P2X7 receptor blockade decreases inflammation, apoptosis, and enteric neuron loss during Clostridioides difficile toxin A-induced ileitis in mice
Ana A Q A Santos, Deiziane v S Costa, Danielle A Foschetti, Antoniella S G Duarte, Conceicao S Martins,Pedro M G Soares,Patricia Castelucci,Gerly A C Brito
Abstract
Key Words: Clostridioides difficile; Clostridioides difficile toxin A; P2X7 receptor; Enteric nervous system;Enteric neuron; Enteric glia
lNTRODUCTlON
Clostridioides difficile(C. difficile) continues to be the leading cause of nosocomial diarrhea worldwide[1].TcdA, TcdB, andC. difficilebinary toxin are the main virulence factors ofC. difficileinfection-related intestinal damage. These toxins have been shown to play an important role in secretory diarrhea and inflammation during the infection[2,3]. The clinical disease ranges from mild diarrhea to toxic megacolon, colonic perforation, and death.
Intestinal dysfunction has been identified in patients after the acute phase ofC. difficileinfection[4-7].Growing evidence suggests that the enteric nervous system (ENS) plays an important role in the regulation of intestinal inflammation. Alterations in the ENS components, including enteric neurons and glia, contribute to the amplification of inflammatory immune response and intestinal dysfunction under inflammatory conditions.
The P2X7 receptor is a low-sensitivity adenosine triphosphate (ATP)-gated cation channel expressed by several cell types, such as macrophages[8], EGCs[9], and enteric neurons[10]. Once activated, the P2X7 receptor increases the intracellular Ca2+concentrations, which in turn promote the release of proinflammatory cytokines and neuromodulators[11,12]. Additionally, high levels of the P2X7 receptor have been reported in enteric neurons during colitis induced by dinitrobenzene sulfonic acid[13] and intestinal ischemia[10].
TcdA and TcdB have been shown to excite enteric neurons, stimulating the release of substance P and vasoactive intestinal peptideviathe inhibition of noradrenergic transmission and the interleukin (IL)-1β pathway, respectively, resulting in neutrophil recruitment and secretory diarrhea[14-16]. However,there is a knowledge gap regarding the population of enteric neurons affected by TcdA and the role of the P2X7 receptor in TcdA-induced alterations in enteric neurons and enteric glial cell (EGC)-derived mediators, particularly S100B.
In the present study, we characterized the population of myenteric neurons affected by TcdA during ileitis in mice. In addition, we investigated the role of the P2X7 receptor in ileal damage, inflammation,and enteric glial and neuronal changes in TcdA-induced ileitis in mice. Our hypothesis was that TcdA affects specific types of neurons and induces reactive gliosis and that activation of P2RX7 is involved not only in ileal damage and inflammation but also in the activation of enteric glia and neuronal loss induced by this toxin.
MATERlALS AND METHODS
Animals
Swiss mice (8-week-old) were provided by the central vivarium of the Federal University of Ceara. All mice were maintained under standard conditions at 24 °C at a 12-h light-dark cycle, and all groups were provided water and foodad libitum. All mouse procedures were conducted according to current regulations regarding animal experiments approved by the local Animals Care and Use Committee(protocol no. 7028200418).
Mouse ileal loop model
A mouse model of TcdA-induced ileitis was established as described previously with[17] some modifications. Swiss mice (n= 5pergroup) were fasted for 4 h with free access to water and deeply anesthetized with an intraperitoneal injection of ketamine (80 mg/kg) and xylazine (10 mg/kg). After a midline laparotomy, a single 4-cm ileal loop was ligated and injected with 50 μg of TcdA in 100 μL of phosphatebuffered saline (PBS). The control loops were injected with 100 μL of PBS alone. After 4 h, the mice were euthanized, and the ileal loops were removed for subsequent analysis. Alternatively, some mice were injected with Brilliant Blue G (BBG, Sigma-Aldrich, 50 mg/kg, i.p.)[10], a nonspecific P2X7 receptor antagonist, or with A438079 (Abcam, 10 μM/200 μL, i.p.), a competitive P2X7 receptor antagonist[18],one hour prior to PBS or TcdA (50 μg) injection in the ileal loops. The experimental groups were as follows: Control (loops were injected with 100 μL of PBS alone), TcdA (loops were injected with 50 μg of TcdA in 100 μL of PBS), BBG (injected with BBG one hour prior to the injection of 100 μL of PBS in the loop), A438079 (injected with A438079 one hour prior to the injection of 100 μL of PBS in the loop), TcdA+ BBG (injected with BBG one hour prior to the injection of 50 μg of TcdA in 100 μL of PBS), and A438079 (injected with A438079 one hour prior to the injection of 50 μg of TcdA in 100 μL of PBS).
TcdA was provided by Prof. Carlos Quesada from the University of Costa Rica. BBG was kindly provided by Dr. Patricia Castelucci from the University of São Paulo. A43807 was kindly provided by Dr. Henning Ulrich from the University of São Paulo.
Analysis of histological damage in the ileum
The ileal samples were fixed in 10% formalin solution for 20 h and processed by the NEMPI-UFC Research Histology Core. The severity of ileal damage was measured by a blinded expert in histopathology based on a scoring system ranging from 0 to 3 as described previously with some modifications as follows: (0) Absence of alterations; (1) Mild loss of the integrity of the villi, mild edema,and neutrophil infiltration; (2) Partial loss of the villi, moderate edema, and neutrophil infiltration; and(3) Complete loss of the villi, extensive edema, and intense neutrophil infiltration[19].
Analysis of enteric neuron population
Fresh ileal samples were flushed with PBS, dissected, and opened along the mesenteric border. Then,the samples were fixed in 4% paraformaldehyde (in 0.2 M sodium phosphate buffer, pH 7.4) overnight at 4 °C. Then, the samples were washed three times with 100% dimethyl sulfoxide for 10 min, followed by three washes with PBS for 10 min each. All samples were stored at 4 °C in PBS containing 0.1%sodium azide. The fixed tissues were dissected to remove the mucosa, submucosa, and circular layers,yielding longitudinal muscle-myenteric plexus whole mounts as described previously[10].

Table 1 Primary and secondary antibodies used
Whole-mount preparations of the ileal myenteric samples were preincubated in 10% horse serum in PBS containing 1.5% Triton X-100 for 45 min at room temperature to reduce nonspecific binding and permeabilize the tissue. The antibodies used in the present study are described in Table 1. Double labeling was achieved using the combinations of primary antibodies (Table 1) overnight at 4 °C. Then,the samples were washed (with PBS three times for 10 min each) and incubated with secondary antibodies (Table 1). After washing with PBS, the samples were mounted in glycerol buffer (in 0.5 M sodium carbonate, pH 8.6). The immunostaining images were acquired using confocal microscopy by a Zeiss confocal scanning laser system installed on a Zeiss Axioplan 2 microscope. The images were acquired at a resolution of 512 × 512 pixels, and the thickness of each optical section was 0.5 μm. The Zstacks of immunoreactive cells were captured as a series of optical sections with a center spacing of 0.2 μm. Confocal images were collected using Zeiss LSM 5 image processing software and further processed using Corel Photo Paint and Corel Draw software[10].
Quantitative analysis of myenteric neuron immunostaining
The antigen colocalization was determined in fluorescently labeled preparations. Initially, the neurons were identified by immunofluorescence. Then, the filter was switched, and the labeling of the second antigen was evaluated. The proportion of the neurons labeled with the antigen pairs was thus determined. The cohort size was 100 neurons, and the data were collected from the preparations obtained from five miceperexperimental group. The percentage of double immunoreactive neurons was calculated and is expressed as the mean ± standard error of the mean (SEM). The density of the neurons immunoreactive (neurons/cm2) to the P2X7 receptor, neuronal nitric oxide synthase (nNOS),calretinin (Calr), and choline acetyltransferase (ChaT) and neuronal morphological profiles were assessed in the whole-mount preparations at 100 × magnification. The number of the cell bodies of immunoreactive neurons in the myenteric ganglia in each visual microscopic field (0.04909 mm2) was estimated. To quantify two whole-mount preparations (1.0 cm2each), the counts were estimated in 40 microscopic fields selected at random for each antigen in each animal. The perikaryon profile areas(μm2) of 50 randomly selected neurons from each animal were obtained using a semiautomatic morphometry device and measured using the Image-Pro Plus software package.
Immunohistochemistry
Immunostaining of S100B (an enteric glia-derived mediator), HuC/D (a neuronal marker), and the P2X7 receptor was performed in paraffin-embedded ileal formalin-fixed sections (4-μm thick) using the streptavidin-biotin-peroxidase method; the sections were mounted on poly(L)-lysine-coated microscope slides as described previously[20]. Briefly, the samples were deparaffinized and rehydrated by incubation with xylene and graded alcohol solutions, respectively. Then, the samples were immersed in antigen retrieval solution (EnVisionTM FLEX target retrieval solution, pH=6.0; Dako, Denmark A/S) for 20 min on a PT Link system (Dako), incubated in 3% hydrogen peroxide (EnVisionTM FLEX peroxidaseblocking reagent; Dako)to block endogenous peroxidase for 15 min at room temperature, and washed with PBS. Then, the samples were incubated with primary antibodies (rabbit anti-P2X7 receptor(Invitrogen), mouse anti-HuC/D (Invitrogen), or goat anti-S100B (Santa Cruz Biotechnology, 1:100) in antibody diluent solution (EnVisionTM FLEX antibody diluent; Dako) overnight at 4 °C. Then, the samples were incubated withEnVisionTM FLEX/HRP (Dako)as recommended by the manufacturer. P2X7 receptor,HuC/D, and S100B were detected using the chromogen 3,3′-diaminobenzidine (DAB,EnVisionTM FLEX DAB+chromogen; Dako). The negative control sections were processed simultaneously as described above; however, the primary antibody was replaced with antibody diluent solution (EnVisionTM FLEX antibody diluent; Dako). The slides were counterstained with Mayer’s hematoxylin. The images were acquired by a Leica DM100 microscope and analyzed using Adobe Photoshop 8.0 software. The percentages of P2X7 receptor-, S100B- and HuC/D-stained tissue sections were measured by using Adobe Photoshop as described previously[20].
Total RNA extraction, reverse transcription, and real-time polymerase chain reaction
Total RNA was isolated from the ileum using anAurum™ total RNA fatty and fibrous tissuekit (Bio-Rad,CA, United States), and 1 μg of the RNA was reverse transcribed using iScript™ (Bio-Rad) according to the manufacturer's instructions. Real-time polymerase chain reaction (qPCR) was performed on a 7900HT fast real-time PCR system (Applied Biosystems) using the following specific primers (IDT,Coralville, IA): P2X7 receptor (forward: GCACGAATTATGGCACCGTC and reverse: CCCCACCCTCTGTGACATTCT) and GAPDH (forward: TGCACCACCAACTGCTTAG and reverse: GGATGCAGGGATGATGTTC)[21]. The reaction mixture was prepared in a final volume of 20 μL as follows: 10 μL of master mix iQTM SYBR®Green (Applied Biosystems), 2 μL of each primer (200 nM), 1 μL of cDNA, and 5 μL of nuclease-free water. The gene amplification included the following steps: 10 min at 95 °C (initial denaturation), 15 s at 95 °C and 60 s at 60 °C for 40 cycles; thus, a melting curve was obtained. Relative gene expression was determined using the 2-ΔΔCt method with GAPDH as a housekeeping gene.
Terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling assay
Ileal samples were processed for terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) using anApopTagR S 7100kit (Merck Millipore, Germany) to quantify apoptotic and necrotic cells. Briefly, paraffin-embedded sections were hydrated and incubated with proteinase K(Sigma, United States, 20 mg/mL) for 15 min at room temperature. Endogenous peroxidase was blocked with 3% hydrogen peroxide in PBS for 5 min at room temperature. After a washing step, the sections were incubated with TdT buffer containing TdT enzyme and reaction buffer in a humidified chamber at 37 °C for 1 h. The specimens were incubated for 10 min at room temperature with stop/wash buffer and then incubated with an anti-digoxigenin-peroxidase conjugate at room temperature in a humidified chamber for 30 min. After washing with PBS, the slides were covered with peroxidase substrate (DAB)to develop the color and were counterstained with methyl green.
Cytokine quantification by enzyme-linked immunosorbent assay
To measure inflammatory markers, the ileal samples were processed to evaluate the levels of IL-1β, IL-6,keratinocyte chemoattractant (KC, a human IL-8 analog), and tumor necrosis factor (TNF)-α by enzymelinked immunosorbent assay (ELISA) using a mouse cytokine kit (R&D Systems) according to the manufacturer’s instructions. The absorbance of the samples was detected at 450 nm using an ELISA plate reader (Biotech Epoch, United States). The data are expressed as pgpermg of tissue.
Statistical analysis
The results are expressed as the mean ± SEM calculated by GraphPad Prism version 9.0 (GraphPad Software, San Diego, CA). The differences between more than two experimental groups were evaluated using one-way analysis of variance (ANOVA) with Bonferroni's multiple comparison test. Student's t test was performed to analyze the differences between two groups. The histopathological score data were compared by using Kruskal-Wallis nonparametric test followed by Dunn’s test. Statistical significance was defined asP< 0.05.
RESULTS
TcdA upregulates the P2X7 receptor transcripts in the ileum of mice and increases the population of myenteric enteric neurons expressing the P2X7 receptor
Initially, we investigated whether TcdA alters the expression of theP2RX7gene in the ileum of mice by using qPCR. We demonstrated that TcdA upregulated the P2X7 receptor in the ileum of mice compared with that in control mice (P< 0.05, Figure 1A). The data of the assay of the P2X7 receptor protein by immunofluorescence showed an increase in the percentage of positive P2X7 receptor immunostaining in the ileal samples of TcdA-challenged mice compared with that in the control samples (P< 0.05,Figure 1B). An increase in the expression of the P2X7 receptor was observed in epithelial cells, the lamina propria, and the myenteric plexus (Figure 1C).
Enteric neurons are an important component of the myenteric plexus, which is a part of the ENS;hence, we investigated whether the level of the P2X7 receptor is increased in these cells in the myenteric plexus by using immunofluorescence analysis. Comparison with the control group indicated an increase in the density of enteric neurons expressing the P2X7 receptor in the ileum myenteric plexus in mice challenged with TcdA (P= 0.01, Figure 1D and E).
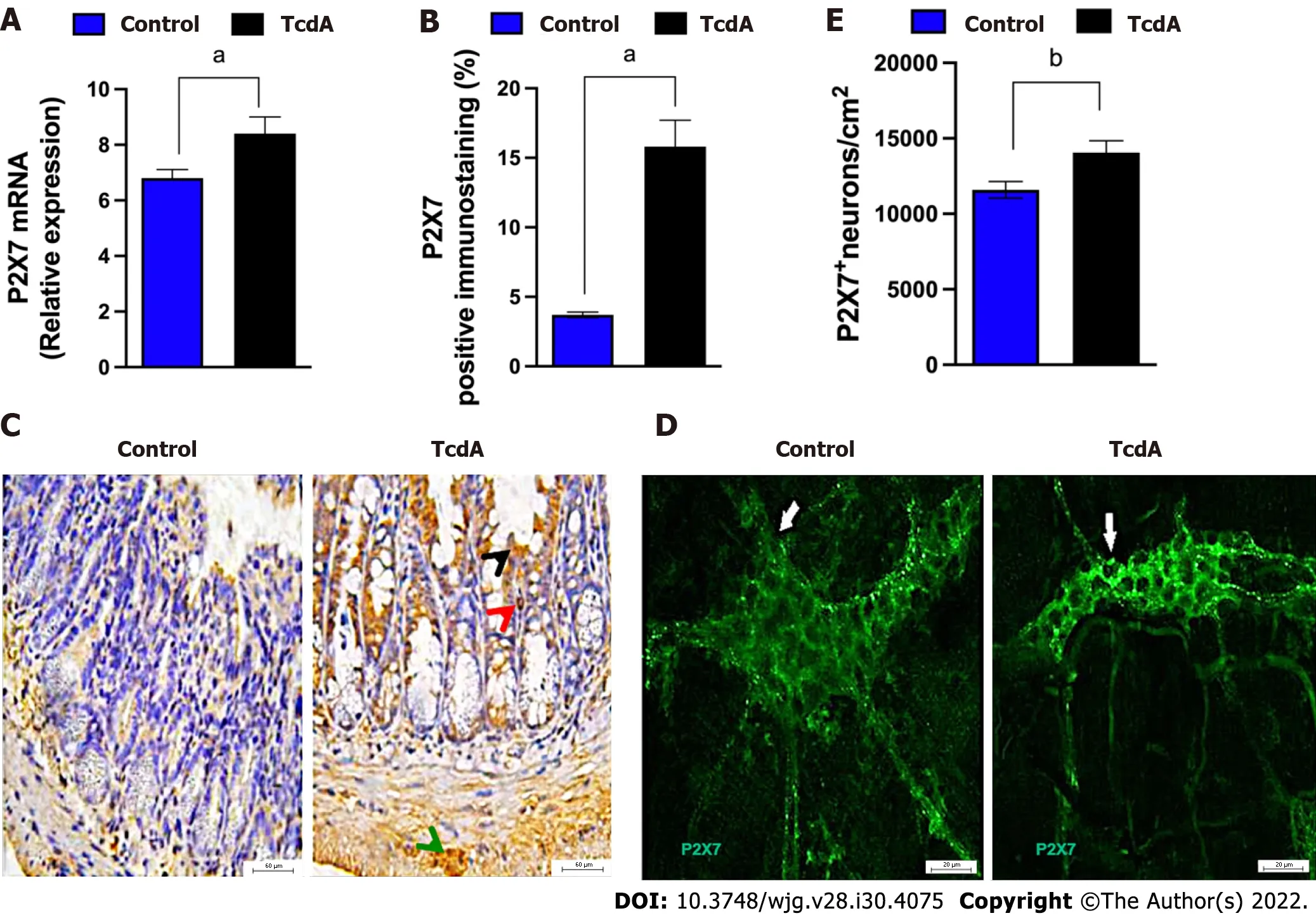
Figure 1 Clostridioides difficile toxin A increases the expression of the P2X7 receptor in the ileum myenteric plexus of mice. A: The expression of the P2RX7 gene [mean ± standard error of the mean (SEM)] assayed by quantitative real-time polymerase chain reaction in the ileal samples of mice injected with TcdA [TcdA, 50 μg in phosphate-buffered saline (PBS)] or PBS alone in the ileal loops (Control) (n = 4); B: Quantification of the percentage (mean ±SEM) of the P2X7 receptor-immunopositive area in the ileum from control and TcdA-challenged mice in 5-6 microscope fields per sample (n = 4 animals per group);C: Representative immunohistochemical images of the expression of the P2X7 receptor in the ileum of control and TcdA-challenged mice. Increased expression of the P2X7 receptor (arrowhead) was detected in the intestinal epithelial layer (black arrowhead), lamina propria (red arrowhead), and myenteric plexus (green arrowhead). Scale bars, 50 μm; D: Representative photomicrographs ofimmunostaining of the P2X7 receptor (arrow indicates the region stained green) in the ileum myenteric plexus from control and TcdA-challenged mice; E: Quantification of the number of P2RX7+ neurons/cm2 (mean ± SEM) in the ileum of control and TcdAchallenged mice (n = 4 animals per group); A, B and E: Unpaired two-tailed Student's t test (aP < 0.05; bP = 0.01).
TcdA decreases the density of enteric Calr+ and ChaT+ neurons in the ileum myenteric plexus of mice
Subsequently, to better understand how TcdA affects the myenteric enteric neuron population, we immunostained the ileum myenteric plexus to detect nNOS, Calr, and ChaT, which were stained in the main population of enteric neurons. As shown in Figure 2A, the density of Calr+ (P< 0.03) and ChaT+neurons (P< 0.002) in the ileum myenteric plexus of mice challenged with TcdA was decreased compared to that in control mice. In addition, all these subtypes of the neurons expressed the P2X7 receptor (Figure 2B).
Comparison with the control group of mice indicated that the enteric neuron population expressing the P2X7 receptor in the ileum myenteric plexus had a higher number of nNOS+P2RX7+ and Calr+P2RX7+ neurons, but not of ChaT+P2RX7+ neurons (P< 0.05, Figure 2C-E).
Overall, these findings indicated that TcdA decreased the enteric neuron population, specifically Calr+ and ChaT+ cells and upregulated the P2X7 receptor in a specific population (nNOS and Calr) of the neurons in the ileum myenteric plexus in mice.
Blockade of the P2X7 receptor decreases ileal damage induced by TcdA in mice
Then, we blocked the P2X7 receptor by pretreating mice using a pharmacological approach, including administration of BBG and A438079 one hour prior to the challenge with TcdA to determine whether P2X7 receptor activity is required for ileal damage induced by TcdA. Hematoxylin and eosin-stained slides of ileum samples were analyzed for evidence of epithelial damage, edema, and neutrophil infiltration, with a maximal severity score of 3 (Figure 3). TcdA induced complete epithelial disruption,extensive edema, and intense neutrophil infiltration in the ileum of mice, resulting in a high damage score (score = 3) compared with those in the undamaged ileum in control mice (P< 0.007, Figure 3).However, both P2X7 receptor antagonists (BBG and 438079) induced a substantial decrease in the ileal damage promoted by TcdA, resulting in a reduction in the damage score (score = 1) compared to that in untreated TcdA-challenged mice (P< 0.04, Figure 3).

Figure 2 Clostridioides difficile toxin A induces alterations in enteric neuronal coding in the myenteric plexus in the ileum of mice. A:Quantification of the number of neuronal nitric oxide synthase+ (nNOS+), calretinin+ (Calr+), and choline acetyltransferase+ (ChaT+) neurons/cm2 [mean ± standard error of the mean (SEM)] in the ileum myenteric plexus in control and TcdA-challenged mice; B: Representative photomicrographs of the P2X7 receptor (green),nNOS (left panels, red), Calr (center panels, red), and ChaT (right panel, red) immunostaining and DAPI (blue) nuclear staining in control and TcdA-challenged mice.White arrows indicate positive immunostaining. Scale bars, 50 μm; C-E: Percentage of NOS+P2X7+ neurons (C), Calr+P2RX7+ neurons (D), and ChaT+P2RX7+neurons (E) (mean ± SEM) in the ileum myenteric plexus of control and TcdA-challenged mice (n = 4); A, C, D and E: Unpaired two-tailed Student's t test (aP < 0.05; b P < 0.03; cP < 0.002 ). NOS: Nitric oxide synthase; Calr: Calretinin; ChaT: Choline acetyltransferase.
Blockade of the P2X7 receptor decreases ileal inflammation and cell death induced by TcdA in mice
Subsequently, we evaluated whether P2X7 receptor activity is involved in ileal inflammation and cell death induced by TcdA in mice. We demonstrated that both P2X7 receptor blockers (BBG and A438079)reversed a TcdA-induced increase in IL-1β (P= 0.008 andP= 0.03, Figure 4A), TNF-α (P= 0.0002 andP= 0.0001, Figure 4B), and IL-6 (P= 0.03 andP< 0.0001, Figure 4C) in the ileal samples of mice. However,comparison with TcdA-challenged mice, which were not pretreated with the blockers, indicated that blockade of the P2X7 receptor by A438079, but not by BBG, decreased the levels of KC (P= 0.01,Figure 4D) and the number of TUNEL+ cells (P= 0.01, Figure 4E) in the ileum of mice challenged with TcdA.
Overall, these data indicated that the P2X7 receptor was involved in intestinal damage, inflammation,and cell death induced by TcdA in mice.
Blockade of the P2X7 receptor decreases enteric neuron loss and S100B synthesis induced by TcdA in mice
Since we demonstrated that TcdA promoted a decrease in ileum enteric neurons in mice, we assessed whether the P2X7 receptor accounts for this alteration. Comparison with TcdA-challenged mice, which were not pretreated with the blockers, indicated that a P2X7 receptor blocker (A438079) increased the percentage of positive immunostaining of HuC/D, which is a pan-marker of enteric neurons, in the ileum of mice challenged with TcdA (Figure 5).
Furthermore, we evaluated whether the activation of the P2X7 receptor is required to induce S100B expression in the ileum of TcdA-challenged mice; high levels of S100B are released by EGCs under inflammatory conditions. As shown in Figure 5, the P2X7 receptor antagonist A438079 induced a considerable decrease in the percentage of S100B-positive immunostaining in the ileum of mice challenged with TcdA compared with that in the ileum TcdA-challenged mice, which were not pretreated with the blockers (P= 0.009).

Figure 3 lnhibition of the P2X7 receptor decreases Clostridioides difficile toxin A-induced ileal damage in mice. Mouse ileal loops were injected with TcdA [TcdA, 50 μg in phosphate-buffered saline (PBS)] or PBS alone (control) (n = 5), and the animals were pretreated with a P2X7 receptor antagonist[Brilliant Blue G (BBG) or A438079] one hour prior to TcdA challenge. A: Representative histological images (magnification of 200 ×) of TcdA-unchallenged (control,BBG, and A438079) and challenged (TcdA, TcdA + BBG, and TcdA + A438079) mice. TcdA induced epithelial disruption (black arrowhead), edema (green arrowhead), and neutrophil infiltration (red arrowhead); B: Histopathological score (median; 0 corresponds to-no damage and 3 corresponds to intense damage)performed by a blinded histopathological expert and based on epithelial damage, submucosal edema, and infiltration of inflammatory cells. Kruskal-Wallis nonparametric test followed by Dunn’s test. aP < 0.04; bP < 0.007. BBG: Brilliant Blue G.
Overall, these data indicated that the P2X7 receptor was involved in enteric neuronal loss and S100B synthesis induced by TcdA in mice.
DlSCUSSlON
The data of the present study indicated that TcdA upregulated the P2X7 receptor in the ileum of mice.An increase in the expression of P2RX7 has been reported in colonic biopsies from Crohn’s disease patients[21] and in preclinical models of intestinal inflammation, such as colitis induced by trinitrobenzene sulfonic (TNBS) acid[22] and sepsis[23]. Thus, the upregulation of the P2X7 receptor is a common phenomenon under inflammatory conditions[24].
In the present study, we also demonstrated that the level of the P2X7 receptor was increased in the epithelial layer, lamina propria, and myenteric plexus. In the myenteric plexus, we detected an increase in the density of neurons expressing the P2X7 receptor, including the nNOS+ and Calr+ subtypes. In addition to enteric neurons, other cell types can express the P2X7 receptors, such as mast cells, T cells,and dendritic cells[21]. However, we focused on enteric neurons in the myenteric plexus because this component of the ENS is a major functional unit of the system that moves the luminal contents along the intestine by coordinating muscle contraction and relaxation[25]. In addition,C. difficileinfection is characterized by intense diarrhea in the acute phase of the disease, and the mechanism of these events is poorly understood.

Figure 4 P2X7 receptor inhibition abrogates Clostridioides difficile toxin A-induced inflammation and cell death in mice. A-D: The levels of interleukin (IL)-1β (A), tumor necrosis factor-α (B), IL-6 (C), and keratinocyte chemoattractant (D) in the ileal samples of TcdA-unchallenged [control, Brilliant Blue G(BBG), and A438079] and challenged (TcdA, TcdA + BBG, and TcdA + A438079) mice measured by ELISA (n = 5); E: The number of TUNEL+ cells per field in the ileal samples of TcdA-unchallenged (control, BBG, and A438079) and challenged (TcdA, TcdA + BBG, and TcdA + A438079) mice. The data are expressed as the mean ± standard error of the mean. ANOVA followed by Tukey’s test was used (aP = 0.03; bP = 0.01; cP < 0.008; dP < 0.0001). IL: Interleukin; TNF: Tumor necrosis factor; BBG: Brilliant Blue G; KC: Keratinocyte chemoattractant; TUNEL: Terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling.
We demonstrated that TcdA promoted neuron loss specifically by reducing the density of the Calr+and ChaT+ neuronal populations. Acetylcholine, which is synthesized in a reaction of choline with acetyl-CoA catalyzed by ChaT, is the primary transmitter in excitatory motor neurons, intrinsic afferent neurons, and interneurons, and Calr is the primary transmitter in excitatory cholinergic neurons[26].Excitatory motor neurons are involved in coordinated muscle contraction[25]; thus, a reduction in the density of Calr+ and ChaT+ neuronal populations induced by TcdA may be involved in the functional disorders manifested afterC. difficileinfection. Accordingly, a study performed in the United States military personnel reported functional gastrointestinal disorders (including gastroesophageal reflux disease, dyspepsia, irritable bowel syndrome, or constipation) afterC. difficileinfection recovery[6]. In the present study, alterations in the myenteric enteric neuron population induced by TcdA could have been related to these post-C. difficileinfection-related intestinal dysfunctions. However, more studies are needed to better understand how these alterations specifically contribute to intestinal dysfunction induced byC. difficileinfection.
In the present study, we also showed that the activation of the P2X7 receptor was involved in the TcdA-induced loss of enteric neurons because inhibition of the receptor by known P2RX7 antagonists(BBG and A438079) resulted in a substantial decrease in the loss of these ENS cells during ileitis induced by TcdA. In agreement with these data of the present study, another study demonstrated that the activation of P2RX7 promotes cell death in mucosal regulatory T cells in colitis induced by TNBS[27].The P2X7 receptor regulates cell death pathways, such as apoptosis, pyroptosis, necrosis, and autophagy[28].
ATP released from dead cells can increase the activation of the P2X7 receptor and promote the secretion of proinflammatory cytokines, such as IL-1β, which in turn can induce the secretion of other cytokines[29,30]. In the present study, the blockade of the P2X7 receptor markedly decreased IL-1β, IL-6,KC, and TNF-α synthesis in the TcdA-challenged mouse ileum, suggesting that this receptor plays an important role in inflammation induced byC. difficiletoxin. Similarly, in a model of TNBS-induced colitis, P2X7 receptor blockade reduces the severity of inflammation by decreasing the infiltration of macrophages in the lamina propria[31]. In contrast, deletion of P2RX7 increases the susceptibility to toxoplasmic ileitis[32], suggesting that the activation of this receptor plays a role in the response against intracellular pathogens. In contrast,C. difficilereleases toxins, which in turn enter the cells to inhibit Rho GTPases, and the P2RX7 antagonists have positive effects in this case.

Figure 5 P2X7 receptor inhibition attenuates Clostridioides difficile toxin A-induced enteric neuron loss and S100B synthesis in mice. A:Representative immunohistochemical images of the expression of HuC/D (neuronal marker) and S100B (enteric glia marker) in the ileum of TcdA-unchallenged(control and A438079) and challenged (TcdA and TcdA + A438079) mice. Black arrows indicate positive immunostaining. Scale bars, 50 μm; B: Quantification of the percentage (mean ± standard error of the mean) of HuC/D- and S100B-immunopositive areas in the ileum of TcdA-unchallenged (control and A438079) and challenged (TcdA and TcdA + A438079) mice (n = 4 animals per group). ANOVA followed by Tukey’s test was used (aP = 0.04; bP = 0.01; cP < 0.009; dP < 0.0001).GFAP: Glial fibrillary acidic protein.
In addition, we demonstrated that blockade of the P2X7 receptor decreased S100B synthesis in the ileum of mice challenged with TcdA. S100B functions as a proinflammatory mediator when released at higher levels by activating the nuclear activation factor-κB[20] and is an important mediator duringC.difficileinfection[33]. In the myenteric plexus, EGCs express S100B[34] and are involved in the control of motility and epithelial barrier[35]. In a rat glioblastoma cell line, IL-6 promotes S100B synthesis[36]. In the present study, a reduction in proinflammatory cytokines related to P2X7 receptor blockade could have contributed to a decrease in S100B synthesis induced by TcdA, which in turn reduced intestinal inflammation and neuronal death.
Additional studies are needed, for example, using aC. difficileinfection model, to explore how P2RX7 blockage can influence theC. difficileinfection outcome and to better understand physiological benefits of this blockade for relief of intestinal permeability and dysmotility during the infection. However, it is important to emphasize that investigations of the role of this receptor in the damaging effects induced by one of the main virulence factors released byC. difficilewill help to understand the pathogenesis of these effects and to develop alternative cotreatments to control the deleterious and exacerbated host response to theC. difficiletoxins.
CONCLUSlON
In conclusion, the results of the present study revealed the mechanism of P2X7 receptor-driven loss of enteric neurons induced by TcdA in the mouse ileum. TcdA promoted the upregulation of the P2X7 receptor, which promoted cell death in enteric neurons and induced the release of proinflammatory mediators (IL-1β, IL-6, KC, and TNF-α) in epithelial/immune cells, which in turn promoted S100B synthesis in EGCs. However, blockade of the P2X7 receptor abrogated ileal damage induced by TcdA(Figure 6). Overall, the findings of the present study open new avenues to better understand howC.difficiletoxins promote the changes in the ENS components that can be related to intestinal dysfunction afterC. difficileinfection.

Figure 6 Scheme of the hypothetical role of the P2X7 receptor during Clostridioides difficile toxin A-induced ileitis. Clostridioides difficile TcdA promotes epithelial damage and the upregulation of the P2X7 receptor in enteric neurons, intestinal epithelial cells, and immune cells. Once activated, the P2X7 receptor promotes the death of enteric neurons and the release of proinflammatory mediators by epithelial/immune cells [interleukin (IL)-1β, IL-6, keratinocyte chemoattractant, and tumor necrosis factor-α]. These proinflammatory mediators induce S100B synthesis by enteric glial cells. Blockade of the P2X7 receptor using a pharmacological approach attenuates these deleterious effects of TcdA. IL: Interleukin; TNF: Tumor necrosis factor; EGC: Enteric glial cell.
ARTlCLE HlGHLlGHTS


Research conclusions
The findings of the present study demonstrated that TcdA induced the upregulation of the P2X7 receptor, which promoted enteric neuron loss, S100B synthesis, tissue damage, inflammation, and cell death in the ileum of mice.
Research perspectives
These findings contribute to future directions in understanding the mechanism involved in intestinal dysfunction reported in patients afterClostridioides difficileinfection.
ACKNOWLEDGEMENTS
The authors would like to thank Darlyane V S Costa for kindly drawing Figure 6 and Socorro França and Flávia A Silva for their technical assistance.
FOOTNOTES
Author contributions:Santos AAQA and Costa DVS contributions equally; Santos AAQA participated in the design and performed the experiments, analyzed the data and wrote the manuscript; Costa DVS analyzed the data and wrote the manuscript; Foschetti DA, Duarte ASG, Martins CS, and Soares PMG helped in the acquisition of the data and review of the manuscript; Castelucci P participated in the initial experimental design and helped to revise the manuscript; Brito GAC, the main investigator of the laboratory where the experiments were performed,conceptualized the main ideas, supervised the study and reviewed the manuscript.
Supported byPRONEX CNPq/FUNCAP, No. PR2-0101-00060.01.00/15; São Paulo Research Foundation (FAPESP),No. 2014/25927-2 and No. 2018/07862-1.
lnstitutional animal care and use committee statement:All mouse procedures were conducted according to current regulations regarding animal experiments approved by the local Animal Care and Use Committee, No. 31/2015.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Data sharing statement:No additional data are available.
ARRlVE guidelines statement:The authors have read the ARRIVE guidelines, and the manuscript was prepared and revised according to the ARRIVE guidelines.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Brazil
ORClD number:Ana A Q A Santos 0000-0001-9512-3182; Deiziane V S Costa 0000-0001-6402-8908; Danielle A Foschetti 0000-0002-4213-9786; Antoniella S G Duarte 0000-0001-6632-6685; Conceição S Martins 0000-0001-8710-1856; Pedro M G Soares 0000-0003-0606-2539; Patricia Castelucci 0000-0002-7475-5962; Gerly A C Brito 0000-0002-8214-4379.
S-Editor:Fan JR
L-Editor:A
P-Editor:Guo X
 World Journal of Gastroenterology2022年30期
World Journal of Gastroenterology2022年30期
- World Journal of Gastroenterology的其它文章
- Role of one-step nucleic acid amplification in colorectal cancer lymph node metastases detection
- Current perspectives on the role of liver transplantation for Langerhans cell histiocytosis: A narrative review
- Gut microbiota, inflammatory bowel disease and colorectal cancer
- Thrombocytopenia in chronic liver disease: Physiopathology and new therapeutic strategies before invasive procedures
- Serological profiling of Crohn’s disease and ulcerative colitis patients reveals anti-microbial antibody signatures
- Trends in medication use and treatment patterns in Chinese patients with inflammatory bowel disease
