Nanomedicine-based approaches for delivery of herbal compounds
Virender Kumar,Vandana Garg*,Harish Dureja*
1Department of Pharmaceutical Sciences,Maharshi Dayanand University,Rohtak 124001,Haryana,India.
Abstract Herbal compounds have enormous potential to enhance the efficacy of current cancer treatments.Several herbal compounds have been reported to enhance the effect of chemotherapy, hormonal therapy and gene therapy on tumor cells.Compared to synthetic formulations, natural products are considered safer and do not have toxic effects at therapeutic doses, thus increasing demand for them.These compounds can significantly effect on signal transduction pathways, reduce side effects, and even inhibit the efflux of anticancer drugs.This review will explain factors influencing herbal nanomedicines,targeting of nanomedicines, nanocarriers used for delivery, herbal nanomedicines in cancer,phytochemical based combinatorial nanomedicines, and challenges to the nanocarriers based cancer therapy.
Keywords: herbal compounds; nanomedicines; targeting; cancer; nanocarriers; drug delivery
Background
Cancer has become such a vulnerable disease that every individual of any age group is prone to it.It has been ranked second among the conditions causing large-scale deaths of people though cardiovascular disorders are in the top position[1].The mortality rate is rising day by day due to cancer, and about 13.1 to 17 million deaths are estimated to be reported by the year 2030 [2].Various methodological strategies and approaches are currently being employed to treat cancer, such as the combination of chemotherapy (United States Food and Drug Administration approved several drugs having antiproliferative,cytotoxic action, ant migratory effect on malignant tumors, several antibodies), radiotherapy, immunotherapy, and surgery [3, 4].However, treatment by chemotherapy produces several severe side effects such as cytotoxicity, nausea, vomiting, hair loss, discomfort,mental distress, etc.It simultaneously affects other normal cells because of the non-selective approach and toxicity on a slight increase in dose and even can be lethal to the patient.The patient’s non-compliance to slight overdosage and relapse of disease post-chemotherapy has been a significant problem in the chemotherapy approach [5].
Nevertheless, surgery is the last option for terminal cancer patients.So, keeping in view the adverse effects caused due to chemicals and drugs mentioned above and also the expensiveness of these drugs,there has been a shift for finding naturally-derived medicinal agents which have anticancer activity and synthesizing the suitable administrable novel formulation from them, which is highly patient-compliance, lesser side effects and more therapeutic action[6].Since these extracts either in the form of volatile oils, resins,gums, alkaloids, terpenoids, saponins, etc.are derived from naturally occurring plants or their explants like stems, leaves, flowers, fruits,shoot, roots, rhizome, bark, etc.They have lesser toxic side effects on patient health, easily affordable, safe to use and produces deemed results [7].Herbal natural products with anti-inflammatory, antiviral activity, chemopreventive & chemosensitization properties have already been revealed in literature as well [8, 9].Similarly, plants having their significant constituents as polyphenols, flavonoids, and xanthanoids are considered more potent for anticancer activity.
The majority of the herbal medicines obtained from the plant extracts usually have either antioxidant action, antiproliferative or anti-migratory activity, as well as selectively induced cellular death(apoptosis) and cytotoxic action by following any of the subcellular mechanisms given below - by decreasing the level of lipid peroxidation and acid phosphatase activity, inhibition of PI3K/Akt pathway, repairing DNA, enhancing body immunity, arrest of the cell cycle at metaphase stage by binding to tubulin protein which is the vital constituent of microtubules, disrupting its functions and hence,mitotic spindle apparatus is abolished [10].In this way, it prevents cell division (antitumor effect), activation of caspases 3 & caspases 9,downregulation of cyclooxygenase-2, by increasing the level of reactive oxygen species, inhibition of topoisomerase-I, etc.[11-13].Although natural products in the form of herbal medicines have been traditionally used by 80% of the population of developing countries,they remained unnoticed until the past few decades because of a lack of knowledge of natural compounds and extracts obtained from plants[14].Due to advancements in research and development of sophisticated technologies for isolating of active compounds from plants, it has been possible to extract out main chemical compounds responsible for anticancer effect with detailed chemical composition and action at the molecular level.However, there is a subtle major challenge in developing formulations/dosage forms of these active compounds, which satisfies all the shortcomings of the conventional dosage regimen already prepared for the treatment of cancerous patients.So, various nanoformulations or nanomedicines (polymeric nanoparticles (NPs), dendrimers, liposomes, nanotubes, magnetic NPs,gold NPs, etc.) have been developed for anticancer agents are in their clinical trial phase/stage for further approval by regulatory authorities[15].
Nanoformulations imply various advantages over shortcomings of conventional dosage formulations like: site-specificity due to very small nano-size , reduced cytotoxicity of normal cells, long half-life so overcomes problems of frequent dosing to the patient, reduction in possible therapeutic side-effects, sustained release of drugs, controlled drug delivery, enhanced drug efficacy, improving upon the hydrophobicity problem of some of the naturally-derived drugs,decrease in cell resistance to therapy with enhanced surface functionalization of a drug herbal medicines aid in chemotherapy in certain aspects because of their high safety, such as activating innate immunity, influencing cytokine regulation (inflammatory mediators),increasing antigen presentation to the immune system, etc.[16-19].There has been immense research for incorporating herbal extracts or compounds of medicinal value into nanomedicines to gain enhanced effects than usual, i.e., synergistic effect [20].Diagrammatic representation of historical development of nanomedicines for cancer treatment is shown in Figure 1 below.
Factors influencing herbal nanomedicines in vivo delivery
Biological medium
Nanomedicines, especially those having charged surface on reaching the systemic circulation or any of the other biological media like interstitial fluid, lymph, etc.via any route, gets incorporated with biomolecules like lipids, proteins, etc., which alters it’s expression i.e.may either accelerate or retard its cellular uptake, may affect or inhibit its pathway for attacking target site, etc.[21].
Immune and metabolic dysfunction
Tumor-associated macrophages are considerably higher than cancer-related inflammatory cells.So, targeting them can indirectly affect tumor growth.For example, doxorubicin-loaded liposomes showed an enhanced effect on incorporating alendronate into them for targeting tumor-associated macrophages [22].
Pathological conditions and structure
Tumour region abnormally differs from the specific body region in many ways, for example, high permeability of tumour vessel, high interstitial fluid pressure, more stiff and denser stromal cells on visualizing their microenvironment [23].These parameters considerably affect the in vivo delivery of nanomedicines to the targeted site.Stromal cells comprising tumor-associated fibroblast are highly impregnated in desmoplastic tumors, which produce a large amount of extracellular matrix and,in this way,inhibit nanomedicines transport.
Other factors
Route of administration, molecular size of nanomedicines, the physiochemical nature of drug entity, chemoselectivity of drug,pharmacological aspects of the drug and biopharmaceutical parameters of the drug, etc., influence the in vivo delivery of nanomedicines to the biological medium in the concerned patient.
Nanomedicines targeting
This technology involves the site specific delivery of nanomedicines i.e.towards tumour cells without affecting or producing very little harm to the normal healthy cells [25].Drug targeting is usually of two types.
Active targeting
In this type of drug targeting, the site-specific (tumor region) delivery of drug from suitable nanocarriers is achieved by changing the nanomedicines entity’s surface characteristics, such as functionalization, conjugation ligand moiety onto the surface, which helps in further binding to the specific receptor.This can be achieved by targeting cancer cells (binding to overexpressed receptors like transferrin, folate, glycoproteins, epidermal growth factor receptor,etc.) or the tumor endothelium (approaching endothelial growth factor receptor) vascular cell adhesion molecule-1, matrix metalloproteinase) or even both.
The key mechanism behind the active targeting is the ligand being recognized by its active target.Ligands include antibodies, proteins,peptides, nucleic acids, sugars, and vitamins.Proteins, sugars, and lipid molecules may be target molecules in diseased organs or on the surface of cells.The specificity of the targeting and the capability of delivery are essential factors to consider when evaluating the efficiency of an active targeting system.Biodistribution and the interactions between the conjugated ligand and nanomedicine system,which regulate specificity, occur during the biodistribution of the ligand-functionalized nanomedicine.Specificity is determined by the properties of the ligand and nanomedicine carrier.Nanomedicine carriers that are actively targeted provide a complementary approach to enhanced cancer nanomedicines that is currently viewed as a promising strategy.
And he carved her a pair of wooden feet and some crutches24, and taught her a psalm which is always sung by sinners; she kissed the hand that guided the axe, and went away over the heath.
Active targeting of nanocarriers like NPs involves interacting with their target antigen.Active-targeted NPs are considered challenging to develop because of that intrinsic property.It is essential to design active-targeted NPs to have long blood circulation times.The enhanced permeability, retention effect enables NPs to reach their targets, since molecular targets are usually located in the extravascular space of the tumor [26].Active targeting of cancer stem cells is shown in Figure 2.
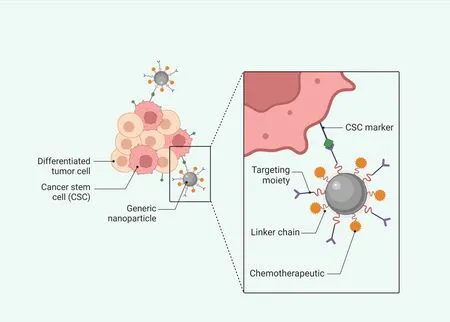
Figure 2 Active targeting of cancer stem cells with NPs.CSC, cancer stem cell; NPs, nanoparticles.
Passive targeting
In this type of drug targeting,nanocarriers are delivered to the specific tumor region by taking advantage of its changed pathological microenvironment, for example, by exploiting enhanced permeability,retention effect of the drug, etc.surrounding tumor region.
Passive targeting involves preparing a drug carrier complex that allows the compound to resist being removed by body mechanisms such as metabolism, excretion, opsonization, or phagocytosis so that the compound can remain circulating in the bloodstream as a result of properties such as power of hydrogen ion temperature, or molecular size that allow it to reach the target receptor.To passively target diseased tissues under various pathological conditions, the physiology of affected tissues is exploited.There is no rupture in normal tissue as the drug travels through the blood vessel.A high concentration of nanomedicine is built up in the later region due to the percolation of the drug through holes in the tumor-affected area, thus starting the healing process [27].
Nanocarriers used in cancer therapy
These are the recently developed formulations for overcoming the shortcomings of conventional dosage techniques by exploiting the advanced ventures of nanotechnology.There are a lot of nanocarriers(liposomes, micelle, dendrimers, polymeric NPs, solid lipid NPs,nanoshells, magnetic nano particles, etc.) available on the market for various formulations for treating various pathological conditions [28].A lot of research and clinical trials are ongoing to approve various nanoformulations for cancer treatment.Numerous types of nanocarriers are used in drug delivery as shown in Figure 3.A description of some of them is mentioned below.
Liposomes
These comprise about 33% of the total nanoformulations under clinical trials for cancer treatment.They consist of a bilayer membrane around a hollow core.Since lipids possess an amphiphilic character (hydrophilic head, hydrophobic tail), they can be used for delivery of both hydrophilic and hydrophobic drugs [29].Examples of liposomal nanoformulations include promitil (under investigation for solid tumor), doxorubicin monotherapy plus trastuzumab,ThermoDox, liposomal daunorubicin and cytarabine for acute myeloid leukaemia, etc.[30-32].
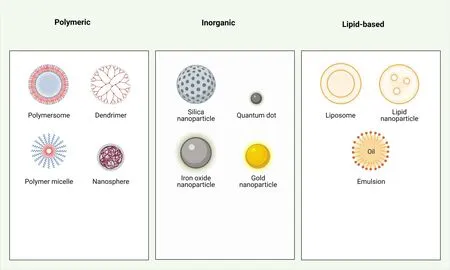
Figure 3 Classification of nanocarriers
Solid lipid NPs
They consist of a core consisting of solid lipid molecules such as fatty acids, phospholipids, mono, di, triglycerides, etc., with simultaneous exhibiting high biodegradability and biocompatibility, most negligible toxicity controlled zero-order drug release.These can be modified to incorporate both hydrophilic and hydrophobic anticancer agents [33].Example includes cucurbitacin solid lipid NPs (for hepatic carcinoma),podophyllotoxin solid lipid NPs, etc.
Nanostructured lipid carriers
These are composed of spatially different lipids which formed by blending solid lipid with liquid lipid(oil)to overcome shortcomings of solid lipid NPs by offering advantage of controlled drug delivery [34].Example includes folic acid-curcumin-nanostructured lipid carrier,isoliquiritigenin solid lipid NPs, etc.Liposome based delivery is shown in Figure 4.
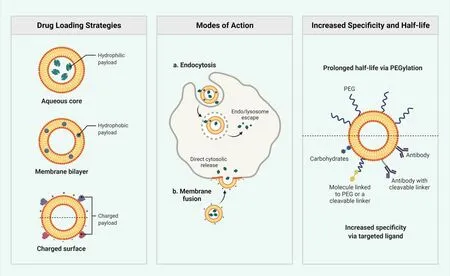
Figure 4 Liposome based delivery polyethylene glycol.PEG, polyethylene glycole.
Lipid micelles
Protein NPs
Albumin is exploited in the formulation of protein NPs Examples includes Ontak (first actively targeted protein nanoparticle approved in 2008 and used for various haematological malignancies treatment)37 Abraxane (around 130 nm in size and encapsulated with paclitaxel(PTX)) etc.[37, 38].
Polymeric NPs
These are also the most widely used and effective nanocarriers for cancer treatment.The drug is encapsulated into a polymeric system having a definite ratio of hydrophilic and hydrophobic portions to release the drug from formulation at a predetermined rate with sustained action.Polymer-like poly lactic-co-glycolic acid (PLGA) is used for preparing NPs as it has both hydrophilic and hydrophobic properties [39].To target cancer cells, surface functionalization can be performed on NPs.Opsonisation can be performed on NPs to stabilize steric hindrance.Examples of polymeric NPs formulation include vivitrol, arestin, Risperdal Consta, opaxio, Etirinotecan Pegol-102 etc.[40].
Polymeric micelles
These are efficient nanocarriers with high stability and biocompatibility and are highly useful for diagnostic and therapeutic applications.Examples of micellar NPs includes i.e.micellar PTX formulation, approved in 2009 by United States Food and Drug Administration for ovarian cancer treatment), nanoplatin,7-ethyl-10-hydroxycamptothecin and polymeric nanoparticle micelle formulation of PTX for lung and metastatic breast cancer, etc.[41].
Polymer drug conjugates
In this type of nanocarriers, one or several drugs can be bonded to functional groups of the polymers via covalent bonds or spacers,which enhance the drug accumulation in tumor cells, prolonged circulation with sustained release of the drug.An example includes high molecular weight polymers of curcumin effective against breast and ovarian cancer cell lines[42].
Dendrimers
These are emerging nanocarriers in nanotechnology research field because of shortcomings of other nanocarriers.They consist of tree-like branches or arms which are radially symmetrical and produce a homogenous monodisperse system.They often form micelles because of their hydrophobic core and hydrophilic surface.Drug release from dendrimers is regulated by the number of branches,rigidity, and density [43].
Carbon nanotubes
They are similar to a seamless cylinder with either open or closed end made by rolling graphene sheets ranging from 1 nm to several micrometers in their size and can be single walled (diameter and length of the cylinder being 1 and 50 nm respectively) or multiwalled consisting of concentrically arranged cylinders which are 10 microns in length, 2-100 nm in diameter and distant apart by about 0.35 nm[44, 45].Site-specific delivery to cancer cells can be achieved via surface functionalization or adding different polymers.
Magnetic NPs
These are the nanocarriers applicable to both molecular and cellular levels and can be used for clinical, therapeutic, and diagnostic applications such as developing magnetic resonance imaging contrast agents and target drug delivery [46].They typically consist of an outer thin layer shell made up of a stabilizer (polyethylene glycol, dextran,chitosan, polyethyleneimine-like polymers) and a magnetically activated core (iron, cobalt), iron oxide, and alloys having magnetic properties) [47].
Gold NPs
They are prepared by reducing gold salts in suitable stabilisers (to prevent agglomeration) to yield monolayer protected clusters which are often functionalised with suitable poly and oligo ethylene glycol molecules [48, 49].They interact with thiols to provide controlled drug release which is their additional advantage [50].They are extensively employed to diagnose cancer and deliver therapeutic agents.
Herbal or bioactive nanomedicines effective against cancer
In many health systems worldwide, traditional medicines play an essential role.Developing and developed countries are beginning to recognize and develop plants’ medical and economic benefits.Herbs are plants or plant components used for their aromas, flavors, and medicinal properties.Medicine made from herbal ingredients is often called phytomedicine or herbal medicine.Herbal medications have traditionally been used to maintain or promote health [1, 2].According to estimates, the current Indian pharmacopeia comprises about one-quarter of plants-based medicines.Herbs that are conventionally recognized as medicinal are plant-derived drugs that have been used in traditional healing systems to treat disease organically, without being chemically altered.Clinical trials are undergoing on plants that contain chemicals with chemoprotective properties[3].Table 1 describes about the type of nanocarriers,active chemical constituent or drug, nanomaterial of formulation, effective against cancer type, biological source of phytonanomedicines which are effective against cancer and are either approved, and pre-clinical testing phase or in clinical trial phase for getting approval by regulatory authorities.
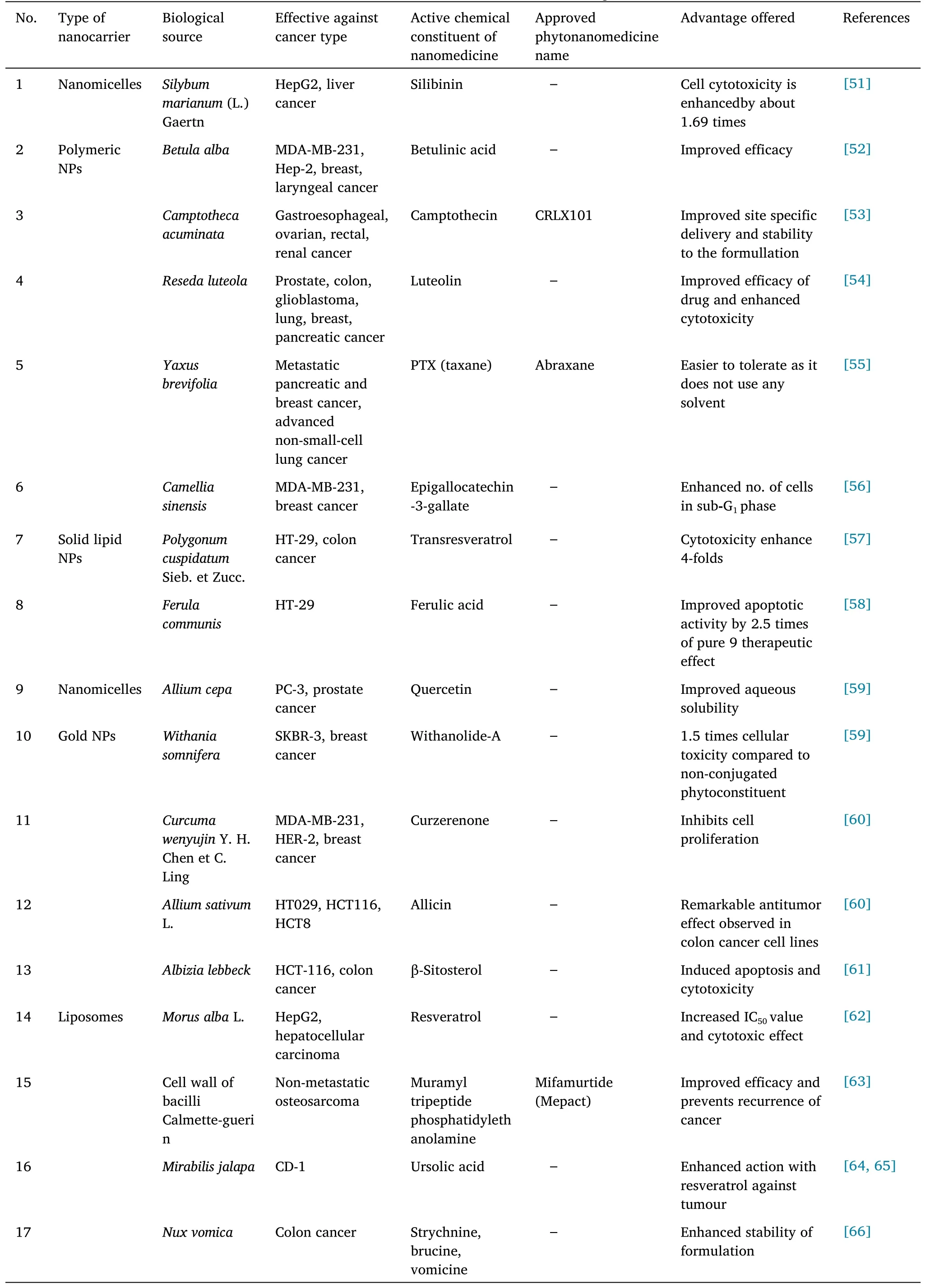
Table 1 Herbal or bioactive nanomedicines effective against cancer
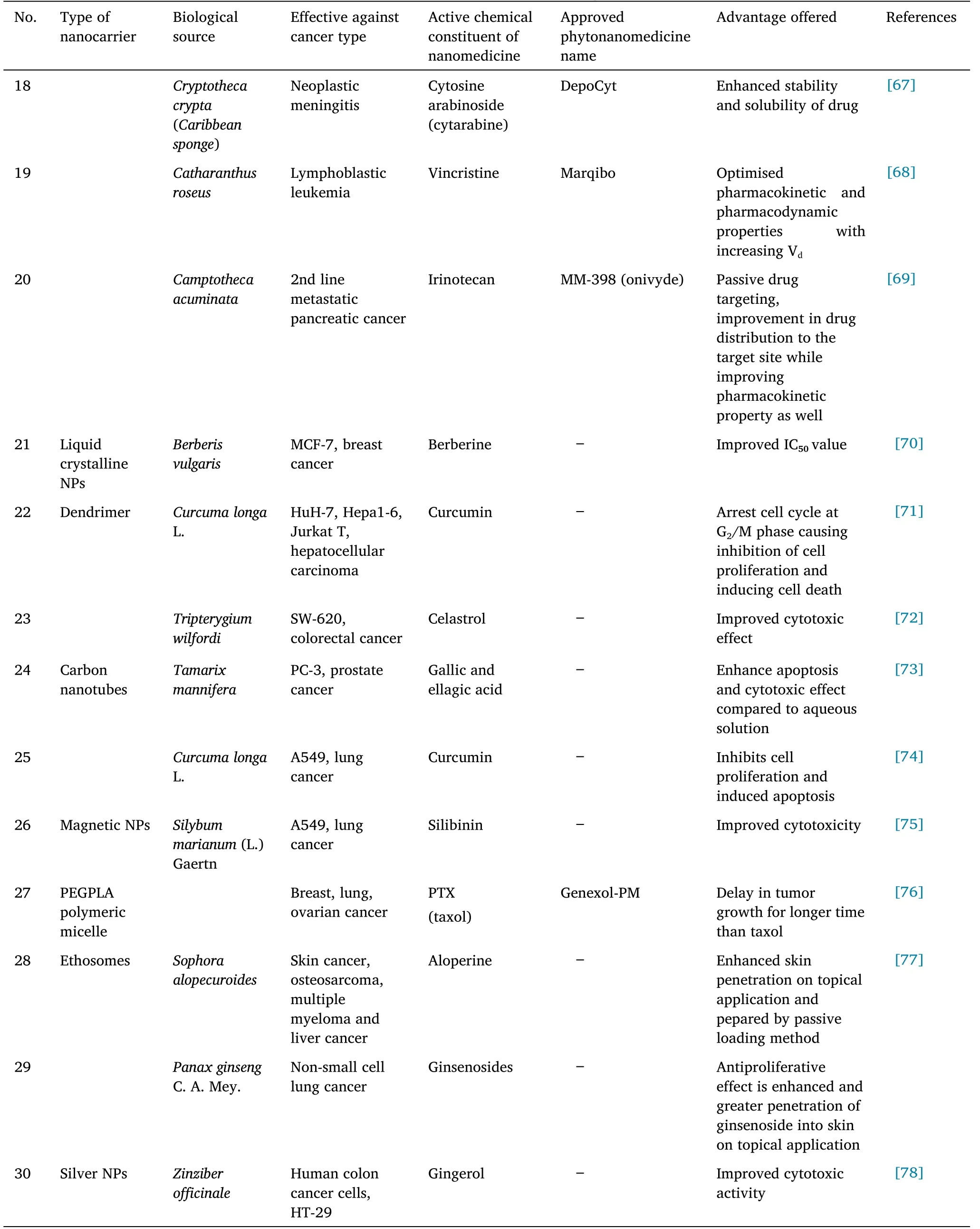
Table 1 Herbal or bioactive nanomedicines effective against cancer(continued)
Phytochemical based combinatorial nanomedicines for cancer
Combinatorial nanomedicines are the nanoformulations prepared by co-administering a chemical drug with a phytochemical to improve the drug’s therapeutic effect and other benefits.In this way, it helps to overcome the limitations of conventional dosage forms, and drug resistance offered by the single chemotherapeutic agent can be abolished.The need for combinatorial nanomedicines because of (a)to overcome drug resistance; (b) to minimize the amount of each drug in the formulation; (c) and to achieve a synergistic or additive therapeutic effect.Chemotherapeutics and phytochemicals are administered simultaneously when the following conditions are met:(a) for co-administration of anticancer agent with phytochemical,emphasis should be on finding a suitable agent with non-overlapping toxicities such that it could be administered without affecting its maximal safe dose; (b) incorporate those anticancer agents together,which have a different mechanism of action and offer significantly less cross-resistance to overcome the natural drug resistance of the concerned anticancer agent.Hence the developed nanomedicines showed various advantages (a) improved patient compliance; (b)improvement of stability of nanoformulations; (c) reduction in a frequent number of drug administrations; and (d) reduction of drug dosage so diminishes the toxicity level of drug in patient [79].Recently various phytocombinatorial nanomedicines are manufactured for the treatment of cancer are shown in Table 2.
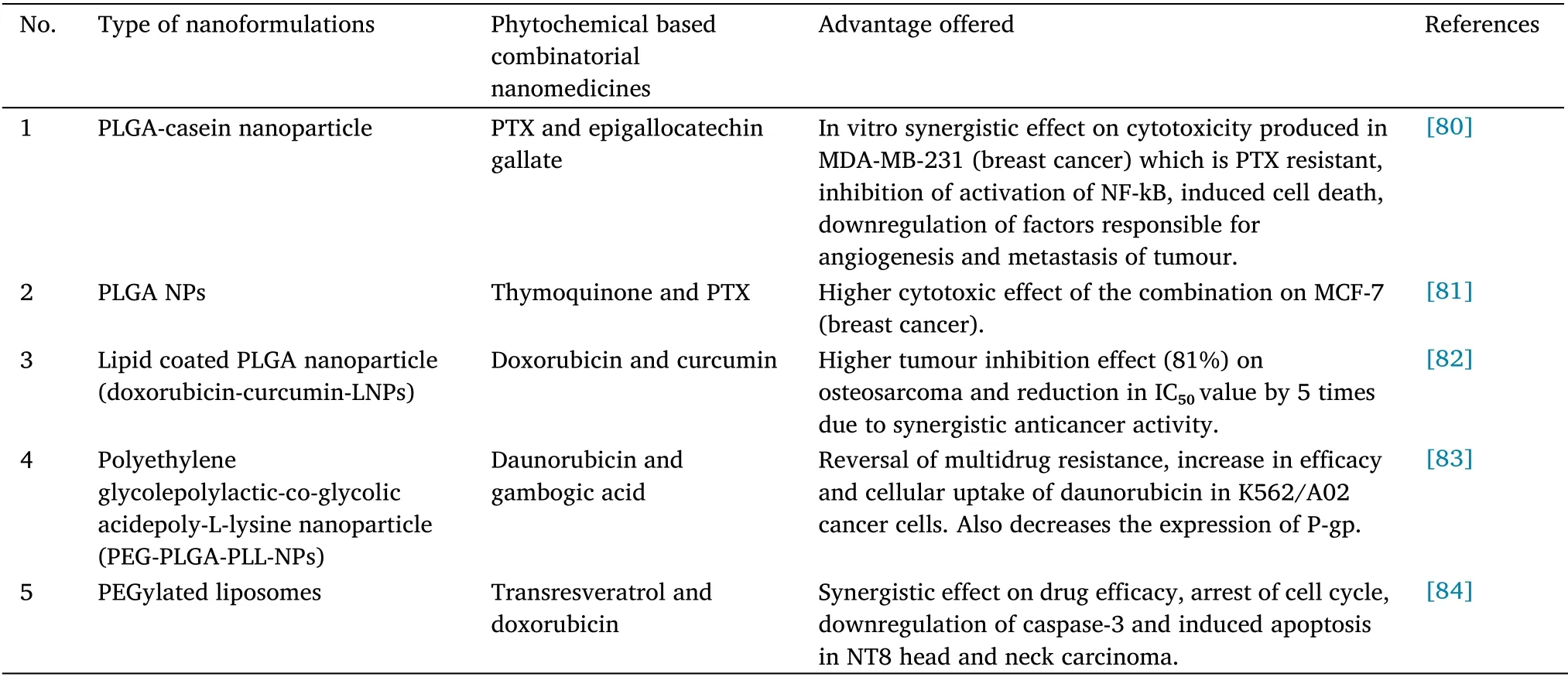
Table 2 Nanomedicines for treatment of cancer
Challenges to the nanocarriers based cancer therapy
The development of nanomedicines for cancer therapy is a very challenging task for both scientists and industrialists.Designing a suitable delivery system having high efficacy, safety, patient compliance is not a cup of tea for scientists.This has to undergo several phases, from synthesizing to testing (pre-clinical and clinical trials) and finally, approval from regulatory bodies.Once nanomedicine is approved, it becomes a herculean task for industrialists to market it, and there remains a constant challenge for its success and acceptance from the public post-marketing.
Lack of specific regulatory guidelines
There is a lack of proper draft policy form regulatory authorities regarding providing proper guidance to researchers in initial phases of formulation development and approval criterion for nanomedicines.The same approval strategy is currently followed by regulatory bodies,including the Food and Drug Administration, for nanoformulations as that of conventional dosage form so [85].However, in June 2014, the Food and Drug Administration also published four guidance documents on nanotechnology after looking over so many nanoformulations developed by several industries and proceeding in the clinical trial phase.
Cost-benefit considerations
A huge lump sum of money is invested in developing nanoformulations and granting their approval from regulatory authorities.The success and profit from the approved formulation depend on the product’s market value, safety, efficacy, acceptance by the patient (economic bias as well), and distribution across the country [86].
Safety issues
It is the point of concern for developing nanoformulations because if they will not be safe and efficacious to use, there is no point in developing them.Some nanoformulations such as NPs are likely to cause immunomodulation effects, biocompatibility issues, etc.
Scale-up
Maintaining consistency and reproducibility within batches is a concern for large-scale manufacturing of nanoformulations.This must be especially sorted upon forecasting the complexity of the disease for which it will be used in patients.
Miscellaneous issues
Significant research activity for studying all the physicochemical properties of drug entity, its behavioural aspect in nanoformulations,in vivo delivery, safety and efficacy profile, etc.must be carried out in order to be nanoformulations to be at a booming pace in the market despite competitive, challenging dosage regimen already available in the market for that particular disease.Some of the issues regarding NPs are evident in post-marketing, which must be studied well before attempting to markets them.Scientists are already researching how to influence the functional relationships and characteristic features like charge, size, surface coatings, composition, etc., in order to attain a favourable risk-benefit ratio for gaining approval from regulatory bodies.
Conclusion
There has been a tremendous increase in active pharmaceuticals derived from plants.These compounds are considered alternatives andadjuvants to conventional chemotherapeutics.Unfortunately,phototherapeutics faces many clinical translation difficulties,including solubility problems, low bioavailability, chemical instability, short half-lives, and systemic side effects.Thus, several approaches have been investigated to overcome the substantial limitations of phytomedicine by nanotechnology combined with a sustained release pattern.However, designing nano-based delivery systems looks at numerous challenges, including the possibility of scale up-processes that can quickly bring original therapies to market and expanding multifunctional systems that can satisfy many biological and therapeutic requirements.In addition, nano’ targeting efficiency must also be tested, and their toxicology and biocompatibility must satisfy international levels.
 Traditional Medicine Research2022年5期
Traditional Medicine Research2022年5期
- Traditional Medicine Research的其它文章
- G1-4A, an arabinogalactan polysaccharide derived from Tinospora cordifolia(Thunb.)Miers:a natural immunomodulator
- Pseudotargeted metabolomics for exploring the changes of neurotransmitters profile in aging rat brain and the potential neuroprotective effect of alkaloids from Uncaria rhynchophylla
- Antioxidant, hepatoprotective and nephroprotective activities of Gazania rigens against carbon tetrachloride-induced hepatotoxicity and nephrotoxicity in rats
- Traditional medicines and experimental analysis methods for Alzheimer’s disease
- Effect of Terminalia chebula Retz.extraction with water on Staphylococcus epidermidis activity and its biofilm formation
- Update on the preclinical and clinical assessment of Withania somnifera: from ancient Rasayana to modern perspectives
