LPS诱导的A1型星形胶质细胞的能量代谢特征*
赵静, 陈茹, 沈桂萍, 张慧丰, 范彦英
LPS诱导的A1型星形胶质细胞的能量代谢特征*
赵静, 陈茹, 沈桂萍, 张慧丰, 范彦英△
(山西医科大学基础医学院药理教研室,山西 太原 030001)
探讨在脂多糖(lipopolysaccharide, LPS)诱导下,小鼠皮层星形胶质细胞转化为A1毒性表型的同时,其能量代谢所发生的变化。小鼠皮层星形胶质细胞培养8~9 d后,分为对照(control, CON)组和LPS组;采用CCK-8细胞增殖及毒性检测试剂盒检测不同LPS处理浓度及不同处理时间下的细胞活力;通过细胞免疫荧光染色技术检测胶质细胞原纤维酸性蛋白(glial fibrillary acidic protein, GFAP)的表达来鉴定星形胶质细胞的纯度;通过补体C3(complement component 3, C3)与GFAP细胞免疫荧光染色共定位,检测C3的表达水平;利用RT-qPCR技术检测C3、鸟苷酸结合蛋白2(guanylate-binding protein 2, GBP2)、S100钙结合蛋白A10(S100 calcium-binding protein A10, S100A10)、转谷氨酰胺酶1(transglutaminase 1, TGM1)及白细胞介素1β(interleukin-1β, IL-1β)的mRNA表达变化;采用Western blot技术检测C3蛋白表达变化。利用Seahorse XFp活细胞生物能量检测技术检测细胞线粒体呼吸功能和糖酵解水平的变化。免疫荧光染色结果显示,GFAP阳性细胞比例达98%以上。CCK-8结果显示,在不同浓度和不同处理时间下,LPS对星形胶质细胞的活力均无显著影响;RT-qPCR结果显示,在LPS剂量为100 μg/L时,C3和GBP2的mRNA表达在24 h均显著升高(<0.01),而S100A10和TGM1的mRNA表达则无显著变化。Western blot和免疫荧光染色结果显示,LPS处理后C3的蛋白表达显著升高(<0.01);RT-qPCR结果显示,IL-1β的mRNA表达水平显著升高(<0.01)。线粒体压力测定结果显示,LPS处理后的线粒体呼吸相关指标——氧消耗速率(oxygen consumption rate, OCR)与CON组相比无显著差异,而糖酵解速率相关指标——细胞外酸化速率(extracellular acidification rate, ECAR)、基础糖酵解质子流出速率(glycolytic proton efflux rate, glycoPER)、代偿性糖酵解、glycoPER百分比及线粒体质子流出速率(mitochondial proton efflux rate, mitoPER)/glycoPER在LPS处理后均显著下降(<0.01)。LPS可诱导星形胶质细胞向A1表型转换,无氧酵解水平显著降低,但对线粒体主导的有氧呼吸无显著影响。
星形胶质细胞;脂多糖;能量代谢
星形胶质细胞是中枢神经系统中最丰富的细胞类型,它们在维持中枢神经系统稳态和功能方面发挥着一系列不可或缺的作用[1],如为神经元提供能量、调节神经元突触发生和消除、维持血脑屏障和传导免疫信号。在感染、急性脑损伤及神经退行性病变中,星形胶质细胞的基因表达、形态和功能均会发生变化,表现为胞体肥大、突起数量和长度的增加等[2],这些变化被称为星形胶质细胞反应性。近年研究发现,反应性星形胶质细胞可被分为A1型(神经毒性表型)和A2型(神经保护表型)[3]。在阿尔茨海默病(Alzheimer disease, AD)、帕金森病(Parkinson disease, PD)、亨廷顿氏病、单侧脊索硬化等疾病中,研究者观察到脑内星形胶质细胞转化为A1表型,这种转化可能是神经元损伤的重要机制[4;5]。与此同时,在这些病理刺激下,星形胶质细胞的能量代谢模式也会发生变化。Ramos-Gonzalez等[5]证实,经Aβ刺激处理后,星形胶质细胞的线粒体功能及ATP生成显著降低。Xie等[6]在PD患者脑内提取的星形胶质细胞上发现,细胞线粒体呼吸功能降低而糖酵解水平升高。此外,在前动力蛋白2(prokineticin 2, PK2)刺激星形胶质细胞向A2表型转化的同时,线粒体呼吸功能增强,且ATP生成增多[7]。脂多糖(lipopolysaccharide, LPS)是炎症模型构建的诱导剂,可以通过诱导促炎介质的生成促进神经炎症反应。最近研究证实,LPS可以诱导培养的星形胶质细胞向A1表型转换,表现为A1标志物H2-T23、H2-D1、鸟苷酸结合蛋白2(guanylate-binding protein 2, GBP2)表达的增加[8]。但在此过程中,其能量代谢模式是否发生变化尚不清楚。
本研究在培养的小鼠皮层星形胶质细胞中,利用LPS刺激模型,观察了星形胶质细胞A1、A2表型的变化,并测定了细胞的线粒体压力和糖酵解速率,以探究星形胶质细胞A1表型转换与能量代谢之间的潜在关系。
材料和方法
1 主要试剂
胎牛血清(fetal bovine serum, FBS)购自CellMax;细胞基础培养液(Dulbecco's modified Eagle medium, DMEM;含4.5 g/L D-葡萄糖,以及L-谷氨酰胺和丙酮酸钠)购自Gibco;不含EDTA的胰蛋白酶、青-链霉素溶液(100×)、D-Hanks缓冲液和过氧化物酶标记的IgG抗体购自博士德生物工程有限公司;D-多聚赖氨酸购自碧云天生物科技有限公司;L-亮氨酸甲酯盐酸盐(L-leucine methyl ester hydrochloride, LME)购自麦克林试剂公司;LPS购自Sigma;Seahorse XF线粒体压力测定试剂盒、Seahorse XF糖酵解速率测定试剂盒以及XF Calibrant、Seahorse XF DMEM Medium、Seahorse XF 1.0M Glucose Solution、Seahorse XF 100mM Pyruvate Solution和Seahorse XF 200mM Glutamine Solution均购自安捷伦科技有限公司;Cell Counting Kit-8(CCK-8)、羊抗小鼠Alexa Fluor 488抗体和羊抗大鼠Alexa Fluor 594抗体购自翌圣生物科技有限公司;小鼠抗胶质细胞原纤维酸性蛋白(glial fibrillary acidic protein, GFAP)抗体和Alexa Fluor 488偶联的小鼠抗GFAP单克隆抗体(GA5)均购自Cell Signaling Technology。大鼠抗补体C3(complement component 3, C3)抗体购自Novus;兔抗C3抗体购自Abcam;UNIQ-10柱式RNA抽提试剂盒购自生工生物工程有限公司;RT Ⅲ All-in-one Mix及SYBR Green PCR Mix试剂购自莫纳生物科技有限公司。
2 实验方法
2.1皮层星形胶质细胞的培养C57BL/6J新生小鼠购自山西医科大学动物中心[许可证号:SYXK(晋)2019-0007]。新生小鼠按照文献中的方法[9-10],完整分离脑组织,将其浸泡在冷的D-Hanks缓冲液中,在体视显微镜下剥离脑膜和血管,去除海马,得到脑皮层组织,将组织用手术刀切碎。在37 ℃、5% CO2的条件下用0.25%不含EDTA的胰酶消化15 min,以含10% FBS的DMEM完全培养液终止消化。将细胞吹散后,收集细胞上清,以每瓶1.0×106个细胞的密度均匀接种在以D-多聚赖氨酸包被过的T25细胞培养瓶中,在37 ℃、5% CO2的细胞培养箱中培养,24 h后换液,之后每2~3 d更换培养液。培养8~9 d后,在室温下以260 r/min的速度摇细胞培养瓶18 h,以去除黏附在星形胶质细胞上的小胶质细胞、神经元及少突胶质细胞,然后弃去原来的培养液,PBS清洗1~2次,向培养液中加入终浓度为50 mmol/L的LME以去除小胶质细胞[11]。45 min后,弃去LME,PBS清洗后,换至完全培养液,继续培养24 h后可传代。
2.2细胞免疫荧光染色培养有星形胶质细胞的细胞爬片,经预冷的4%多聚甲醛固定,含0.1% Triton的PBS破膜后,加入10%的牛血清白蛋白封闭45 min,封闭后加小鼠抗GFAP抗体(1∶1 000)于4 ℃孵育过夜,PBS漂洗3次后,与羊抗小鼠Alexa Fluor 594抗体(1∶400)在室温下孵育2 h,经DAPI染色并封片固定;GFAP与C3免疫荧光染色共定位时,用Alexa Fluor 488偶联的小鼠抗GFAP单克隆抗体(GA5; 1∶200)和大鼠抗C3抗体(1∶400),在4 ℃下共同孵育过夜,PBS漂洗后与羊抗大鼠Alexa Fluor 594抗体(1∶400)在室温孵育2 h,DAPI染色封片后,于荧光显微镜下观察并拍照。
2.3CCK-8检测细胞活力星形胶质细胞按照每孔5 000个细胞接种在预先以100 mg/L多聚赖氨酸包被过的96孔板上,待其汇合度达到80%~90%,分别检测LPS浓度为100、50及10 μg/L时星形胶质细胞的活力。之后,在LPS浓度为100 μg/L的条件下,分别检测处理6、12和24 h后星形胶质细胞的活力。待测孔内每100 μl培养液中加入10 μl CCK-8溶液,继续在细胞培养箱中培养2 h,最后在酶标仪上检测450 nm处的吸光度。
2.4RT-qPCR检测mRNA表达按照试剂盒说明书操作提取星形胶质细胞的总RNA,使用RT Ⅲ All-in-one Mix及SYBR Green PCR Mix试剂进行mRNA的逆转录及扩增。内参照GAPDH的上游引物序列为5'-GTCGGTGTGAACGGATTTGG-3',下游引物序列为5'-GCTCCTGGAAGATGGTGATGG-3';白细胞介素1β(interleukin-1β, IL-1β)的上游引物序列为5'-CGTGGACCTTCCAGGATGAG-3',下游引物序列为5'-CATCTCGGAGCCTGTAGTGC-3';C3的上游引物序列为5'-GGCTAGACAAGGCTTGTGAGC-3',下游引物序列为5'-CCTGCACCTCATCTGAGCC-3';GBP2的上游引物序列为5'-GGAGGAGCTGTGTGGTGAAT-3',下游引物序列为5'-TTAGACGTGGCCCATTGACT-3';S100钙结合蛋白A10(S100 calcium binding protein A10,S100A10)的上游引物序列为5'-GTGCTCATGGAACGGGAGT-3',下游引物序列为5'-AAAGCTCTGGAAGCCCACTT-3';转谷氨酰胺酶1(transglutaminase 1, TGM1)的上游引物序列为5'-CCCTGGATGACAATGGAGTT-3',下游引物序列为5'-GAATAGCCGGTGCGTAGGTA-3'。IL-1β、C3、GBP2、S100A10及TGM1的mRNA水平归一化为内参照GAPDH的表达,并采用2-ΔΔCt法进行计算。
2.5Western blot检测蛋白表达将星形胶质细胞用细胞裂解液收集并超声后,提取总蛋白。总蛋白采用BCA法定量,经8%的聚丙烯酰胺凝胶电泳分离蛋白后,在70 V电压下转至聚偏氟乙烯膜上,以5%的脱脂奶粉在室温下封闭2 h,加入兔抗C3抗体(1∶2000),在4 ℃条件下孵育过夜。次日,TBST洗膜3次,每次5 min,将膜与辣根过氧化物酶标记的山羊抗兔IgG抗体(1∶6 000)在37 ℃条件下孵育1 h,并用ECL超敏化学发光液进行成像,Image Lab测定分析条带灰度值。
2.6线粒体压力和糖酵解速率的测定利用Seahorse XFp能量代谢分析仪测定星形胶质细胞在不同处理条件下线粒体压力和糖酵解速率的变化情况。在线粒体压力测定中,基础呼吸的氧气消耗速率(oxygen consumption rate, OCR)代表细胞在基础状态下的能量需求,与ATP产生相关的OCR代表了线粒体满足细胞能量需求的ATP合成能力。在测量基线OCR后,依次加入1.5 μmol/L oligomycin、4 μmol/L羰基氰-4-(三氟乙氧基)苯腙[carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone, FCCP]和0.5 μmol/L鱼藤酮/抗霉素(rotenone/antimycin A, Rot/AA)来检测基础呼吸值(细胞基础状态下的能量需求)、最大呼吸值、剩余呼吸容量(细胞适应能量变化的能力)及与ATP产生相关的OCR等指标,依此来反映线粒体的呼吸功能。在糖酵解速率测试中,测量基线细胞外酸化速率(extracellular acidification rate,ECAR)后,依次加入0.5 μmol/L Rot/AA和50 mmol/L 2-脱氧葡萄糖(2-deoxyglucose, 2-DG),最后一步添加的2-DG能够通过竞争性结合糖酵解途径的己糖激酶引起ECAR降低,依此来反映细胞的糖酵解水平降低。通过检测基础糖酵解质子流出速率(glycolysis proton efflux rate, glycoPER)、代偿性糖酵解、glycoPER百分比、线粒体质子流出速率(mitochondial proton efflux rate, mitoPER)/glycoPER等指标来反映细胞的糖酵解水平,其中glycoPER能够反映乳酸产生的质子流出速率。星形胶质细胞按照每孔1.5×104个接种在多聚赖氨酸包被过的8孔细胞培养板上进行培养,用LPS处理24 h后,将培养液更换为含10 mmol/L葡萄糖、1 mmol/L丙酮酸和2 mmol/L谷氨酰胺的37 ℃ DMEM无酚红培养液(pH 7.4),培养1 h后,上机检测。本实验测试了所有参数值,且进行了三次重复,数据采用WAVE软件进行分析。
3 统计学分析
采用软件GraphPad Prism 7.0进行数据处理分析。所有实验数据均采用均数±标准误(mean±SEM)表示。两组样本之间选用Student's检验法进行比较,三组或三组以上选用单因素方差分析进行比较分析。以<0.05为差异有统计学意义。
结果
1 星形胶质细胞纯度的鉴定
通过细胞免疫荧光染色检测星形胶质细胞的标志物GFAP,结果显示培养的皮层胶质细胞中GFAP阳性率达98%以上(图1),表明星形胶质细胞的纯度达98%以上,符合实验要求。

Figure 1. The measurement of purity of cortical astrocytes. The GFAP expression was measured by immunofluorescence. Scale bar=50 μm.
2 不同处理条件下LPS对小鼠皮层星形胶质细胞活力及向A1表型转换的影响
CCK-8检测结果显示,不同浓度和不同处理时间的LPS对细胞的活力均无显著影响(图2A、B)。RT-qPCR的结果显示,LPS处理后A1表型标志物C3和GBP2的表达呈浓度依赖性升高,在LPS浓度为50和100 μg/L时,C3(图2C)和GBP2(图2D)的表达均显著升高(<0.01),A2表型标志物S100A10(图2E)及TGM1(图2F)则无显著变化。与50 μg/L组相比,100 μg/L浓度下A1表型标志物C3和GBP2的表达更高,且Zhang等[8]证实LPS(100 μg/L)可诱导星形胶质细胞向A1表型的转换,因此本研究确定LPS的处理剂量为100 μg/L。LPS(100 μg/L)处理24 h后,A1表型标志物的mRNA表达最高,其中C3的mRNA表达(图2G)显著升高(<0.01),约为CON组的53.40倍,GBP2的mRNA表达(图2H)亦显著升高(<0.01),约为CON组的6.19倍,A2表型标志物S100A10(图2I)和TGM1(图2J)的表达则无显著变化。综合上述结果,我们在后续研究中选择LPS(100 μg/L)持续刺激24 h诱导星形胶质细胞的表型转换。

Figure 2. Effects of LPS treatment on phenotypic transformation and viability of primary mouse cortical astrocytes. The astrocytes were treated with different concentrations of LPS for 24 h or 100 μg/L LPS for different time. Cell viability was detected by CCK-8 assay (A and B;n=10). The mRNA expression of C3, GBP2, S100A10 and TGM1 in the astrocytes were detected by RT-qPCR (C to J,n=3). Mean±SEM. **P<0.01 vs CON group.
在此条件下,RT-qPCR结果表明,与CON组相比,在LPS的刺激下IL-1β的mRNA表达(图3A)显著升高(<0.01),约为CON组的4.56倍,表明LPS模型已成功建立。Western blot检测结果显示,LPS组C3的蛋白表达(图3B)显著升高(<0.01),约为CON组的20.99倍。星形胶质细胞GFAP与C3的免疫荧光染色结果表明,LPS诱导后,GFAP阳性的星形胶质细胞中检测到C3表达的细胞占90%以上,表明星形胶质细胞转变为A1表型的转换率达90%以上(图3C)。

Figure 3. Effects of LPS on expression of IL-1β and C3 in primary mouse cortical astrocytes. The astrocytes were treated with LPS (100 μg/L) for 24 h. The mRNA expression of IL-1β was detected by RT-qPCR (A;n=3). The relative expression of C3 protein was detected by Western blot (B;n=4). The co-localization of C3 and GFAP was detected by immunofluorescence staining (C; scale bar=50 μm). Mean±SEM. **P<0.01 vs CON group.
3 LPS对星形胶质细胞能量代谢模式转变的影响
在线粒体压力测试中,通过依次加入oligomycin(1.5 μmol/L)、FCCP(4 μmol/L)和Rot/AA(0.5 μmol/L)测量细胞的OCR(图4A)。测试结果显示,LPS不能诱导基础呼吸值(图4B)、最大呼吸值(图4C)、与ATP产生相关的OCR(图4D)和剩余呼吸能力(图4E)发生显著变化,表明LPS对星形胶质细胞以线粒体为主的有氧呼吸功能无显著影响。
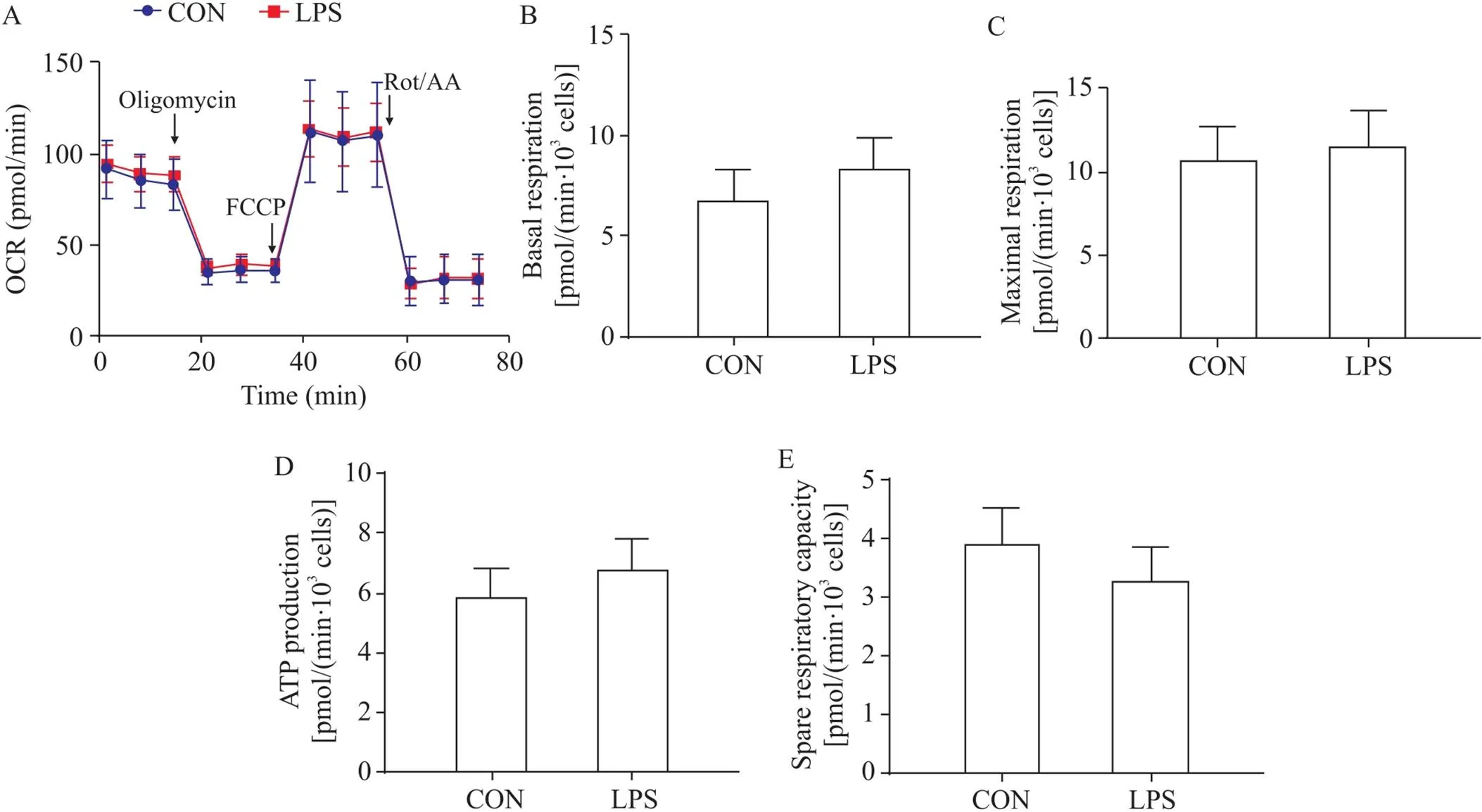
Figure 4. Effects of LPS on mitochondrial respiration function of primary mouse cortical astrocytes. The astrocytes were treated with LPS (100 μg/L) for 24 h, and then the oxygen consumption rate (OCR; A), basal respiration (B), maximal respiration (C), ATP generation (D) and spare respiratory capacity (E) were detected by Seahorse XF Cell Mito Stress Test Kit. Mean±SEM. n=6.
在糖酵解速率测试中,在加入2-DG前,LPS能够显著降低ECAR,在加入2-DG后,2-DG能够通过竞争性结合糖酵解途径的己糖激酶,从而引起ECAR进一步降低(<0.01),见图5A。测试结果显示,LPS能够诱导基础glycoPER降低,表明LPS能够显著降低星形胶质细胞乳酸的产生(<0.01),见图5B;LPS能够诱导代偿性糖酵解(图5C)和糖酵解途径产生的PER百分比(图5D)显著降低(均<0.01),表明LPS可使星形胶质细胞糖酵解水平显著降低;mitoPER/glycoPER比值(图5E)显著升高(<0.01),表明经过LPS处理后,星形胶质细胞的代谢方式以有氧糖酵解为主,无氧糖酵解水平显著降低。

Figure 5. Effects of LPS on the glycolysis rate of primary mouse cortical astrocytes. The astrocytes were treated with LPS (100 μg/L) for 24 h, and then the extracellular acidification rate (ECAR; A), basic glycolytic proton efflux rate (glycoPER; B), compensatory glycolysis (C), percentage of PER produced by glycolysis (D) and the ratio of mitochondrial PER (mitoPER) to glycoPER (E) were measured by Seahorse XF Glycolytic Rate Assay Kit. Mean±SEM. n=6. **P<0.01 vs CON group.
讨论
尽管星形胶质细胞在AD、PD等病理过程中发生的能量代谢变化已被研究,但在LPS刺激模型下,星形胶质细胞的能量代谢模式是否会发生变化尚不清楚。本研究证实,在LPS诱导星型胶质细胞向A1毒性表型转化的同时,线粒体呼吸功能并未发生显著变化,而糖酵解功能显著降低,提示星形胶质细胞的糖酵解活性对LPS炎性刺激更为敏感。星形胶质细胞被认为是一种“糖酵解”细胞,会消耗大量的葡萄糖并产生乳酸,具有很高的有氧糖酵解能力[12]。星形胶质细胞糖酵解活性的降低会导致其为自身提供能量的不足并减少乳酸释放,而乳酸作为神经元的主要能量来源,是维持神经元活动所必须的[12],对谷氨酸引起的神经元兴奋性毒性损伤有抑制作用。本课题组研究发现LPS处理的星形胶质细胞无氧糖酵解水平降低,乳酸产生减少,因此我们推测,糖酵解活性的降低可能是LPS诱导A1型星形胶质细胞损伤神经元的机制之一。
已有研究证实,在同样可诱导星形胶质细胞向A1型转化的Aβ刺激AD的模型中,星形胶质细胞存活率显著降低,同时线粒体呼吸功能被抑制,线粒体产生的ATP显著减少[6, 13]。相似地,从PD患者脑内提取的星形胶质细胞(A1型)与正常细胞相比,线粒体呼吸功能也显著下降,但无氧糖酵解活性显著增强[5]。上述研究与本研究观察到的A1型星形胶质细胞的线粒体呼吸功能不变而糖酵解活性降低的现象并不一致。由于本研究中LPS处理对细胞存活率并无显著影响,我们推测,在不同模型中,A1型星形胶质细胞线粒体呼吸和糖酵解功能的不同变化可能与不同模型所致的细胞损伤程度的差异有关。A1型星形胶质细胞的能量代谢在不同模型中可能具有异质性。
此外,Voloboueva等[14]证实,LPS(1 mg/L)处理BV2细胞3 h后,M1促炎表型显著增加,基础OCR值和ATP生成等表征线粒体能量代谢的指标均显著低于对照组,而ECAR表征的糖酵解代谢增强[15]。Namwanje等[16]发现,LPS可使树突细胞的代谢模式从高效的氧化磷酸化转向糖酵解。以上结果与本研究中星形胶质细胞在LPS刺激后所发生的糖酵解活性降低的结果相反,提示LPS刺激不同的细胞,其所发生的能量代谢模式的变化存在差异,这可能在一定程度影响了这些细胞在炎症反应中所发挥的不同作用。
综上所述,LPS诱导的A1型星形胶质细胞的能量代谢特征为线粒体呼吸功能不变而糖酵解活性降低。这种能量代谢模式的变化进一步补充了A1型星形胶质细胞产生神经元毒性作用的机制。该研究为利用LPS模型探讨星形胶质细胞活性及功能变化提供了新的指标。
[1] Miller SJ. Astrocyte heterogeneity in the adult central nervous system[J]. Front Cell Neurosci, 2018, 12:401.
[2] Fan YY, Huo J. A1/A2 astrocytes in central nervous system injuries and diseases: angels or devils?[J]. Neurochem Int, 2021, 148:105080.
[3] Zamanian JL, Xu L, Foo LC, et al. Genomic analysis of reactive astrogliosis[J]. J Neurosci, 2012, 32(18):6391-6410.
[4] Goetzl EJ, Schwartz JB, Abner EL, et al. High complement levels in astrocyte-derived exosomes of Alzheimer disease[J]. Ann Neurol, 2018, 83(3):544-552.
[5] Ramos-Gonzalez P, Mato S, Chara JC, et al. Astrocytic atrophy as a pathological feature of Parkinson's disease with LRRK2 mutation[J]. NPJ Parkinsons Dis, 2021, 7(1):31.
[6] Xie Y, Zheng J, Li S, et al. GLP-1 improves the neuronal supportive ability of astrocytes in Alzheimer's disease by regulating mitochondrial dysfunction via the cAMP/PKA pathway[J]. Biochem Pharmacol, 2021, 188:114578.
[7] Neal M, Luo J, Harischandra D S, et al. Prokineticin-2 promotes chemotaxis and alternative A2 reactivity of astrocytes[J]. Glia, 2018, 66(10):2137-2157.
[8] Zhang HY, Wang Y, He Y, et al. A1 astrocytes contribute to murine depression-like behavior and cognitive dysfunction, which can be alleviated by IL-10 or fluorocitrate treatment[J]. J Neuroinflammation, 2020, 17(1):200.
[9]乔圆,廖雁,南方,等. 组胺对星形胶质细胞Egr-1表达的调节作用[J]. 中国病理生理杂志, 2016, 32(4):680-685.
Qiao Y, Liao Y, Nan F, et al. Effects of histamine on mRNA expression of Egr-1 in astrocytes[J]. Chin J Pathophysiol, 2016, 32(4):680-685.
[10] Mccann MS, Fernandez HR, Flowers SA, et al. Polychlorinated biphenyls induce oxidative stress and metabolic responses in astrocytes[J]. Neurotoxicology, 2021, 86:59-68.
[11] Hamby ME, Uliasz TF, Hewett SJ, et al. Characterization of an improved procedure for the removal of microglia from confluent monolayers of primary astrocytes[J]. J Neurosci Methods, 2006, 150(1):128-137.
[12] Takahashi S. Neuroprotective function of high glycolytic activity in astrocytes: common roles in stroke and neurodegenerative diseases[J]. Int J Mol Sci, 2021, 22(12):6568.
[13] Phatnani H, Maniatis T. Astrocytes in neurodegenerative disease[J]. Cold Spring Harb Perspect Biol, 2015, 7(6):a020628.
[14] Voloboueva LA, Emery JF, Sun X, et al. Inflammatory response of microglial BV-2 cells includes a glycolytic shift and is modulated by mitochondrial glucose-regulated protein 75/mortalin[J]. FEBS Lett, 2013, 587(6):756-762.
[15] Kucic N, Racki V, Sverko R, et al. Immunometabolic modulatory role of naltrexone in BV-2 microglia cells[J]. Int J Mol Sci, 2021, 22(16):8429.
[16] Namwanje M, Bisunke B, Rousselle TV, et al. Rapamycin alternatively modifies mitochondrial dynamics in dendritic cells to reduce kidney ischemic reperfusion injury[J]. Int J Mol Sci, 2021, 22(10):5386.
Characteristics of energy metabolism in lipopolysaccharide-induced A1-type astrocytes
ZHAO Jing, CHEN Ru, SHEN Gui-ping, ZHANG Hui-feng, FAN Yan-ying△
(,,030001,)
To investigate the change of energy metabolism during transformation of mouse cortical astrocytes to the A1 toxic phenotype induced by lipopolysaccharide (LPS).Primary mouse cortical astrocytes were divided into control (CON) group and LPS group after cultured for 8 to 9 d. Cell Counting Kit-8 (CCK-8) was used to detect the cell viability. The expression of glial fibrillary acidic protein (GFAP) was detected by immunofluorescence. The expression of complement component 3 (C3) was detected by co-staining with GFAP. The mRNA levels of C3, guanylate-binding protein 2 (GBP2), S100 calcium-binding protein A10 (S100A10), transglutaminase 1 (TGM1) and interleukin-1β (IL-1β) after LPS treatment were detected by RT-qPCR. The expression of C3 protein was assessed by Western blot. The levels of cellular mitochondrial respiratory function and glycolysis were detected by Seahorse XFp live-cell bioenergy detection technology.Immunofluorescence staining showed that the percentage of GFAP reached more than 98%. Treatment with LPS did not change the viability of astrocytes. The mRNA levels of C3 and GBP2 were significantly increased at 24 h after treatment with LPS at the concentration of 100 μg/L (<0.01), while the expression of S100A10 and TGM1 did not change. Both Western blot and immunofluorescence staining showed C3 was significantly increased after treated with LPS (<0.01). The results of RT-qPCR showed that the mRNA level of IL-1β was significantly increased (<0.01). Mitochondrial pressure measurement showed that there was no significant difference in oxygen consumption rate (OCR), an indicator of mitochondrial respiration, between control group and LPS group. Glycolysis rate-related indicators such as extracellular acidification rate (ECAR), basal glycolytic proton efflux rate (glycoPER), compensatory glycolysis, the percentage of glycoPER, and mitochondial proton efflux rate (mitoPER)/glycoPER were decreased significantly after LPS treatment (<0.01).LPS induces the transformation of astrocytes to A1 phenotype and reduces the level of anaerobic glycolysis, but did not change the mitochondrial aerobic respiration.
Astrocytes; Lipopolysaccharides; Energy metabolism
R741.02; R363.2
A
10.3969/j.issn.1000-4718.2022.06.002
1000-4718(2022)06-0970-08
2022-01-20
2022-03-16
国家自然科学基金资助项目(No. 81872854; No. 81202520)
Tel: 0351-4135172; E-mail: fyanying6@hotmail.com
(责任编辑:卢萍,罗森)

