 or 4 LNs, 20 min for a single LN.The stage of the tumor and the number of lymph nodes analyzed correlates with upstaging. Notably,the OSNA upstaging rate in Croner’s investigation for stage UICC I and II patients was 16.2% and 30.3%, respectively. Therefore, it was suggested that stage UICC I and II patients, who suffer from recurrent disease, were understaged by conventional HE analyses[9,124-126].
In a study of Yamamotoet al[13] OSNA-positive patients (2.0% of stage I CRC and 17.6% of stage II CRC) had more advanced features of CRC, such as deeper invasion to the colonic wall and severe invasion to lymphatic invasion compared with OSNA-negative cases. They found a 95% concordance rate between OSNA and classical histological analyses with HE and IHC. Yamamotoet al[13] concluded that OSNA is comparable to a 2-mm interval histopathological examination in its ability to detect LNMs.
In our previous published study[127], OSNA was superior to HE in identifying LNMs, with a false negative rate of 0%vs44.4% and accuracy of 100%vs76.4%, respectively (Table 2). As represented in Tables 2 and 3, few studies evaluated HE and IHC, few performed multi-sliced tissue sections using HE and the remaining single slice HE tissue sectionvsOSNA[11,128-131]. While the detection of small metastatic foci in LNs is influenced by the skill and experience of the pathologists, the advantage of the OSNA assay is the possibility to perform standard evaluations without being influenced by operator skill or experience. This explains the reason why the use of OSNA has recently garnered interest for detecting MMs[9,16,17,128].
Methods of LN division and pooled OSNA
Previous reports have detailed three major methods to compare LN status between pathological examination and the OSNA assay (Table 3). The first method[14,125] involves dividing LNs in half and sending each 50% portion for pathology and OSNA (half-division method). The second method[11,12,15] involves dividing LNs into four equal sections and sending two of these sections (50%) for pathology and OSNA (four-section method). In the third method[14,124], only 1 mm from the center of LNs are sent for pathological examination and the rest are used for OSNA measurement (center-cut method). The latter two methods described above are thought to be technically difficult for evaluating small LNs. By contrast, dividing in half and sending each 50% portion for pathology and OSNA is the simplest method.
In previous studies using classic OSNA (cOSNA), 50% of each LN was submitted for pathologic examination, followed by evaluation of each remaining half by OSNA. The obstacles for clinical applications of cOSNA include a need to simplify the procedure for halving the dissected LNs and reducing the operating costs associated with the equipment used for OSNA analyses.
Rakislovaet al[125] conducted a study comparing two methods of LN evaluation by OSNA in CRC:an individual analysis of each LN (cOSNA) and a new approach involving pooling several LNs, known as the "pooling method". The diagnostic performance of pooled OSNA (pOSNA) was comparable to that of cOSNA. In the pOSNA method, the LNs are pooled together in a test tube for OSNA analysis. The weight limitation for the LNs per tube was ≤ 600 mg, with those exceeding this limit placed in anothertube for measurement. In the study by Taniet al[33], pOSNA and the half-division method were combined and used in the diagnosis of pericolic LNMs and applied in clinical practice[33]. These results revealed that the pOSNA with the half-division method might be useful as a clinical molecular staging method.
In this study, the upstaging rate for early-stage CC patients was 9.1% (6/66). The upstaging rates of the study by Taniet al[33] were slightly lower than those previously reported (Table 3).
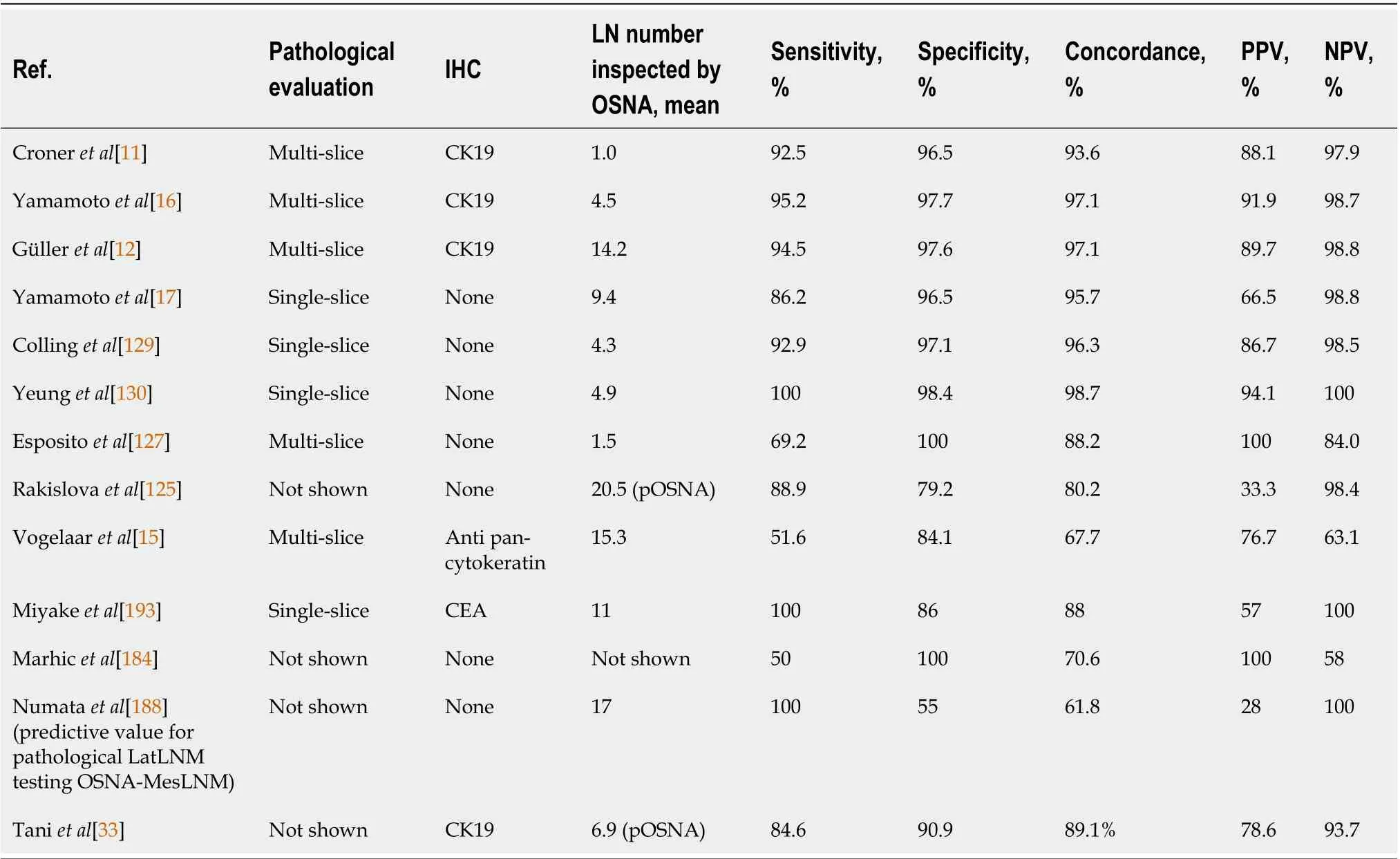
Table 2 A comparison of the diagnostic accuracy of the one-step nucleic acid amplification assay in colorectal cancer patients
COMPARlSON OF THE NUMBER OF POSlTlVE NODES AND QUANTlTATlVE OSNA RESULTS
The OSNA assay of retrieved LNs does not allow the number of involved LNs typically used for TNM staging, and therefore cannot be used for conventional cancer staging. Nevertheless, the OSNA assay can potentially be used to infer the size of metastatic foci based on the detected copy numbers[113,132].Patient’s total tumor load (TTL) resulted from the sum of all CK19 mRNA tumor copies/μL of each positive LN from the colectomy specimen. Yamamotoet al[17] found that the sum of CK19 mRNA increased as the number of histologically positive LNs increased. Indeed, the median value of CK19 mRNA was significantly smaller in patients with < 3 regional LNMs than in those with ≥ 4 regional LNMs. The median TTL values of pN0, pN1 (1-3 positive LNs), and pN2 (4 or more positive LNs) were 1550 copies/mL (300-320000 copies/mL), 24050 copies/mL (250-890000 copies/mL), and 90600 copies/mL (7700-1635100 copies/mL), respectively. The TTL significantly increased as the node status increased.
In the study of Aldecoaet al[133] the TTL was related to pT stage (P= 0.01) and tumor size (P< 0.01)in low-grade tumors. In that study TTL, correlated with classical high-risk factors in stage I-II CC patients. These findings indicate that the sum of CK19 mRNA assessed by OSNA displays a trend compatible to the current pathological diagnosis system. These findings suggest the future possibility of novel molecular staging using OSNA, based on metastasis volume (amount of CK19 mRNA) rather than the number of LNMs. It has been suggested a correlation between CRC risk factors[11,12,16,18,122,126,134] such as pN, pT, tumor grade, male sex, tumor size, lymph vascular invasion (LVI), poor prognosis,worse DFS and TTL.
Correlation of node TTL with TB and poorly differentiated clusters
The physiological process that can lead epithelial cells to acquire mesenchymal properties and the potential for migration and stromal invasion, essential for the development of metastases, is the morphological manifestation of the epithelial-mesenchymal transition phenotype that can lead to the formation of TB and clusters poorly differentiated (PDCs). The presence of isolated tumor cells or cell clusters of ≤ 4 cells on the invasive front of the tumor is termed tuberculosis[134]. 5 or more neoplastic cells in the tumor stroma not organized into glandular structures constitute the PDCs. In stage II CC,both PDCs and TB are independent prognostic factors[134-136], associated with LNMs, distantmetastases, extramural vascular invasion, LVI, perineural invasion (PNI), tumor grade and high pT stage[136-142].
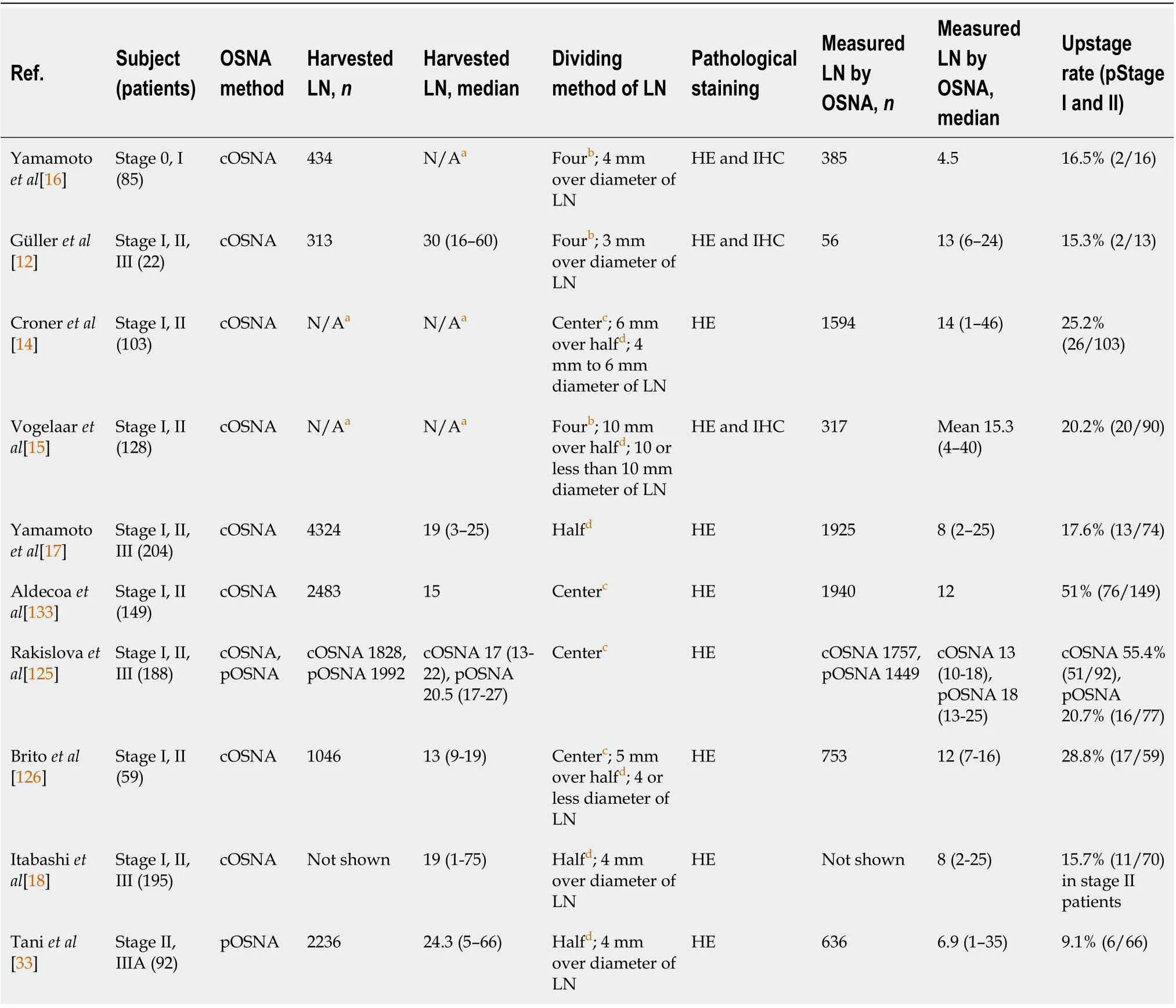
Table 3 Differences in lymph node processing methods and upstage rates of previous reports
International Consortium on TB Recommendations indicates how to count the number of TB at the invasive front of the tumor[135]. The classification for TB was as follows: Bd1/low (≤ 4 buds),Bd2/intermediate (5-9 buds), and Bd3/high (10 one more buds). The classification for PDCs evaluated[143] at the invasive front or in the center of the tumor was as follows: G1 (≤ 4 clusters), G2 (5-9 clusters), and G3 (10 or more clusters). Barresiet al[144] suggested not classifying tumor cells within mucin pools in mucinous carcinomas as TB, considering only tumor cells infiltrating the stroma with minimal extracellular mucin. While, the PDCs were evaluated within mucin lakes.
Recently Archillaet al[145] suggested the correlation of the TTL with patient outcome, TB, and PDC.The use of molecular methods to assess LN status, together with other pathological risk factors, could help improve risk stratification and management of patients with early-stage CRC.
Indeed, OSNA positivity was found in 38.3% of the cases (131/342) with a mean TTL of 36662 copies/mL among positive cases. The TTL present in the LNs evaluated by the OSNA test correlated positively, with both PDC (r = 0.266 by IHC; r = 0.257 by HE) and TB (r = 0.249 by IHC; r = 0.243 by HE)(P = 0.001). Low and intermediate TB had similar mean TTL (Bd1: 3292 copies/mL and Bd2: 18002 copies/mL), with no significant differences between both groups (P= 0.154). The mean TTL of high-Bd3 TB was 45331 copies/mL, and. it was significantly different from Bd1 and Bd2. Likewise, the mean TTL of PDC G1, with 4962 copies/mL and G2, with 13146 copies/mL did not show significant differences (P= 0.068), while PDC G3 had 61108 copies/mL, significantly different than low and intermediate grades.Thus, the authors grouped low and intermediate grades of TB and PDC into one category, obtaining two groups with significant differences for both TB and PDC (P< 0.001) as well. The authors also concluded that TTL can be used as an alternative method to to better stage patients compared to the classic HE because it is able to identify real stage II or III patients, thereby selecting those who are candidates for adjuvant therapy[145].
SURVlVAL ANALYSES
In the meta-analysis of Wildet al[146] it is emphasized that long-term outcomes and the use of adjuvant therapy in those upstaged by OSNA should be clarified before routine use of OSNA test.
Itabashiet al[18] showed that pStage II patients with OSNA positive LN had a lower 3-year DFS than negative patients (55%vs86%; P = 0.005), with no significant differences in 3-year OS (P= 0.914). In this study, the upstaging occurred for patients with pStage II, of whom 11 of 70 patients (15.7%) were OSNA-positive. Most of the OSNA positive LNs were located in the peri-colic or peri-rectal area (10 out of 11 OSNA-positive stage II CRC patients).
Weixleret al[147] showed that the detection of positive LN by HE staining but not by OSNA as significant predictors of cancer-specific survival, cancer-specific and recurrence-free survival, and DFS.He concluded that in patients with CC, OSNA offers no prognostic advantage compared to conventional LN staging with HE contrasting findings in other cancers. It is important to highlight that the methodology of the histopathological evaluation for detection of the LNMs differed among studies. In Weixler’s multicenter study[147] all harvested LNs > 3 mm in greatest dimension or a short axis ≥ 10 mm was cut into four slices: two were stored for later OSNA analysis and two were allocated to conventional standard HE staining, multilevel HE staining, and IHC for CK19. Multilevel sectioning with IHC leads to relevant upstaging of 15.4%-26% of otherwise negatively classified patients[148,149]. In addition, stage I-III patients were included in this study, whereas most of the OSNA studies focused on stage I-II patients. Therefore this HE + IHCvsOSNA study including stage I-III patients, although well conducted and of great value and interest, is not amenable to a comparison with studies in which HEvsOSNA in stage I-II patients are evaluated.
ORGAN-PRESERVlNG SURGERY
CC
Early CRC can be treated by endoscopic mucosal resection or endoscopic submucosal dissection (ESD).In determining the indication for endoscopic treatment and the treatment method, information on the depth of invasion and morphology of the tumor is essential. Colorectal ESD is an “endoscopic resection technique, which enables en bloc resection of a tumor, regardless of size” and avoids piecemeal resection. It is of great importance to differentiate Tis and T1a cancers from T1b cancers (T1 cancer with≥ 1000 μm submucosal invasion depth), as the former can be treated by endoscopy while the latter requires surgical operation with nodal status assessment[29,150-154]. Moreover, innovative organpreserving procedures such as endoscopic full-thickness resection or transanal minimally invasive surgery have been proposed to perform high-quality resections with decreased incidence of specimen fragmentation without resorting to demolitive interventions[155]. Nevertheless, it is estimated that overall 10%-20% of patients in stage T1 will have LNMs, and such patients subjected to a localized resection are undertreated[151].
Laparoscopic endoscopic cooperative surgery (LECS) can also lead to full thickness local resection by means of combined use of laparoscope and endoscope. The development of modified LECS procedures,such as non-exposed endoscopic wall-inversion surgery and closed LECS has almost resolved these drawbacks. This has led to a recent increase in the indication of modified LECS to include patients with gastric epithelial neoplasms. The LECS concept is also beginning to be applied in other organs such as the duodenum, colon and rectum. Further evolution of LECS procedures is expected in the future. SLN mapping could also be combined with LECS, resulting in a portion of early gastrointestinal cancers being treated by LECS with SLN mapping[156].
Rectal cancer
TEM, first described by Gerhard Buess[157-161], due to its ability to perform high-quality resections with decreased incidence of fragmentation, is superior to standard transanal excision for treating benign and malignant rectal lesions, most notably[162,163].
Transanal minimally invasive surgery (TAMIS) was initially born as a fusion between trans anal endoscopic microsurgery (TEM) and single-site laparoscopy. This technique was designed as a costeffective and easily reproducible alternative to TEM without specialized equipment.
The indications for TAMIS are similar to standard transanal resection for benign and malignant lesions determined by EUS or MRI[164,165]. TAMIS is also indicated for early malignant neoplasms confined to the submucosa[166]. T1 neoplasms of the rectum can still be divided into low-risk lesions[167] and at high risk for poor histopathological features (TB, poor differentiation, LVI or perineural invasion). Studies[155,168-170] identified a higher risk of LN metastasis in T1 sessile tumors with deeper submucosal invasion (sm2or sm3).
THE OSNA ASSAY FOR THE DETECTlON OF CRC METASTASlS lN SLN
LN status plays a crucial role in oncologic therapeutic strategies, and despite the use of increasingly sophisticated imaging techniques, pre-operative metastatic LN identification in patients with CRC is unsatisfactory[171,172].
The study of the SLN is gaining more and more popularity because it can avoid extensive lymphadenectomies, reduce operating times and morbidity. This can change the surgical strategy in patients with an apparent early stage of CRC, as patients with intraoperative positive SLN are submitted, during the same surgical procedure, to an adequate lymphadenectomy, whereas those with negative SLN can be treated with an organ-preserving surgery, avoiding unnecessary lymphadenectomy. The extemporaneous intraoperative examination performed on frozen specimen has a lower sensitivity than the classic postoperative analysis. The problem is mainly due to the low detection rate of MMs and ITC[173,174]. The disease detection rate increased with the technique of multi-step formalinfixed tissue sections (FFTS) stained by HE with or without IHC[6,175-179]. Nonetheless, a significant number of MMs can still be overlooked, as during histological workup usually only small parts of LNs are screened.
Therefore, there are two methods for SLN mapping: staining and radioguided[180]. Three tracers have been used to detect SLNs: Dye, radioisotope, and indocyanine green (ICG). Each tracer has its respective disadvantages. The use of the ICG fluorescence method has officially been approved in Japan for LNs of BC and malignant melanoma; thus, it appears that ICG can be an acceptable tracer for the detection of LNs in gastric and CC.
ICG tattooing method is very useful for the marking of early gastric and CCs, especially when using a laparoscopic approach[181]. It has been suggested that SLN mapping with fluorescent dye can play an important role in the treatment of CC, particularly those at early stages, and can lead to ultraconservative surgery[182]. Because the results of OSNA are available in a relatively short time compared to the conventional technique, intraoperative OSNA analysis of SLNs may be employed easily in clinical practice.
In the study by Vogelaaret al[15] OSNA proved to be a promising method for the detection of SLN metastases in CC patients afterex vivoSLN mapping. OSNA appeared to outperform routine pathological examination with HE-stained slides with an upstaging rate of 20.2%. In the study by Yeunget al[130] OSNA was used intraoperatively, together with the technique of retrieving colorectal LNs by fluorescence imaging, to analyze the status of these specific LNs. In this study, OSNA is highly concordant with standard histology.
The results of the meta-analysis by Tranouliset al[183] indicate that the the use of OSNA can allow to identify the status of the LNs even when applied intraoperatively. Marhicet al[184] proposed that the OSNA technique may be a new method to reduce time to adjuvant chemotherapy after surgery for CC.In this study, SLN status was determined intraoperatively with the OSNA assay; when positive, a porta-cath was placed during the procedure for upcoming adjuvant chemotherapy. In this study, there was no difference between the groups regarding cancer staging, duration of hospitalization, and major morbidities but the time interval between surgery and adjuvant chemotherapy was significantly shorter in the OSNA group at 35 ± 8 dvs67 ± 36 d (P= 0.021).
In our previous study[127], we showed that SLN analysis with OSNA in combination with ICG-near infrared (NIR) lymphangiography is feasible and may allow intraoperative prediction of LN status in patients with CRC (Table 4). Patients with SLN positive by the OSNA method were considered pNpositive and subjected to adjuvant chemotherapy. The time to start chemotherapy was lower in OSNA(+) patients [39.1 ± 1.9 dvs50.2 ± 4.1 d in the OSNA (−) group;P= 0.01].
Bothex vivoandin vivoICG fluorescence imaging are feasible for the detection of SLNs in CRC. The submucosal injection technique and subserosal were both used. A NIR 30° laparoscope (Olympus,Tokyo, Japan) was used to inspect the mesocolon. The LNs found using fluorescence were considered SLNs and analyzed intraoperatively. More work needs to be done to define protocols, indications for its use, a standard number of LNs that need to be removed and to test its efficacy in larger patient populations.
Implementation SLN analysis with OSNA in combination with ICG-NIR lymphangiography could allow more precise staging, reducing the delay between surgery and the onset of adjuvant chemotherapy. SLN evaluation by intraoperative OSNA analysis combined with a LECS approach may allow, in case of OSNA-negative early CRC, to apply an organ-preserving surgery avoiding the complic-ations related to an unnecessary lymphadenectomy.

Table 4 Studies analyzing colorectal cancer metastasis in sentinel lymph nodes with one-step nucleic acid amplification
By contrast, in intraoperative OSNA-positive early CRC, a colorectal resection associated with selective lymphadenectomy can be performed, also taking into consideration the presence of aberrant lymphatic drainage and skip metastases. Despite the attractiveness of the previously exposed concept,studies in this field are lacking or very few (Table 4). However, several studies underline the role of OSNA SLN evaluation in more advanced stages and in case of positivity, the consequent upstaging and access to adjuvant treatment (Table 4).
LATERAL PELVlC LNs AND OSNA
There is disagreement in the international literature regarding the use of prophylactic lymphadenectomy in comparison with preoperative radiochemotherapy to improve prognosis in patients with locally advanced rectal cancer, due in part to the complex anatomy of the pelvic floor which makes diagnosis of lateral pelvic LNs (LPLNs) metastasis difficult. In Japan, the evolution in the surgical oncology approach has been toward LN clearance and, as a result, LPLNs have been considered localregional disease from the outset[185].
Nevertheless, the rate of pathological lateral (Lat) LNM (p-LatLNM) in patients without clinical LatLNM (c-LatLNM) remained low at 7%, and lateral LN dissection (LatLND) is generally considered technically demanding and can prolong the operative time[186-188]. As Sammouret al[189] proposed,patients who are candidates for curative-intent treatment should be stratified depending on their risk to have LPLN metastasis in high, moderate, and low risk in order to select the best option to manage the pelvic compartment. Nevertheless, with preoperative images or traditional criteria, it is difficult to predict LatLNM. Obtaining a preoperative or intraoperative diagnosis is essential to select patients with LatLNM.
If SLN navigation surgery could be applied in cases of middle and lower rectal cancer, unnecessary LatLND procedures could be avoided. SLN anlysis may be useful in deciding both the indication of LatLND and which side of the lateral pelvic wall should be dissected[190]. In the study of Nouraet al[191] the existence of a lateral pelvic region SLN in 53 lower rectal cancer patients was investigated. The lateral pelvic region was observed using a NIR camera system (photodynamic eye) after the ICG has been injected into the submucosa along the dentate line. If SLNs were positive for metastasis a Bilateral LatLND was performed, if instead SLNs were negative for metastasis mesorectal excision only was performed. In 49 (92.5%) of the 53 patients the lateral SLNs were successfully identified, 4 of these patients (8.2%) had lymph node metastases; the mean number of lateral SLNs per patient was 2.0 (range,1-4).
The results of Yasuiet al[192] suggested the potential use of SLN with ICG strategy to identify cases with non-metastatic LPLN, and to omit LatLND in such cases, and thereby avoid both LatLND-related surgical complications and radiation-induced adverse events. Moreover, the author suggested that further studies are needed to shorten the required time and improve the accuracy of SLN biopsy by the intraoperative rapid diagnosis with different methods such as molecular biological diagnosis.
Miyakeet al[193] attempted to perform an intraoperative OSNA assay to detect perirectal LNMs to predict LPLN metastasis in rectal cancer patients undergoing surgical resection plus LatLND. In their study, LPLN metastases were present in 16% of patients (4/25), and all of these patients were positive on an OSNA for perirectal LNMs. The sensitivity of OSNA was 100%, specificity 86%, positive predictive value 57%, and negative predictive value 100% for predicting LPLN metastasis, and the authors concluded that the OSNA of perirectal LNs might be useful for selecting candidates for omission of LatLND in rectal cancer surgery. OSNA can be associated with SLN to intraoperatively identify foci of metastasis in LPLNs.
With respect to risk factors for p-LatLNM, three previous studies reported that pathological mesorectal LN (MesLN) metastasis (p-MesLNM) is a consistent risk factor for p-LatLNM[194-196].Furthermore, previous studies have shown that p-LatLNM rarely occurs without p-MesLNM[16,197].Negative OSNA diagnosis for mesorectal LNM (MesLNM) (OSNA-MesLNM) is highly correlated with negative p-LatLNM; hence, negative confirmation of OSNA-MesLNM may be useful in selecting patients in whom LatLND can be omitted[189].
In conclusion, the role of LatLND is still under discussion. Nevertheless, it has been suggested a selective and mono- or bilateral LatLND in advanced low and middle rectal cancer, based on OSNA positive mesorectal nodes or OSNA-positive ICG-stained sentinel LPLNs.
COSTS
A disadvantage of cOSNA is that it is more costly than pathologic examination. Depending on the number of samples analyzed in each lot, there is a variation in the cost of consumables and additional reagents. For example, a 12 LN analysis would have an indicative cost of single use products is £ 550-£590 per patient [excluding value added tax (VAT)]. If consumables are maximized and samples from more than 1 patient are tested in one batch, the cost could be as high as £ 33.50 per LN. The annual maintenance contract for the system (which would apply from the 2ndyear after installation) is priced at£ 6628.48 (excluding VAT) with a 12 mo warranty.
The lifespan of the OSNA system declared by the manufacturer is at least 6 years. The duration of the analysis is approximately 90 min for 12 LNs, therefore it is possible to analyze samples of approximately 5 patients per day (7.5 h), and 1200 in 1 year (considering 240 annual working days). Average cost per patient (including capital, maintenance and disposable costs) ranges from £ 568 to £ 608, using the standard annuity method with a 3.5% discount rate[198]. Nonetheless, the OSNA use may reduce the reinterventions and allow earlier commencement of adjuvant treatment. The financial implications of OSNA have been previously investigated in BC, with an estimated saving between 400 and 700 £ per patient[199,200].
With pOSNA, only one measurement is required, at a cost of $ 225 (¥ 24000). In contrast, cOSNA requires three or more measurements, at a cost of $ 670 (¥ 72000) or more. In pOSNA, multiple LNs can be measured in one tube, taking into consideration the upper limit of the LN weight that can be assayed in one tube by the OSNA method is 600 mg. However, in cOSNA, only one LN can be measured in a single tube (max 50 mg). Using cOSNA to measure 12 or more halved LNs would require at least 12 tubes and three or more measurements per tube, whereas if 12 pericolic LNs are measured, pOSNA would require only one or two tubes for each case. The OSNA measuring device can measure up to four tubes at once; however, because pOSNA requires only one or two measurements per case, this allows the device to take measurements for two cases simultaneously depending on the number and weight of the removed LNs.
Diaz-Mercedeset al[109] analyzed the budget impact of introducing an OSNA assay in early-stage CRC patients and suggested that OSNA might have not only an economic benefit but also a clinical benefit in CRC patients, since it enabled more accurate staging, thereby avoiding unnecessary treatment.The results of Diaz-Mercedeset al[109] indicate that the Spanish National Health System would have saved over € 19 million from 2017 to 2019 if OSNA had been introduced in clinical practice for surgically treated CRC patients. In this study, HE-positive patients and OSNA-positive patients, both underwent adjuvant therapy. Savings are explained by the fact that OSNA ensures a more accurate diagnosis in CRC patients, allowing a reduction in treatment costs after initial surgery, as well as costs of adjuvant treatments and surgery after recurrence, compared with HE techniques. Although patients’ LN staging is more expensive with OSNA than with HE, savings regarding treatment costs after surgery and treatment costs due to recurrence are high enough to justify the investment in OSNA analysis.
Although the costs of OSNA are high, the speed, simplicity, and reproducibility could allow a reduction in the hours of work of individual pathologists. Furthermore, two cases can be studied during a single procedure using the pOSNA method. Adding, as demonstrated by Diaz-Mercedeset al[109], the reduction in treatment costs after surgery and the reduction in costs relating to the treatment of recurrences, this method could be also attractive for developing countries.
ADVANTAGES AND DlSADVANTAGES
Advantages
The innovative aspects of OSNA are that unlike standard histopathology, OSNA can analyze the whole LN as well as partial LNs. This may improve cancer staging accuracy. It can detect metastatic foci regardless of their size or location. It is seemingly superior to conventional FFTS in detecting MMs and ITC, as it can identify metastatic foci as small as 0.35 mm[201]. Yamamotoet al[17] found that the sum of CK19 mRNA increased as the number of histologically positive LNs increased. Indeed, the median value of CK19 mRNA was significantly smaller in patients with < 3 regional LNMs than in those with ≥4 regional LNMs. In the study of Aldecoaet al[133], the TTL was related to pT stage (P= 0.01) and tumor size (P< 0.01) in low-grade tumors. In this study, TTL correlates with classical high-risk factors in stage I-II CC patients. These findings indicate that the sum of CK19 mRNA assessed by OSNA displays a trend compatible to the current pathological diagnosis system. These findings suggest the future possibility of novel molecular staging using OSNA, based on metastasis volume (amount of CK19 mRNA) rather than the number of LNMs.
Archillaet al[145] have suggested the correlation of TTL with TB and PDCs. TTL could be used as a new prognostic factor in CRC as it is related to the outcome and. The combination of the TTL as a new prognostic factor, TB and PDC, could help to better stratify and manage patients with early-stage CRC at risk of recurrence.
The application of OSNA in addition to the current standard pathology would likely provide an additional risk factor for disease recurrence regarding patients with stage II CRC. LNM is a reliable prognostic marker of CRC; it is used as the ‘‘gold standard’’ for post adjuvant chemotherapy after curative surgery. One may question whether OSNA-positive CRC patients should receive post-adjuvant chemotherapy after curative surgery. Further clinical trials are needed to determine if adjuvant therapy is beneficial in this upstaged group. On the other hand, the short turnaround time renders OSNA an attractive intra-operative method. Based upon the BC accumulated experience, the turnaround time is less than 40 min for one LN, whereas it ranges from 50 to 62 min for assessing four LNs[7]. The OSNA rapid turnover time may potentially be useful in circumventing the major issues associated with SLN biopsy. Moreover, OSNA is automated and the results are quantifiable; hence, easily reproducible, less operator-dependent with short learning curve[201-203]. Therefore, implementation of OSNA in routine clinical practice may ease the burden on pathologists.
Finally, it is laborious and rather expensive to perform molecular tests. Nonetheless, the OSNA use may reduce the re-interventions and could allow earlier commencement of adjuvant treatment. The financial implications of OSNA have been previously investigated in BC[199,200]. The results showed that pOSNA can simplify the process of cutting harvested LNs in half while reducing the equipmentrelated costs associated with OSNA assays used in clinical practice. Additionally, pOSNA demonstrated an upstaging rate for pNNCC equivalent to that reported in previous studies, suggesting its feasibility for molecular staging in clinical practice. With pOSNA, the possibility of fewer measurements per patient and of studying more cases simultaneously with the same panel further reduces costs[33,125]. In an era of stringent economics, health systems should undergo cost-effectiveness analyses upon which a progressive integration of OSNA in their daily clinical practice could be based on[109,200].
Limitations
The few OSNA limitations should be acknowledged. OSNA can be used to analyze LNs more than 50 mg; if the LN diameter is inferior to 3-4 mm, it cannot be divided and analyzed with OSNA and conventional histology (Table 3). The examination of the whole LN by OSNA inevitably precludes FFTS examination. One limitation of all OSNA studies performed in CRC, is that the analysis has been performed using only a part of the LNs, while using the rest of the LN tissue for conventional histological analysis and pN staging[145].
The technique of nodal division must be taken into account when evaluating the results because it can vary widely between different studies. Discordant results between OSNA and FFTS were reported. The three main reasons for these discrepancies are: tissue allocation bias, low CK19 expression, and tissue contamination. Moreover, discordances may also arise from the presence of metastases from primary tumors that do not express CK19[147]. The latter is considered an important limitation of OSNA. The accuracy of OSNA is seemingly higher amongst CK19 positive primary tumors by IHC compared to those with CK19-negative primary tumors[183]. As such, the positive CK19 IHC in primary tumors has been proposed as a prerequisite for the OSNA use by some authors[183]. An additional challenge is the fact that 36% and 49% of CK19-negative primary tumors have CK19-positive LNs[183]. Peignéet al[203]also reported that CK19 mRNA can also be detected by OSNA even in cases with CK19-negative primary lesion.
The tendency toward a loss of CK19 expression in poorly differentiated cancers may represent a challenge for assays using CK19 IHC or PCR for detecting MMs. It is of note that upregulation of CK19 in tumors derived from cells that are CK19-negative can also be linked to unfavorable tumor features.CK19 is highly expressed in positive LNs from BC patients even when its expression is not observed in primary tumors. Targeted studies on the upregulation of CK19 mRNA in LNM of CRC are needed[13].
In light of the potential for false-negative results, the incorporation of additional markers would be a possible direction in improving the diagnostic performance of OSNA. The cut-off point of 250 copies/mL is established in BC and seemingly sensitive in CRC while the optimal diagnostic cut-off point is a matter of debate[184]. The aforementioned pitfalls remain to date a field of contention necessitating a shift in the focus of future research into incorporation of novel biomarkers and evaluation of the optimal diagnostic cut-off point.
At this time there is no exact definition of true LN positives or negatives, cancer-related relapse and death were used as real positive LN indicators, disease-free survival as negative real LN[147]. The falsenegative rate of pOSNA is a point to be considered when applying OSNA in clinical practice (Table 2).However, Aldecoaet al[133] observed that high-grade (G3) tumors or tumors with vascular invasion presented lower levels of TTL making it not a reliable prognostic tool for these specific pathologic features.
Finally, in this review, we found few reports dealing with CRC and SLN evaluation and there is obviously the need for future research in this field.
CONCLUSlON
OSNA analysis is potentially more accurate than conventional pathologic methods for identifying metastasis because it solubilizes the entire LN and analyzes CK19 mRNA levels in the resulting sample.The advantages of OSNA include a short analysis time of approximately 30-40 min from start to completion, and the ability to automate the OSNA assay eliminates interlaboratory differences based on pathologist skill and experience. The short turnaround time renders OSNA an attractive intra-operative method. Patients with pN0 OSNA-positive CRC might also need chemotherapy after curative surgery.To achieve this goal, it needs several studies to compare the recurrence rate between the groups of no treatment or adjuvant chemotherapy after surgery both in OSNA-positive pStage II CRC patients. The result would clarify whether adjuvant chemotherapy is beneficial to patients with OSNA-positive pStage II CRC. Anyway, it can be suggested that OSNA may be considered as the route to tailormade surgery.
ACKNOWLEDGEMENTS
We thank the members of the Department of Surgery at San Giuseppe Moscati Hospital for carefully reading and examining the manuscript.
FOOTNOTES
Author contributions:Crafa F wrote and edited the manuscript and collected the clinical data; Vanella S reviewed the discussion section of the manuscript; Baiamonte M, Catalano OA and Pomykala KL revised the manuscript and provided recommendations for the clinical diagnosis paragraph.
Conflict-of-interest statement:Dr Crafa has nothing to disclose.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Italy
ORClD number:Francesco Crafa 0000-0002-2038-625X; Serafino Vanella 0000-0002-6599-8225; Onofrio A Catalano 0000-0001-7733-4138; Kelsey L Pomykala 0000-0001-8922-9597; Mario Baiamonte 0000-0001-8323-8118.
S-Editor:Zhang H
L-Editor:A
P-Editor:Zhang H
 World Journal of Gastroenterology2022年30期
World Journal of Gastroenterology2022年30期
- World Journal of Gastroenterology的其它文章
- Current perspectives on the role of liver transplantation for Langerhans cell histiocytosis: A narrative review
- Gut microbiota, inflammatory bowel disease and colorectal cancer
- Thrombocytopenia in chronic liver disease: Physiopathology and new therapeutic strategies before invasive procedures
- P2X7 receptor blockade decreases inflammation, apoptosis, and enteric neuron loss during Clostridioides difficile toxin A-induced ileitis in mice
- Serological profiling of Crohn’s disease and ulcerative colitis patients reveals anti-microbial antibody signatures
- Trends in medication use and treatment patterns in Chinese patients with inflammatory bowel disease
