E2F1调节肾小管上皮细胞自噬对小鼠糖尿病肾病肾纤维化的作用研究*
邹琴, 李清璇, 屈玲玲, 安小敏, 黄靖惠, 王金艳, 龙天华, 石明隽
E2F1调节肾小管上皮细胞自噬对小鼠糖尿病肾病肾纤维化的作用研究*
邹琴, 李清璇, 屈玲玲, 安小敏, 黄靖惠, 王金艳, 龙天华, 石明隽△
(贵州医科大学病理生理学教研室,贵州省常见慢性疾病发病机制及药物研究重点实验室,贵州 贵阳 550025)
探讨E2F转录因子1(E2F transcription factor 1, E2F1)是否可以通过调节肾小管上皮细胞(renal tubular epithelial cells, RTECs)自噬而影响糖尿病肾病(diabetic nephropathy, DN)小鼠肾纤维化进程,并探讨其可能机制。将12只C57BL/6小鼠随机分为正常对照(normal control, NC)组和糖尿病(diabetes mellitus, DM)组,每组各6只,用链脲佐菌素(55 mg/kg,腹腔注射,每天1次,连续5 d)复制小鼠1型DM模型。16周时处死小鼠后检测血糖和24 h尿总蛋白;HE和Masson染色观察肾组织病理形态改变;免疫组织化学染色观察肾组织E2F1表达情况;Western blot和RT-qPCR检测肾组织E2F1、哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin, mTOR)、自噬及纤维化相关指标表达的变化。高糖培养RTECs并分别转染敲减或过表达的质粒,Western blot法检测相关指标蛋白的表达情况。与NC组相比,DM组小鼠血糖和24 h尿总蛋白均显著增高(<0.05)。HE和Masson染色观察到DM组小鼠肾小管管腔塌陷,肾小球基底膜增厚,且肾间质有大量胶原纤维沉积。RT-qPCR结果显示,与NC组相比,DM组小鼠E2F1、III型胶原(collagen type III, Col III)和α-平滑肌肌动蛋白(α-smooth muscle actin,α-SMA)的mRNA表达上调(<0.05),上皮钙黏素(E-cadherin)的mRNA表达下调(<0.05)。Western blot结果显示,与NC组比较,DM组小鼠肾组织及高糖培养的RTECs中E2F1、mTOR、自噬降解底物蛋白P62、Col III和α-SMA的蛋白表达上调(<0.05),E-cadherin蛋白表达水平和微管相关蛋白1轻链3-II(microtubule-associated protein 1 light chain 3-II, LC3-II)/LC3-I比值降低(<0.05)。与空载体组相比,在高糖状态下转染敲减表达的质粒后,RTECs中mTOR、P62、Col III和α-SMA蛋白表达下调(<0.05),E-cadherin蛋白表达水平和LC3-II/LC3-I比值升高(<0.05);而过表达后,mTOR、P62、Col III和α-SMA的蛋白表达增加(<0.05),E-cadherin蛋白表达水平和LC3-II/LC3-I比值降低(<0.05)。E2F1/mTOR可通过抑制RTECs自噬水平而促进小鼠DN肾纤维化进程。
E2F转录因子1;自噬;肾间质纤维化;糖尿病肾病
糖尿病(diabetes mellitus, DM)是一种常见的慢性内分泌性疾病,而糖尿病肾病(diabetic nephropathy, DN)是DM最严重的全身性微血管并发症之一,已成为DM患者终末期肾衰竭及死亡的主要原因[1]。肾小管间质纤维化是DN发展至终末期肾衰竭的重要病理改变,而肾小管上皮细胞(renal tubular epithelial cells, RTECs)的上皮-间充质转化(epithelial-mesenchymal transition, EMT)在肾小管间质纤维化的发生与发展过程中发挥着重要作用[2-5],但其具体发生机制迄今尚未充分阐明。
自噬是细胞中蛋白质降解的2种主要途径之一[6],即细胞通过降解自噬小体内包裹的受损细胞器、侵入的病原体及衰老的蛋白质等,以维持细胞稳态、能量生成和细胞器的更新。研究显示,在DN大鼠RTECs中自噬水平明显受到抑制[7],当提高自噬水平后,可明显抑制RTECs的EMT进程及DN的发生与发展[8]。以上研究提示,自噬与DN时RTECs EMT的关系密切,但其具体调控机制尚不清楚。E2F转录因子1(E2F transcription factor 1, E2F1)是细胞周期相关转录因子家族成员之一,可以诱导肺癌和骨肉瘤细胞发生EMT[9-10]。另外,在大鼠心肌细胞中通过敲减的表达可以明显提高细胞自噬水平[11]。然而在高糖(high glucose, HG)状态下,E2F1对RTECs自噬水平及EMT的作用尚不清楚。因此,本项工作拟以1型DM小鼠模型及HG状态下小鼠RTECs为研究对象,观察E2F1对HG状态下RTECs自噬水平及EMT的影响,探讨其对DN肾纤维化的作用及可能机制,为进一步阐明DN的发生机制提供一定的实验依据。
材料和方法
1 实验动物与细胞
健康清洁级雄性6周龄C57BL/6小鼠12只,体重(24±5) g,购自斯贝福(北京)生物技术有限公司,许可证号为SCXK(京)2016-0002;小鼠RTECs株购自上海中乔新舟生物科技有限公司。
2 药品与试剂
链脲佐菌素(streptozotocin, STZ)购自Sigma;逆转录试剂盒、实时荧光定量PCR试剂盒和免疫组织化学染色试剂盒购自北京中杉金桥生物技术有限公司;胎牛血清(fetal bovine serum, FBS)购自BIOIND;过表达和敲减质粒购自上海毅乐生物科技有限公司;质粒小提中量提取试剂盒购自北京天根生化科技有限公司;抗β-actin抗体及辣根过氧化物酶标记的羊抗兔和羊抗小鼠IgG购自武汉普美克生物技术有限公司;兔抗III型胶原(collagen type III, Col III)和哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin, mTOR)抗体购自北京博奥森生物技术有限公司;鼠抗上皮钙黏素(E-cadherin)、α-平滑肌肌动蛋白(α-smooth muscle actin, α-SMA)和P62抗体购自Abcam;兔抗微管相关蛋白1轻链3(microtubule-associated protein 1 light chain 3, LC3)抗体购自Cell Signaling Technology;兔抗E2F1抗体购自Absin;所用引物由上海生物工程股份有限公司根据设计合成。
3 主要方法
3.1动物模型复制和分组C57BL/6小鼠适应性喂养1周后,参考相关文献方法复制DM小鼠模型[12]。小鼠随机分为正常对照(normal control, NC)组和DM组,每组6只。模型复制前小鼠禁食不禁水4 h,DM组小鼠给予腹腔注射2%的STZ(55 mg/kg;采用高压灭菌、0.1 mol/L、pH 4.5的柠檬酸-柠檬酸钠缓冲液配制),连续5 d,NC组予以腹腔注射等体积STZ溶媒。给药2周后检测小鼠空腹血糖,血糖≥16.7 mmol/L即为模型复制成功,继续饲养小鼠16周,每周检测小鼠体重和空腹血糖各1次,2组小鼠均予普通饲料喂养。
3.2动物标本采集糖尿病模型复制成功16周后处死各组小鼠,处死前1 d接取24 h尿液,处死当天禁食不禁水4 h,测空腹血糖和体重;行乙醚麻醉,摘取眼球取血,室温离心(92 r/min,5 min)取血清,置于-80 ℃冰箱保存;取双侧肾脏,去除包膜脂肪,记录肾脏重量,留取部分组织切片约1 mm于4%多聚甲醛溶液中固定,其余部分于-80 ℃冰箱保存。
3.3生化指标检测葡萄糖氧化酶GOD法检测血清血糖;邻苯三酚红钼比色法测尿总蛋白(urinary total protein, UTP)。以UTP乘以24 h尿量计算24 h UTP。
3.4肾组织形态观察取4%多聚甲醛溶液中固定的肾组织经梯度乙醇脱水、透明、石蜡包埋后制成3 μm厚的石蜡切片,进行常规HE和Masson染色,普通光学显微镜下观察肾组织病理形态改变情况。
3.5免疫组织化学染色参照北京中杉金桥生物技术有限公司小鼠二步法试剂盒操作,石蜡切片脱蜡后,梯度乙醇水化,PBS洗涤,3% H2O2阻断内源性过氧化物酶,微波抗原修复,加兔抗E2F1抗体(1∶100),4 ℃冰箱孵育过夜,复温,PBS洗涤后,滴加即用型辣根过氧化物酶标记的山羊抗兔IgG,37 ℃恒温孵育30 min,PBS洗涤,DAB显色,自来水冲洗终止显色;苏木精染核,梯度乙醇脱水,二甲苯透明,自然晾干后封片,光学显微镜观察并拍照保存。
3.6细胞培养及分组用含10%FBS的正常糖(normal glucose, NG; 5.5 mmol/L葡葡糖)DMEM培养液培养RTECs,置于5% CO2、37 ℃培养箱中培养。待细胞密度达到40%时,加入无血清的DMEM培养液同步化培养细胞12 h,再将细胞分为NG组(5.5 mmol/L葡葡糖+1% FBS)和HG组(30 mmol/L葡葡糖+1% FBS),继续培养48 h后收集细胞蛋白标本进相关检测。
3.7激光扫描共聚焦显微镜检测将RTECs进行爬片,分别用NG和HG培养液培养细胞48 h后,吸弃培养液,PBS洗涤后,用4%多聚甲醛溶液室温固定15 min,预冷的PBS洗涤,加入0.2% Triton X-100室温放置6 min,PBS洗涤,加入3% BSA室温孵育封闭30 min,PBS洗涤,滴加Ⅰ抗(LC3-I和LC3-II抗体,1∶100),4 ℃冰箱孵育过夜;次日室温复温1 h,PBS洗涤后,加入荧光Ⅱ抗FITC-IgG(1∶100),37 ℃避光孵育1 h,PBS洗涤,抗荧光淬灭剂封片后,使用激光扫描共聚焦显微镜观察图像并拍摄保存。
3.8细菌接种和质粒提取将含有敲减和过表达质粒的甘油菌Z型划线接种于已加氨苄青霉素的LB固体培养基上,37 ℃培养15~18 h后可见单克隆菌落,挑取单克隆菌落接种于含氨苄青霉素的LB培养液中,37 ℃摇菌培养12~15 h,根据质粒小提中量提取试剂盒说明书提取质粒。
3.9质粒转染待RTECs密度达到50%时,将细胞分为HG组、HG+空载体转染组(HG+vector组)和HG+敲减质粒转染组(HG+E2F1-shRNA组),以及NG组、NG+空载体转染组(NG+vector组)、NG+过表达质粒转染组(NG+OE-E2F1)、HG组、HG+vector组和HG+过表达质粒转染组(HG+OE-E2F1)组,用聚乙烯亚胺转染法将空载质粒、敲减及过表达的质粒分别转染相应分组的目的细胞,待质粒转染细胞6 h后均换为含1% FBS的对应NG或HG培养液继续培养48 h,收集细胞标本进行相应的检测。
3.10RT-qPCR实验取出-80 ℃冰箱中冻存的肾脏组织,各取50 mg肾皮质于1.5 mL EP管中,加入500 μL的Trizol液,置于高通量研磨机中研磨,根据Trizol法提取肾脏皮质总RNA,测定总RNA浓度,用逆转录试剂盒将RNA逆转录合成cDNA,存放于-20 ℃冰箱备用。以cDNA为模板扩增E2F1、α-SMA、E-cadherin和Col III。PCR总体系为20 μL,其中Hieff UNICON qPCR SYBR Green Master Mix 10 μL,上、下游引物各0.4 μL,模板DNA 1 μL,RNase-Free ddH2O 8.2 μL。扩增上机程序为:95 ℃ 30 s;95 ℃ 10 s,58 ℃ 20 s,72 ℃ 20 s,40次循环。以β-actin为内参照,E2F1、α-SMA、E-cadherin和Col III的mRNA相对表达量用2-ΔΔCt表示。引物序列见表1。
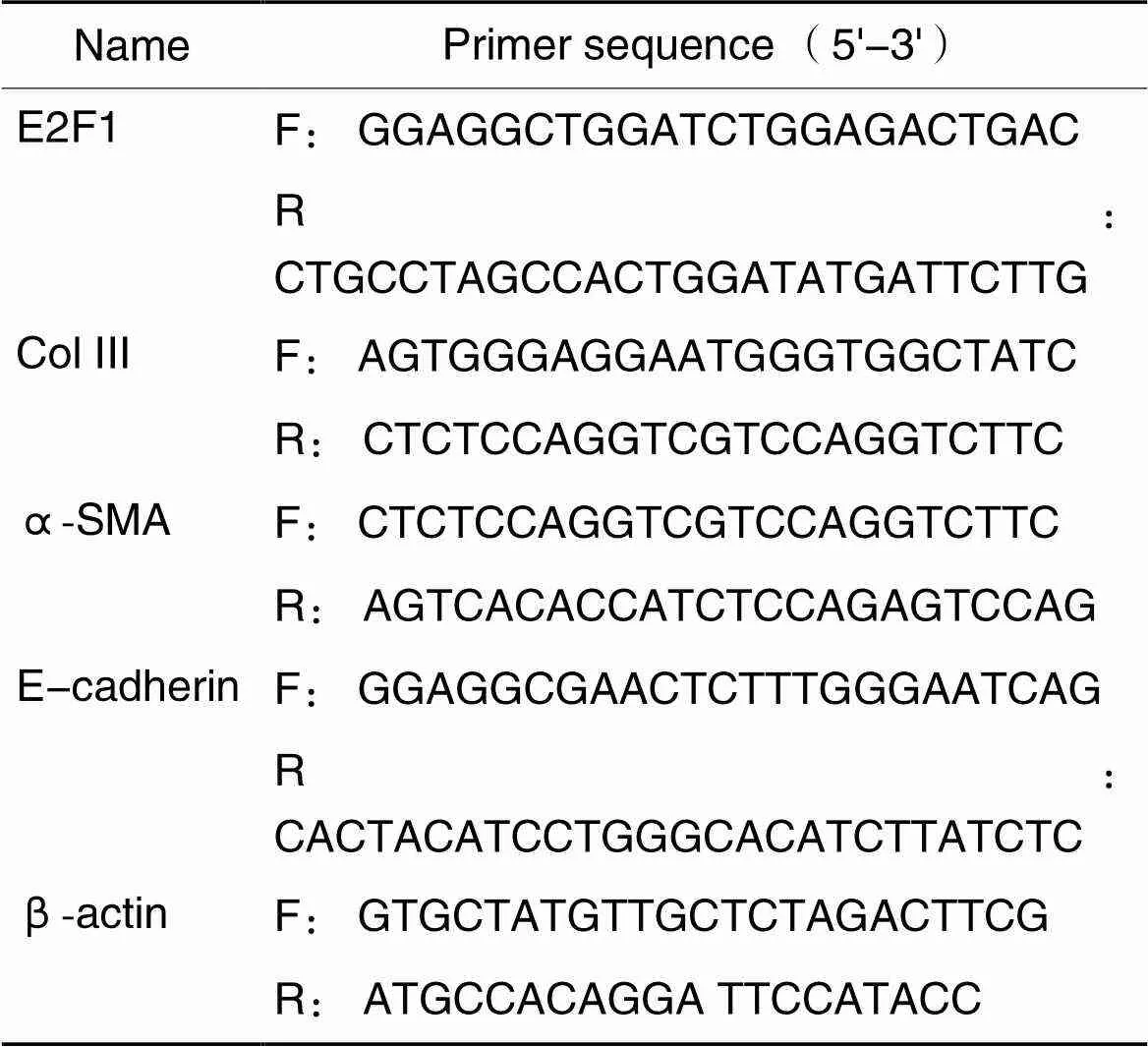
表1 引物序列
F: forward; R: reverse.
3.11Western blot实验取出-80 ℃冰箱中肾组织,称量100 mg肾皮质组织转移入1.5 mL EP管中,加1 mL蛋白裂解液,经匀浆研磨后离心,取上清液制成蛋白样本进行聚丙烯酰胺凝胶电泳、转膜、封闭,分别加入小鼠抗β-actin抗体(1∶5 000),鼠抗α-SMA(1∶1 000),兔抗P62、LC3、Col III和E-cadherin抗体(1∶1 000),兔抗E2F1和mTOR抗体(1∶1 500),置于4 ℃冰箱中孵育过夜;TBST洗膜,加入相应的辣根过氧化物酶标记的山羊抗鼠Ⅱ抗(1∶6 000)和辣根过氧化物酶标记的山羊抗兔Ⅱ抗(1∶7 000),室温摇床孵育1 h;TBST洗涤后加入Smart-ECL发光试剂,凝胶成像仪进行化学发光曝光,结果用Image Lab 5.0程序软件对蛋白条带进行分析处理,并统计数据。
4 统计学处理
采用SPSS 17.0软件进行统计学分析。数据均以均数±标准差(mean±SD)表示。两组间比较采用检验,多组间比较采用单因素方差分析。以<0.05为差异有统计学意义。
结果
1 DM小鼠肾组织及HG培养的RTECs中Col III、α-SMA及E-cadherin的表达
NC组小鼠生长正常,状态良好,DM组小鼠出现“三多一少”的症状。与NC组相比,DM组小鼠血糖和24 h UTP水平均显著升高(<0.05),见图1A、B。HE染色结果显示,NC组小鼠肾小球及肾小管结构较为清晰,上皮细胞排列较为整齐,基底膜完整,肾间质中未见到炎性细胞浸润,而DM组小鼠肾组织中可见肾小管间质周围增宽,有炎性细胞浸润,肾小管管腔扩张,基底膜增厚;Masson染色可见DM组小鼠肾小球及肾间质有大量蓝色胶原沉积,见图1C。此外,Western blot结果显示,DM组小鼠肾组织Col III和α-SMA蛋白表达显著升高(<0.05),E-cadherin蛋白表达显著降低(<0.05),RT-qPCR结果与蛋白表达结果一致,见图1D、E。在HG培养的RTECs中上述蛋白表达的趋势与DM小鼠肾组织的Western blot实验结果一致,见图1F。

Figure 1. The expression levels of collagen type III (Col III), α-smooth muscle actin (α-SMA) and E-cadherin in kidney tissues of diabetes mellitus (DM) mice and mouse renal tubular epithelial cells (RTECs) cultured with high glucose (HG). A: the blood glucose level in normal control (NC) group and DM group; B: 24 h urinary total protein (UTP) in NC group and DM group; C: the images of HE and Masson staining of renal tissues in NC group and DM group; D: the mRNA levels ofCol III, α-SMA and E-cadherin in kidney tissues of DM mice were detected by RT-qPCR; E and F: the protein levels of Col III, α-SMA and E-cadherin in kidney tissues of DM mice and HG-stimulated mouse RTECs were determined by Western blot, respectively. Mean±SD. n=6. *P<0.05 vs NC group;#P<0.05 vs normal glucose (NG) group.
2 DM小鼠肾组织及HG培养的RTECs的自噬情况
与NC组小鼠和NG培养的RTECs相比,DM组小鼠肾组织中和HG培养的RTECs中LC3-II/LC3-I比值显著降低(<0.05),而P62蛋白累积增多(<0.05),见图2A、B。激光扫描共聚焦显微镜观察到,NG组RTECs中有大量自噬小体形成,而HG培养细胞后,自噬小体形成明显减少,见图2C。
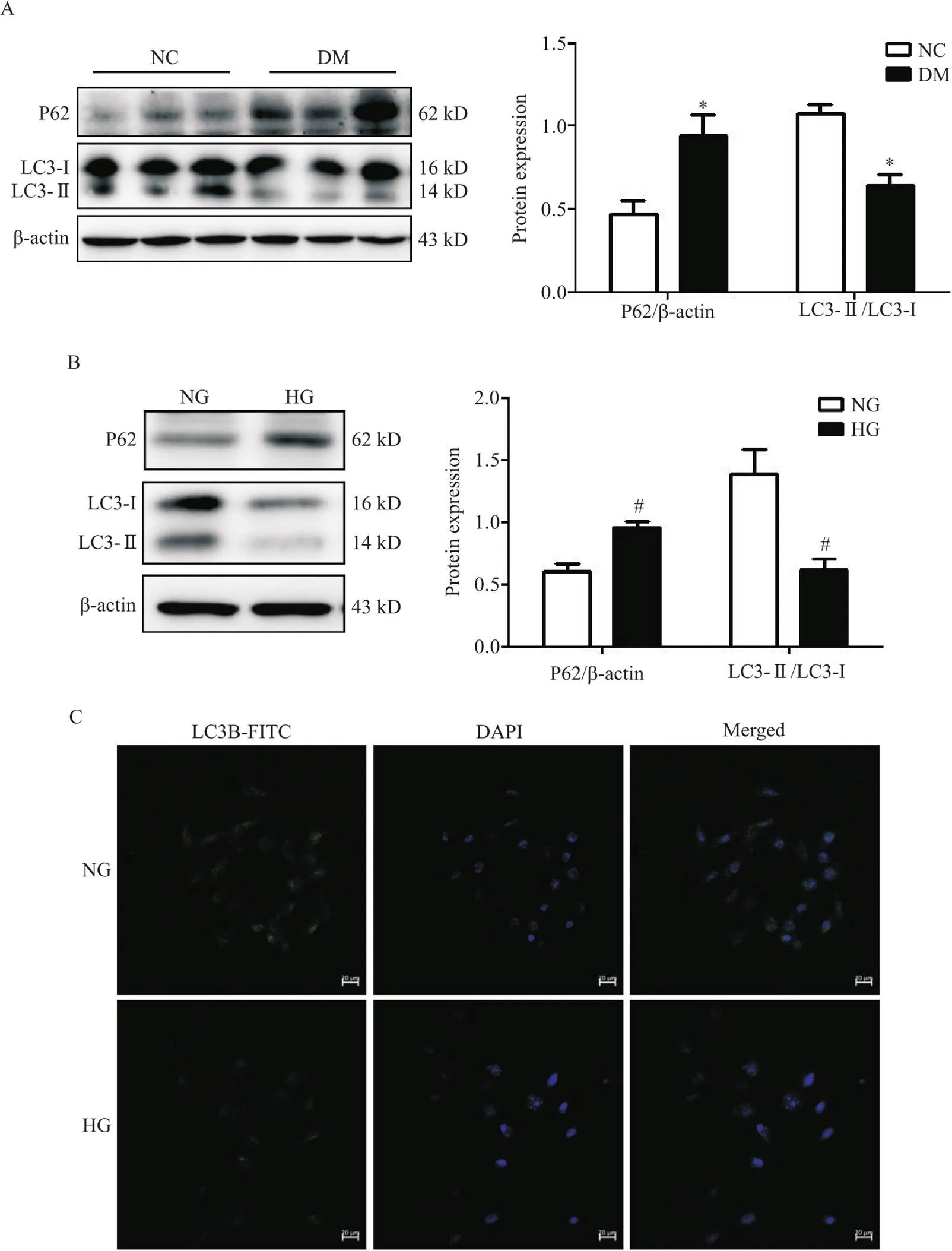
Figure 2. The autophagy levels in kidney tissues of diabetes mellitus (DM) mice and mouse renal tubular epithelial cells (RTECs) cultured with high glucose (HG) were inhibited. A: the protein levels of P62, microtubule-associated protein 1 light chain 3-I (LC3-I) and LC3-II in normal control (NC) group and DM group were determined by Western blot; B: the protein levels of P62, LC3-I and LC3-II in HG-stimulated mouse RTECs were determined by Western blot; C: laser scanning confocal microscopy was used to detect autophagosomes. Mean±SD. n=6. *P<0.05 vs NC group;#P<0.05 vs normal glucose (NG) group.
3 DM小鼠肾组织及HG培养的RTECs中E2F1和mTOR的表达
免疫组织化学染色结果显示,NC组小鼠肾小管间质几乎无E2F1的阳性表达,而DM组小鼠肾小管细胞质中颗粒状E2F1蛋白阳性表达显著增多,见图3A。与NC组相比,DM组小鼠肾组织E2F1的mRNA表达水平显著升高(<0.05),见图3B。Western blot结果显示,与NC组小鼠和NG培养的RTECs相比,DM组小鼠肾组织和HG培养的RTECs中E2F1和mTOR的蛋白表达水平显著升高(<0.05),见图3C、D。
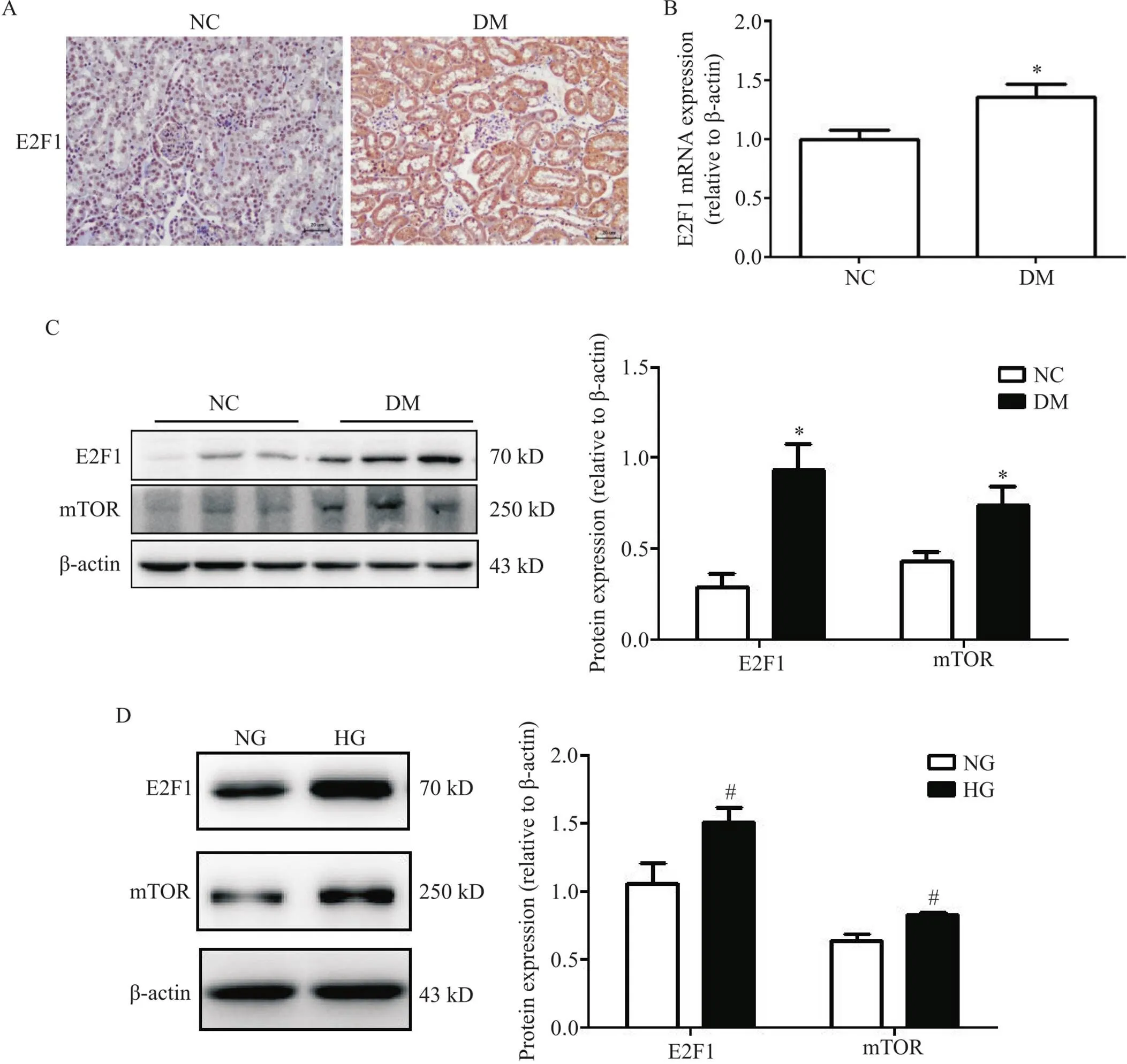
Figure 3. The expression levels of E2F transcription factor 1 (E2F1) and mammalian target of rapamycin (mTOR) were increased in kidney tissues of diabetes mellitus (DM) mice and mouse renal tubular epithelial cells (RTECs)cultured with high glucose (HG). A: immunohistochemical staining was used to detect the expression of E2F1 in the kidney tissue; B: the mRNA level of E2F1 was detected by RT-qPCR; C: the protein levels of E2F1 and mTOR in normal control (NC) group and DM group were determined by Western blot; D: the protein levels of E2F1 and mTOR in HG-stimulated mouse RTECs were determined by Western blot. Mean±SD. n=6. *P<0.05 vs NC group;#P<0.05 vs normal glucose (NG) group.
4 E2F1对RTECs自噬、EMT及细胞外基质沉积的影响
在HG培养的RTECs中敲减内源性表达后,mTOR的蛋白表达显著减少(<0.05),自噬指标LC3-II/LC3-I比值显著升高(<0.05),P62蛋白表达显著减少(<0.05),同时E-cadherin的蛋白表达显著增多(<0.05),α-SMA和Col III的蛋白表达显著减少(<0.05),见图4。
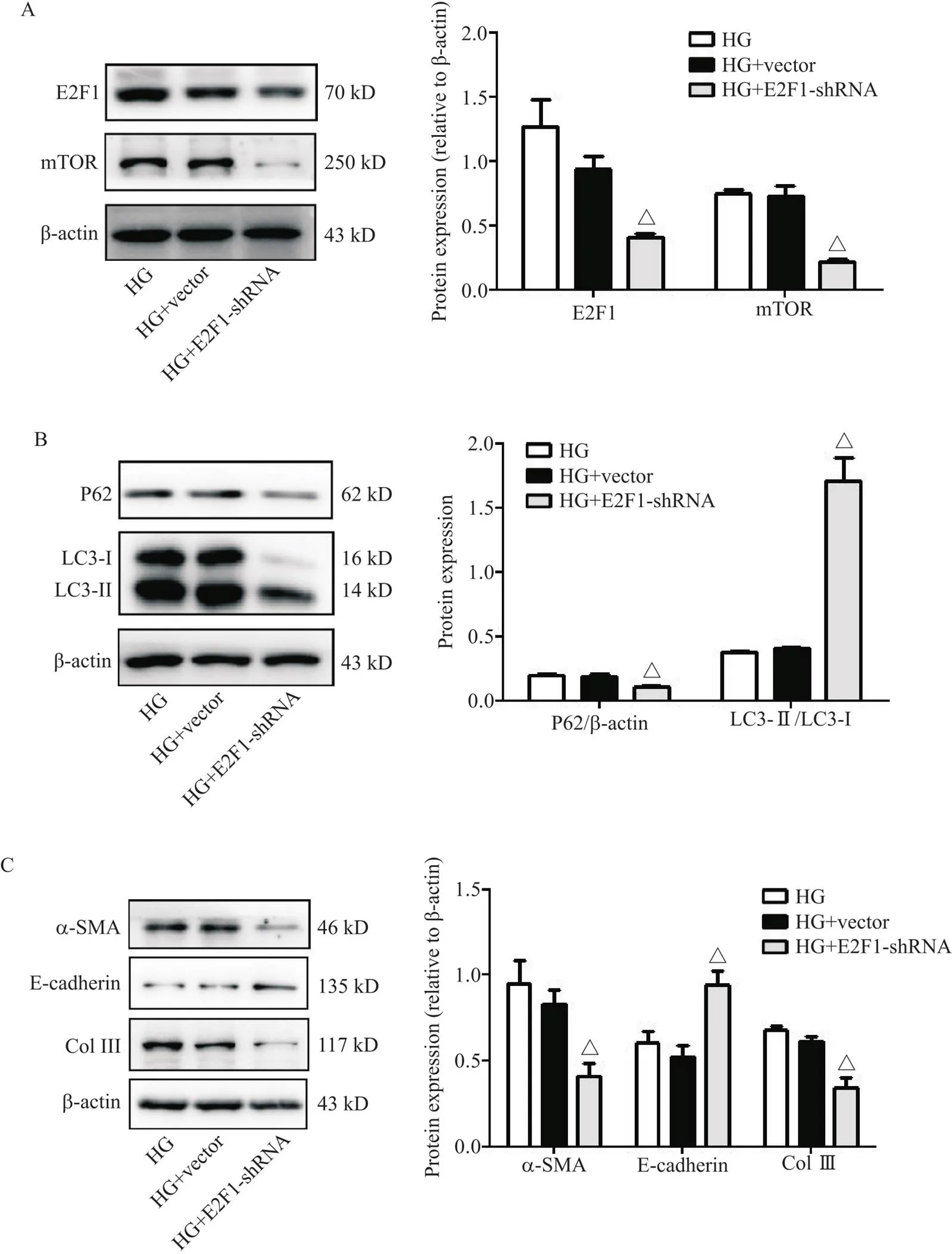
Figure 4. The protein levels of E2F transcription factor 1 (E2F1), mammalian target of rapamycin (mTOR), P62, microtubule-associated protein 1 light chain 3-I (LC3-I), LC3-II, α-smooth muscle actin (α-SMA), E-cadherin and collagen type III (Col III) in high glucose (HG)-stimulated mouse renal tubular epithelial cells transfected with E2F1 knockdown (shRNA) plasmid were determined by Western blot. A: E2F1 and mTOR; B: P62, LC3-I and LC3-II; C: α-SMA, E-cadherin and Col III. Mean±SD. n=3. △P<0.05 vs HG+vector group.
在HG状态下过表达后,RTECs中mTOR的蛋白表达显著增加(<0.05),P62蛋白表达显著增加(<0.05),LC3-II/LC3-I比值显著降低(<0.05),同时α-SMA和Col III蛋白表达显著增多(<0.05),E-cadherin蛋白表达显著减少(<0.05),见图5。
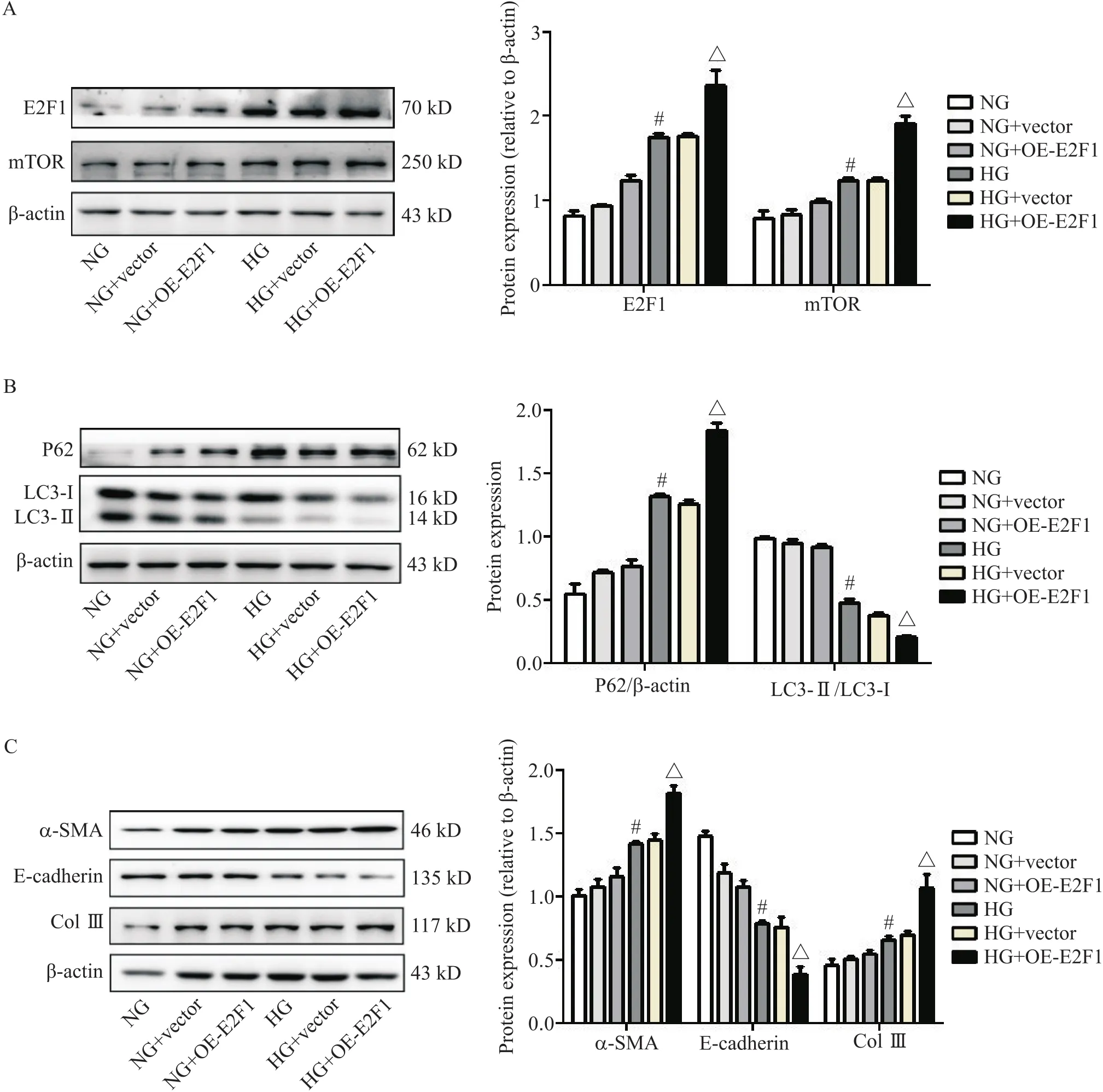
Figure 5. The protein levels of E2F transcription factor 1 (E2F1), mammalian target of rapamycin (mTOR), P62, microtubule-associated protein 1 light chain 3-I (LC3-I), LC3-II, α-smooth muscle actin (α-SMA), E-cadherin and collagen type III (Col III) in high glucose (HG)-stimulated mouse renal tubular epithelial cells transfected with E2F1 over-expression (OE) plasmid were determined by Western blot. A: E2F1 and mTOR; B: P62, LC3-I and LC3-II; C: α-SMA, E-cadherin and Col III. Mean±SD. n=3. #P<0.05 vs normal glucose (NG) group;△P<0.05 vs HG+vector group.
讨论
DN主要临床病理改变为肾小球硬化与肾小管间质纤维化,肾小管间质纤维化主要表现为肾间质内肾实质细胞损伤以及大量细胞外基质沉积[12],而EMT在DN肾小管间质纤维化中扮演着重要角色。RTECs发生EMT后,丢失上皮细胞表型(如E-cadherin表达减少等),获得间充质细胞表型(如α-SMA表达增加等),同时合成和分泌细胞外基质的能力明显增强。在本研究中,DM组小鼠生化指标血糖及24 h UTP均较NC组小鼠显著升高,且DM组肾组织病理结果显示,肾小管间质周围增宽,肾小管管腔扩张,基底膜增厚,肾间质可见炎性细胞浸润,Masson染色显示肾小球及间质有大量蓝色胶原沉积,说明小鼠DM模型复制成功,且发生了DN表现。同时Western blot及RT-qPCR结果还观察到DM组小鼠肾组织中α-SMA和Col III表达增加,E-cadherin的表达减少,提示DM小鼠肾组织发生了EMT及细胞外基质沉积增多,但其机制并不清楚。
有研究报道,自噬可以调控RTECs的EMT过程[13]。自噬是一种保守的蛋白降解过程,在代谢、退行性变和恶性疾病等方面发挥重要的作用[14-15]。在大鼠RTECs株NRK-52E中,二甲双胍能通过促进细胞自噬从而抑制由血清白蛋白所诱导的EMT[16]。在人近端RTECs中,脂联素能促进自噬进而抑制由胆固醇诱导的EMT,而自噬抑制剂氯喹可拮抗该效应[17]。但HG状态下自噬水平如何变化及是否可以通过调控EMT而影响DN的发病过程,需要进一步研究证实。本研究显示,DM小鼠肾组织以及HG培养的RTECs细胞中P62蛋白累积增加,LC3-II与LC3-I蛋白比值显著减小,激光扫描共聚焦显微镜观察到NG培养的RTECs中有较多自噬小体形成,而HG刺激RTECs细胞后,自噬小体形成明显减少。这些结果提示HG状态下RTECs的自噬水平受到抑制,但其原因并不清楚。
近年来研究显示,E2F1/mTOR信号通路与自噬关系密切,细胞周期相关转录因子E2F1的基因是一种原癌基因,可以诱导肿瘤细胞发生EMT[9-10];mTOR是一种丝氨酸/苏氨酸蛋白酶,可以调节细胞增殖和生长,促进蛋白质、脂质、细胞器等合成代谢,抑制自噬等分解代谢[8]。有研究报道,在骨肉瘤细胞中,E2F1能通过激活mTOR而抑制自噬[18];另外,在大鼠心肌细胞中增加E2F1的表达能促进mTOR的表达,进而抑制心肌细胞的自噬水平,而敲减的表达能明显抑制mTOR的表达,自噬水平明显升高[11]。在本研究中,DM小鼠肾组织及HG培养的RTECs中E2F1和mTOR蛋白表达增加自噬水平明显下调,且伴有EMT的发生。HG状态下在小鼠RTECs转染敲减质粒后,mTOR表达减少,细胞自噬水平增加,EMT现象减轻,细胞外基质沉积减少;而转染过表达质粒后mTOR蛋白表达增加,细胞自噬受抑,促进了EMT发生和细胞外基质沉积增多。这些结果提示,E2F1/mTOR可以通过调控表达,进而调节自噬水平,参与DN小鼠肾小管-间质纤维化过程。
综合文献及本实验研究结果,我们认为HG状态下E2F1/mTOR表达增多后抑制了自噬相关蛋白的表达,加剧了DM小鼠肾纤维化进程,从而促进其DN的发生与发展。因此深入研究E2F1/mTOR在DN肾纤维化中的调控机制,对DN的防治具有重要意义。
[1] Sagoo MK, Gnudi L. Diabetic nephropathy: an overview[J]. Method Mol Biol, 2020, 2067:3-7.
[2] Zhao Y, Yin Z, Li H, et al. MiR-30c protects diabetic nephropathy by suppressing epithelial-to-mesenchymal transition in/mice[J]. Aging Cell, 2017, 16(2):387-400.
[3] Zhang X, Guan T, Yang B, et al. Effects of ZnT8 on epithelial-to-mesenchymal transition and tubulointerstitial fibrosis in diabetic kidney disease[J]. Cell Death Dis, 2020, 11(7):544.
[4] Catalano M, Alessandro G, Lepore F, et al. Autophagy induction impairs migration and invasion by reversing EMT in glioblastoma cells[J]. Mol Oncol, 2015, 9:1612-1625.
[5]张昌志,石明隽,王圆圆,等. 丹酚酸B对高糖培养肾小管上皮细胞转分化的影响及意义[J]. 贵阳医学院学报, 2015, 40(4):337-345.
Zhang CZ, Shi MJ, Wang YY, et al. Effect and significance of salvianolic acid B on transdifferentiation of renal tubular epithelial cells cultured in high glucose[J]. J Guiyang Med Coll, 2015, 40(4):337-345.
[6] Du L, Chen E, Wu T, et al. Resveratrol attenuates hydrogen peroxide-induced aging through upregulation of autophagy in human umbilical vein endothelial cells[J]. Des Devel Ther, 2019, 13:747-755.
[7] LuQ, Wang WW, Zhang MZ, et al. ROS induces epithelial-mesenchymal transition via the TGF-β1/PI3K/Akt/mTOR pathway in diabetic nephropathy[J]. Exp Ther Med, 2019, 17(1):835- 846.
[8] Laplante M, Sabatini DM. mTOR signaling at a glance[J]. J Cell Sci, 2009, 122(20):3589-3594.
[9] Wang T, Chen X, Qiao W, et al. Transcription factor E2F1 promotes EMT by regulating ZEB2 in small cell lung cancer[J]. BMC Cancer, 2017, 17(1):719.
[10] Wang Z, Sun X, Bao Y, et al. E2F1 silencing inhibits migration and invasion of osteosarcoma cells via regulating DDR1 expression[J]. Int J Oncol, 2017, 51(6):1639-1650.
[11] Gu J, Fan YQ, Zhang HL, et al. Resveratrol suppresses doxorubicin-induced cardiotoxicity by disrupting E2F1-mediated autophagy inhibition and apoptosis promotion[J]. Biochem Pharmacol, 2018, 150:202-213.
[12]赵俊琳,张莹莹,段玲弟,等. 白藜芦醇对糖尿病小鼠肾脏自噬水平及肾间质纤维化的影响[J]. 中国病理生理杂志, 2020, 36(5):893-898.
Zhao JL, Zhang YY, Duan LD, et al. Effects of resveratrol on renal autophagy and interstitial fibrosis in diabetic mice[J]. Chin J Pathophysiol, 2020, 36(5):893-898.
[13] Wu W, Zhang M, Liu Q, et al. Piwil 2 gene transfection changes the autophagy status in a rat model of diabetic nephropathy[J]. Int J Clin Exp Pathol, 2015, 8(9):10734-10742.
[14] Kim KH, Lee MS. Autophagy: a key player in cellular and body metabolism[J]. Nat Rev Endocrinol, 2014, 10(6):322-337.
[15] Rohatgi RA, Shaw LM. An autophagy-independent function for Beclin 1 in cancer[J]. Mol Cell Oncol, 2016, 3(1):e1030539.
[16] Allouch S, Munusamy S. Metformin attenuates albumin-induced alterations in renal tubular cells[J]. J Cell Physiol, 2017, 232(12):3652-3663.
[17] Ruan CC, Li Y, Ma Y,et al. Adiponectin-mediated epithelial autophagy attenuates hypertensive renal fibrosis[J]. J Hypertens, 2016, 34(Suppl 1):e204.
[18] Meo-Evoli N, Almacellas E, Massucci FA, et al. V-ATPase: a master effector of E2F1-mediated lysosomal trafficking, mTORC1 activation and autophagy[J]. Oncotarget, 2015, 29, 6(29):28057-28070.
E2F1 promotes renal fibrosis in mouse diabetic nephropathy by regulation autophagy of renal tubular epithelial cells
ZOU Qin, LI Qing-xuan, QU Ling-ling, AN Xiao-min, HUANG Jing-hui, WANG Jin-yan, LONG Tian-hua, SHI Ming-jun△
(,&,,550025,)
To investigate whether E2F transcription factor 1 (E2F1) modulates the process of renal fibrosis in diabetic nephropathy, and to explore its possible mechanism.Twelve C57BL/6 mice were randomly divided into normal control (NC) group and diabetes mellitus (DM) group. Type 1 DM mouse model was established by injection of streptozotocin (55 mg/kg) for 5 d. At 16 weeks, the mice were sacrifaced to detect the blood glucose and 24 h urinary total protein. The pathological changes of the kidney were observed by HE and Masson staining. The expression of E2F1 was observed by immunohistochemical staining. Western blot and RT-qPCR were used to detect the changes in E2F1, mammalian target of rapamycin (mTOR), autophagy levels and fibrosis-related indicators. Renal tubular epithelial cells (RTECs) were transfected withknockdown or over-expression plasmids, and the changes of related proteins were detected by Western blot.Higher blood glucose and 24 h urinary total protein were observed in DM group than NC group (<0.05). The results of HE and Masson staining showed that the renal tubules of DM mice collapsed, the glomerular basement membrane thickened, and a large number of collagen fibers deposited in the renal interstitium. Compared with NC group, the mRNA expression of E2F1, collagen type III (Col III) and α-smooth muscle actin (α-SMA) was increased in DM group (<0.05), but the expression of E-cadherin was decreased (<0.05). The protein levels of E2F1, mTOR, P62, Col III and α-SMA were also increased in kidney tissues of DM mice and high glucose-induced RTECs (<0.05), while the protein expression of E-cadherin and the ratio of microtubule-associated protein 1 light chain 3-II (LC3-II)/LC3-I were decreased (<0.05). The protein levels of mTOR, P62, Col III and α-SMA were increased (<0.05), and the protein expression of E-cadherin and the ratio of LC3-II/LC3-I were decreased (<0.05) after knockdown ofin the RTECs. Moreover, over-expression ofincreased the protein expression of mTOR, P62, Col III and α-SMA (<0.05), and decreased the expression of E-cadherin and the ratio of LC3-II/LC3-I.E2F1/mTOR promotes the progression of renal fibrosis in diabetic nephropathy by inhibiting the autophagy of RTECs.
E2F transcription factor 1; Autophagy; Renal interstitial fibrosis; Diabetic nephropathy
R587.2; R363.2+1
A
10.3969/j.issn.1000-4718.2022.06.012
1000-4718(2022)06-1046-10
2021-07-08
2022-01-06
国家自然科学基金资助项目(No. 81860135; No. 82060142);贵州省科技计划项目(黔科合平台人才[2019]5801号)
Tel: 0851-86908348; E-mail: 2396272113@qq.com
(责任编辑:余小慧,李淑媛)
——一道江苏高考题的奥秘解读和拓展

