Effect of Terminalia chebula Retz.extraction with water on Staphylococcus epidermidis activity and its biofilm formation
Shi-Qian Zheng,Xiao-Shi Wu,Zhi-Ling Cai,Rong-Rong Deng,Zhi-Bin Shen,2,3*
1School of Traditional Chinese Medicine, Guangdong Pharmaceutical University, Guangzhou 511400, China.2Guangdong Provincial Engineering Technology Research Center of Localized Precision Drug Delivery Preparation,Guangzhou 510006,China.3Cosmetics Engineering Technology Research Center of Guangdong Province,Guangzhou 510006,China.
Abstract Background: Based on modern pharmacological studies, Terminalia chebula Retz.exhibits antimicrobial activity against a variety of bacteria.Previously,we found Terminalia chebula Retz.exhibited excellent antibacterial activity against Malassezia restricta. Methods: We determined the minimal inhibitory concentration (MIC) and minimum bactericidal concentration of Terminalia chebula Retz.extraction with water (TRW) against Staphylococcus epidermidis(including Staphylococcus epidermidis 1-15) using the microdilution method. Staphylococcus epidermidis 1 (SE11), which was the most sensitive to TRW, was selected as the test bacterium for subsequent experiments.The time-kill curve of TRW on SE11 was generated using the viable count method.Further, an in vitro biofilm model of SE11 was constructed using the 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-((phenylamino)carbonyl)-2H-tetrazolium hydroxide test, semi-quantitative crystal violet experiment, and scanning electron microscopy.The effects of TRW on the total amount of biofilm formation, the number of viable biofilm bacteria, and biofilm microstructure of SE11 were also determined using a semi-quantitative crystal violet experiment, viable count method, and scanning electron microscopy.Finally, the electrical conductivity and soluble protein content of the SE11 suspensions were determined. Results:The average MIC of TRW against SE11 was 0.75 ± 1.09 mg/mL.TRW (1/2MIC and 2MIC) and zinc pyrithione (1/2MIC and 2MIC) had no significant effect on total biofilm inhibition in the adhesion stage (P >0.05) and the aggregation stage (P >0.05).Further, TRW (1/2MIC and MIC) and zinc pyrithione (1/2MIC and MIC) had no significant effect on viable biofilm bacteria in the adhesion stage(P >0.05)and aggregation stage(P >0.05).TRW destroyed the integrity of the SE11 cell membrane, resulting in leakage of intracellular substances. Conclusion: TRW inhibits SE11 biofilm formation and is similar to zinc pyrithione in the adhesion and aggregation stages, which provides a theoretical basis for its application in the field of antimicrobial additives.
Keywords:Terminalia chebula Retz.; zinc pyrithione; biofilm;Staphylococcus epidermidis
Background
Staphylococcus epidermidisis an important pathogen in scalp seborrheic dermatitis [1-4].Staphylococcus epidermidistends to form biofilms at the infected site of the scalp, which increases the difficulty of treating scalp seborrheic dermatitis [5-7].To alleviate the symptoms of greasy dandruff and mild itching of scalp seborrheic dermatitis,zinc pyrithione(ZPT)has been used as a bacterial inhibitor in daily chemical dandruff removal products [8].Although ZPT has antibacterial and dandruff removal effects, its long-term use can lead to strong scalp defatting, dry scalp, brittle hair, and other problems.ZPT also exhibits reproductive toxicity in aquatic organisms [9-12].Therefore, identifying safe and effective inhibitors of bacteria and their biofilms is of great significance for scalp seborrheic dermatitis treatment.
Based on ancient research records,Terminalia chebulaRetz.can be used to treat some infectious diseases and has been recorded in the Chinese medicinal book,Synopsis of Golden Chamber, written by a famous medical scientist named Zhong-Jing Zhang in the Han Dynasty(25-220 C.E.) [13].During the Tang Dynasty (618-907 C.E.),Terminalia chebulaRetz.was used to treat dysentery, clear stool,nourish hair, eliminate phlegm, and treat eye diseases [14].In the traditional Indian system of Ayurveda medicine, a herbal formula containingTerminalia chebulaRetz.is very important for the treatment of various gastrointestinal disorders [15].Some folklore people useTerminalia chebulaRetz.to treat diarrhea, dysentery, ulcers, and skin infections[16].
According to modern pharmacological studies,Terminalia chebulaRetz.can treat diseases caused by bacterial or fungal infections, such asEscherichia coli,Vibrio cholera,Staphylococcus aureus,Shigella, etc.Terminalia chebulaRetz.can be used to treat acute diarrhea, mainly caused byenterotoxigenic Escherichia coliandVibrio cholera[17];chronic ulcer wounds and skin and soft tissue infections, mainly caused byStaphylococcus aureus[18-20]; and dysentery caused byShigella[21].Terminalia chebulaRetz.possesses antimicrobial properties as it contains compounds with antibacterial properties,such as gallic acid, ethanedioic acid, ellagic acid, chebulinic acid,chebulagic acid, ethanedioic acid, and ellagic acid [22, 23].Modern pharmacological studies have shown thatTerminalia chebulaRetz.has antimicrobial activity against various gram-positive and gram-negative bacteria [24-26].The minimal inhibitory concentration (MIC) ofTerminalia chebulaRetz.against six types of gram-positive bacteria ranges from 1.95-7.81 mg/mL, while that against seven types of gram-negative bacteria ranges from 15.63-125 mg/mL [27].The MIC ofTerminalia chebulaRetz.against the predominant bacteria in pulp periapical disease and periodontitis ranges from 10-20 mg/mL [28], while that against multidrug-resistantStaphylococcus aureusranges from 0.195-0.78 mg/mL [29].
In recent years,the antimicrobial activity ofTerminalia chebulaRetz.was mainly assessed via in vitro drug sensitivity experiments.However, no reports have been published on the effects ofTerminalia chebulaRetz.extraction with water (TRW) onStaphylococcus epidermidisand its biofilms in vitro.Therefore,we sought to determine the inhibitory effect of TRW againstStaphylococcus epidermidisand its biofilm to lay a solid theoretical foundation for the application ofTerminalia chebulaRetz.in the field of antimicrobial additives.
Methods
Bacterial strains
Fifteen clinically isolated strains ofStaphylococcus epidermidis(includingStaphylococcus epidermidis1-15), which were donated by Guangdong Lewwin Pharmaceutical Research Institute Co., Ltd.(Guangzhou, China) were used.The strains were identified by Jing-Hui Lu at the First Affiliated Hospital of Guangdong Pharmaceutical University (Guangzhou, China).The quality control strain,Staphylococcus aureus(ATCC@29213), was purchased from the Guangdong Microbial Culture Collection Center (Guangzhou, China).
Source of Terminalia chebula Retz.
Dry ripe fruits ofTerminalia chebulaRetz.(lot number: 181120) used in this study were purchased from Guangxi Wuzhou Guikang Pharmaceutical Co., Ltd.(Guangxi, China) and identified by Professor Han-Jing Yan of Guangdong Pharmaceutical University.
Reagents
ZPT (96%) was purchased from Macklin Biochemical Technology Co.,Ltd.(Shanghai, China); trypticase soy broth was purchased from Guangdong Huankai Microbial Technology Co., Ltd.(Guangdong,China); Caton-adjusted Mueller-Hnton broth (CAMHB) was purchased from Guangdong Huankai Microbial Technology Co.,Ltd.(Guangdong,China);dimethysulfoxide was purchased from Tianjin Baishi Chemical Co., Ltd.(Tianjin, China); 95% ethanol was purchased from Tianjin Zhiyuan Chemical Reagent Co., Ltd.(Tianjin, China);2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-((phenylamino)carbonyl)-2H-tetrazolium hydroxide was purchased from Beijing Kulaibo Technology Co., Ltd.(Beijing, China); crystal violet was purchased from Shanghai Aiyan Biotechnology Co., Ltd.(Shanghai, China);glutaraldehyde fixing solution was purchased from Shanghai Yuanye Biotechnology Co., Ltd.(Shanghai, China); phosphate buffered saline was purchased from Dalian Meilun Biotechnology Co., Ltd.(Dalian,China); tert-butyl alcohol was purchased from Tianjin Zhiyuan Chemical Reagent Co.Ltd.(Tianjin, China); and Bradford kit was purchased from Shanghai Beyotime Biotechnology Co., Ltd.(Shanghai,China).
Instruments
Instruments included the Lab-1A-80E freeze dryer (Beijing Bo Yikang Experimental Instrument Co., Ltd., Beijing, China), FA2104 electronic scales (Shanghai Precision Scientific Instrument Co., Ltd., Shanghai,China), DHP-9032B electric heating constant temperature incubator(Shanghai Yiheng Scientific Instrument Co., Ltd., Shanghai, China),SW-OJ-1F clean bench (Suzhou Antai Air Technology Co., Ltd.,Suzhou, China), BKQ-Z301 vertical pressure steam sterilizer(Shandong Boco Disinfection Equipment Co., Ltd., Shandong, China),iMark microplate reader (BIO-RAD Corporation, Hercules, California,United States), CHA-S constant temperature oscillator (Jiangsu Jintan Ronghua Instrument Manufacturing Co., Ltd., Jiangsu, China), and JEM-2100HR cold field emission scanning electron microscope (JEOL Co., Ltd., Beijing, China).
Preparation of bacterial suspension and drugs
Preparation of bacterial suspension.An appropriate amount of sterile normal saline was added to the culture medium containing mature strains, and the bacterial suspension was rinsed using the turbidimetric method.The bacterial suspension was diluted with sterile normal saline to adjust the turbidity of the 0.5 turbidimetric tubes, and the bacterial suspension concentration was 1.0 × 108colony-forming unit (CFU)/mL.The bacterial suspension was then diluted to 1.0 ×106CFU/mL with CAMHB.
Preparation of drugs.Dry ripe fruits ofTerminalia chebulaRetz.were crushed, passed through an 80 mesh sieve, and weighed (50 g).Thereafter, the sample was mixed with 500 mL distilled water and decocted gently for 1.5 h.After collection of the filtrate, the residue was added to 400 mL of distilled water and decocted for 1 h.The filtrate was then collected and combined with the filtrate two times.The filtrate was concentrated and freeze-dried to obtain a freeze-dried powder (brownish-yellow powder, extraction rate of 65.34%), and then stored at 4 °C.Dimethyl sulfoxide was used to prepare stock solutions of TRW(600 mg/mL) stored at 4°C in the dark.The positive control drug, ZPT (96%), was dissolved in dimethyl sulfoxide as a drug stock solution (100 mg/mL) and stored at 4 °C in the dark.
Determination of MIC and minimum bactericidal concentration(MBC)
MIC and MBC were determined according to the double dilution method described by the Clinical and Laboratory Standards Institute[30].The steps involved in the double dilution method are described below [31-33].
The control group contained 100 μL of bacterial suspension (the final concentration of bacterial suspension was 1.0 × 106CFU/mL)and 100 μL of CAMHB.TRW and ZPT were added to 100 μL bacterial suspension(the final concentration of the bacterial suspension was 1.0×106CFU/mL)and 100 μL CAMHB containing TRW or ZPT.Different concentrations of TRW and ZPT were diluted with CAMHB in a 96-well microplate using the double-dilution method.The maximum concentrations of TRW and ZPT in the 96-well microplate were 12 and 50 μg/mL, respectively.TRW and ZPT were diluted with CAMHB to minimum concentrations of 0.023 μg/mL and 0.098 μg/mL,respectively.The final concentrations of TRW and ZPT were 0.023-12 mg/mL and 0.098-50 μg/mL, respectively.A 96-well microplate was incubated at 37 °C for 18-24 h, and the lowest concentration of the agent that inhibited the growth ofStaphylococcus epidermidiswas considered the MIC.Accordingly, 50 μL of high-concentration MIC-containing bacterial liquid was adsorbed onto the surface of the agar medium.After incubation at 37 °C for 18-24 h, the MBC was observed.All MICs and MBCs were confirmed using repeated tests.
According to the M07-A9 regimen formulated by the Clinical and Laboratory Standards Institute, the test process and environment must be tested for quality control during the drug susceptibility test.Staphylococcus aureuswas used as the quality control strain and cefoxitin was used as the quality control drug.If the MIC of the quality control strain existed within the range of 0.25-1 µg/mL under the conditions of parallel operation, the determination result was considered valid and reliable.
Time-kill curve assay
Based on the above experiments,Staphylococcus epidermidis1 (SE11)was selected as the test strain.The time-kill curve of TRW on the growth of SE11 was generated using the viable count method [34].
TRW (1/2MIC, MIC, 2MIC), ZPT (1/2MIC, MIC, 2MIC), and control were used to determine the time-kill curve.TRW and ZPT were diluted to three concentrations of 2MIC, MIC and 1/2MIC, and added to a 96-well microplate containing CAMHB and a bacterial suspension of 1.0 × 106CFU/mL.The control group was added to a CAMHB-containing bacterial suspension of 1.0 × 106CFU/mL.The 96-well microplate was then placed in a constant temperature shaking box for culture.A total of 50 µL bacteria-containing culture solution from each well was collected at an interval of 2 h.Thereafter,reasonable dilutions were prepared.A 20-µL diluted bacteria-containing culture solution was evenly placed in the culture dish, added to the culture medium for solidification, and incubated at 37°C in a constant temperature incubator for 18-24 h.Thereafter,the number of colonies was calculated using the plate counting method.
Establishment of the Staphylococcus epidermidis biofilm model in vitro
Determination of the biofilm growth dynamic curve of Staphylococcus epidermidis by the 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-((phenylamino)carbonyl)-2H-tetrazolium hydroxide test (XTT-test).Staphylococcus epidermidisgrowth and control groups were established to determine the biofilm growth dynamic curve using the XTT-test [35, 36].The specific experimental steps are presented in Supplementary Materials.
Determination of the biofilm total amount growth curve of Staphylococcus epidermidis using the semi-quantitative crystal violet experiment.Staphylococcus epidermidisgrowth group and control group were established to determine the biofilm total amount growth curve using a semi-quantitative crystal violet experiment[37].The bacterial suspension inoculation and biofilm establishment were similar to the above XTT-test, with minor modifications.The specific experimental steps are presented in Supplementary Materials.
Observation of the biofilm microstructure of Staphylococcus epidermidis by scanning electron microscopy(SEM).Staphylococcus epidermidisbiofilms were cultured on a 48-well microplate with sterile round slides and observed under an SEM.The specific experimental steps are presented in Supplementary Materials.
Determination of the effect of drugs on Staphylococcus epidermidis biofilm using semi-quantitative crystal violet experiment
TRW (1/2MIC, MIC, and 2MIC), ZPT (1/2MIC, MIC, and 2MIC) and control groups were established.Based on the experimental results of SE11 biofilm construction, the stages of biofilm adhesion and aggregation were determined, and 6 h and 12 h were selected as the time points of drug treatment.The SE11 biofilm was constructed as described above.Bacterial suspensions (1 × 106CFU/mL) were inoculated into a 96-well microplate and incubated at 37 °C for 6 h and 12 h.
After constructing the biofilm and rinsing it with phosphate buffer saline (PBS), the biofilm was treated with the drugs.TRW (1/2MIC,MIC,and 2MIC) and ZPT(1/2MIC, MIC, and 2MIC)were added to the 96-well microplates.Thereafter, the cells were incubated at 37 °C for 24 h.A control group was simultaneously established.
After treatment with the drugs and washing with PBS, the biofilm was fixed with a 2.5%glutaraldehyde fixative solution for 30 min.The glutaraldehyde fixative solution was then discarded, and 200 μL of 0.1% crystal violet staining solution was added to each well.The unadhered dye was removed via washing after staining for 15 min.Finally, 95% ethanol was added to dissolve the dye bound to the biofilm; the mixture was gently shaken to ensure full dissolution.Bacterial growth was measured at 570 nm optical density with a microplate reader.Thereafter, the effect of the drugs on the biofilm of SE11 was determined.
Determination of the effect of drugs on Staphylococcus epidermidis biofilm using the viable count method
TRW (1/2MIC, MIC, 2MIC), ZPT (1/2MIC, MIC, 2MIC), and control groups were established.The SE11 biofilm was constructed as described above,with minor modifications.Based on the experimental results of SE11 biofilm construction, the stages of biofilm adhesion,aggregation, and maturity were determined, and 6, 12, and 24 h were selected as the time points of drug treatment.The treatment of biofilms with drugs was performed as described above.
The viable count method was performed as below.Briefly, the bacterial solution was diluted with an appropriate amount of sterile normal saline, and 100 μL of the diluted bacterial solution was coated onto the solid medium.After cultivation at 37°C for 24 h,the number of bacterial colonies on the medium was determined, and the number of viable bacteria was calculated as log10CFU/mL.
Observation of the effect of drugs on biofilm microstructure of Staphy lococcus epidermidis by SEM
TRW (1/2MIC, MIC, 2MIC), ZPT (1/2MIC, MIC, 2MIC), and control groups were established.The SE11 biofilm was constructed as described above, with minor modifications.Sterile round slides were placed in a 48-well microplate with tweezers.The bacterial suspension(1 × 106CFU/mL) was inoculated into the 48-well microplate and incubated at 37 °C for 6, 12, and 24 h.The treatment of biofilms with drugs was performed as described above.
The microplate was rinsed three times with sterile PBS.Thereafter,2.5% glutaraldehyde fixative solution was added for overnight fixation at 4°C.After rinsing with sterile PBS,different concentrations(30%,50%,70%,80%,90%,95%,and 100%)of ethanol were used to perform gradient dehydration.Thereafter, 100% tert-butanol was added to replace the ethanol.The samples to be tested were freeze-dried, glued to the stage with a conductive adhesive, sprayed with gold for 50 s using a vacuum ion sputtering instrument, and observed under an SEM.
Effect of drugs on Staphylococcus epidermidis cell membranes
Effect of drugs on the electrical conductivity of Staphylococcus epidermidis suspension.The TRW (1/2MIC, MIC, and 2MIC), ZPT(1/2MIC, MIC, and 2MIC), and control groups were established.The bacterial suspension (1 × 106CFU/mL) was inoculated on a 12-well microplate, and the corresponding concentrations of TRW and ZPT were added into the 12-well microplate.A control group was also established.The cells were incubated at 37°C for 0,1,2,4,6,and 8 h.In the corresponding period, the samples to be tested were collected from each well and centrifuged at 5,000 rpm for 10 min.Thereafter,the electrical conductivity was measured.
Effect of drugs on the soluble protein content of Staphylococcus epidermidis suspension.The TRW (1/2MIC, MIC, and 2MIC), ZPT(1/2MIC, MIC, and 2MIC), and control groups were established.A standard protein curve was generated according to the manufacturer’s instructions.The bacterial suspension (1 × 106CFU/mL) was inoculated on a 12-well microplate.Thereafter, the corresponding concentrations of TRW and ZPT were added to the 12-well microplate.Simultaneously, a control group was established.After incubation at 37°C for 0, 1, 2,4, 6,and 8 h,the samples to be tested were collected from each well, centrifuged at 5,000 rpm for 10 min, and measured using the Bradford kit.
Statistical analysis
GraphPad Prism software (version 8.0.1) was used to carry out a statistical analysis of the data.Mean ± standard deviation (± SD)was used for the measurement data.Thet-test was used for comparisons between two groups, while a one-way analysis of variance was used for comparisons between multiple groups.
Results
MIC and MBC of the drugs against Staphylococcus epidermidis
The MIC and MBC results are presented in Table 1.The MIC of TRW and ZPT againstStaphylococcus epidermidisranged from 0.75-5.99 mg/mL and 1.3-3.12 μg/mL, respectively.The MIC of TRW against SE11 was 0.75 mg/mL.This result indicates that TRW had the best antibacterial activity against SE11.Therefore, SE11 was selected as the test bacterium.
Table 1 MIC and MBC of tested drugs against Staphylococcus epidermidis (± SD, n=8)
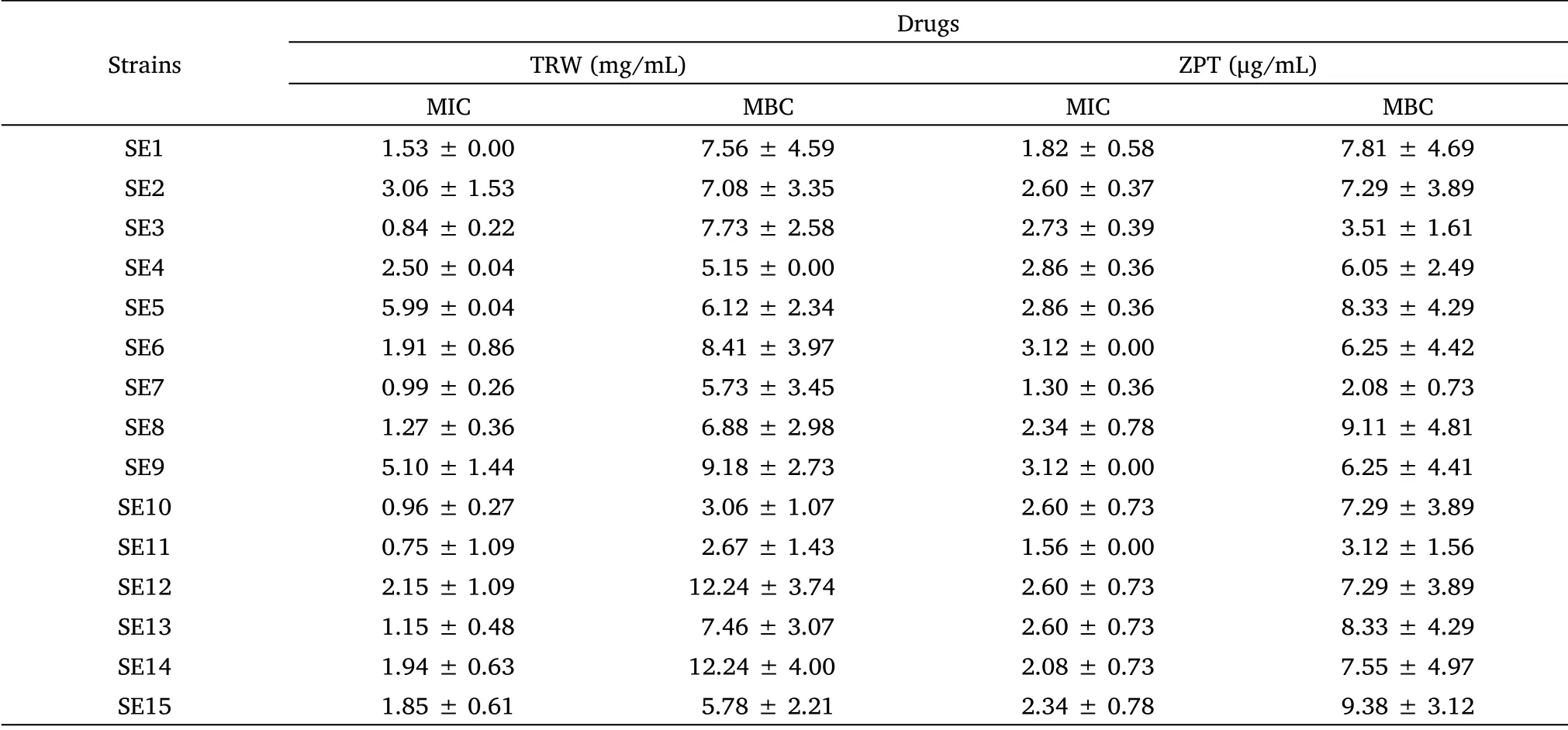
Table 1 MIC and MBC of tested drugs against Staphylococcus epidermidis (± SD, n=8)
According to the calculation of the original data,after the data is rounded to two decimal points,there is a case where the standard deviation value is 0.00.± SD, mean ± standard deviation; TRW, Terminalia chebula Retz.extraction with water; ZPT, zinc pyrithione; MIC, minimal inhibitory concentration;MBC, minimum bactericidal concentration; SE1-SE15, Staphylococcus epidermidis 1-Staphylococcus epidermidis 15.
Strains Drugs TRW (mg/mL) ZPT (μg/mL)MIC MBC MIC MBC SE1 1.53 ±0.00 7.56 ± 4.59 1.82 ± 0.58 7.81 ± 4.69 SE2 3.06 ±1.53 7.08 ± 3.35 2.60 ± 0.37 7.29 ± 3.89 SE3 0.84 ±0.22 7.73 ± 2.58 2.73 ± 0.39 3.51 ± 1.61 SE4 2.50 ±0.04 5.15 ± 0.00 2.86 ± 0.36 6.05 ± 2.49 SE5 5.99 ±0.04 6.12 ± 2.34 2.86 ± 0.36 8.33 ± 4.29 SE6 1.91 ±0.86 8.41 ± 3.97 3.12 ± 0.00 6.25 ± 4.42 SE7 0.99 ±0.26 5.73 ± 3.45 1.30 ± 0.36 2.08 ± 0.73 SE8 1.27 ±0.36 6.88 ± 2.98 2.34 ± 0.78 9.11 ± 4.81 SE9 5.10 ±1.44 9.18 ± 2.73 3.12 ± 0.00 6.25 ± 4.41 SE10 0.96 ±0.27 3.06 ± 1.07 2.60 ± 0.73 7.29 ± 3.89 SE11 0.75 ±1.09 2.67 ± 1.43 1.56 ± 0.00 3.12 ± 1.56 SE12 2.15 ±1.09 12.24 ± 3.74 2.60 ± 0.73 7.29 ± 3.89 SE13 1.15 ±0.48 7.46 ± 3.07 2.60 ± 0.73 8.33 ± 4.29 SE14 1.94 ±0.63 12.24 ± 4.00 2.08 ± 0.73 7.55 ± 4.97 SE15 1.85 ±0.61 5.78 ± 2.21 2.34 ± 0.78 9.38 ± 3.12
Time-kill curves assay
Based on the above results, SE11 was selected as the test bacterium.To evaluate the dynamic bactericidal effect of drugs, time-kill curves were established using the viable count method [38].The results are presented in Table 2 and Figure 1.
Table 2 Time-kill curves assay of tested drugs against SE11 (± SD, n=6)
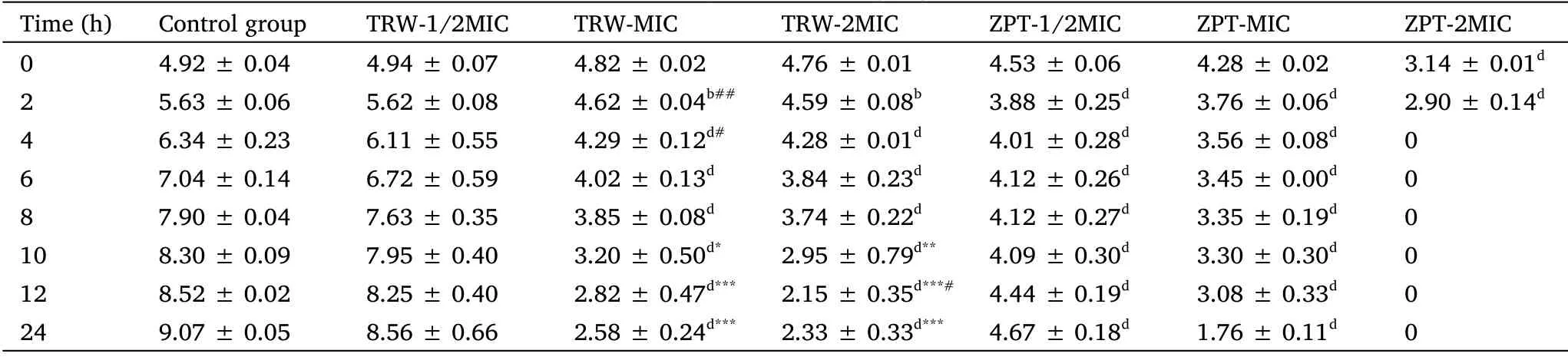
Table 2 Time-kill curves assay of tested drugs against SE11 (± SD, n=6)
According to the calculation of the original data,after the data is rounded to two decimal points,there is a case where the standard deviation value is 0.00.Compared with the control group,aP <0.05,bP <0.01,cP <0.001,dP <0.0001;compared with the ZPT group(1/2MIC),*P <0.05,**P<0.01,***P <0.0001; compared with the ZPT group (MIC),#P <0.05,##P <0.01.± SD, mean ± standard deviation; SE11, Staphylococcus epidermidis 11; TRW, Terminalia chebula Retz.extraction with water; ZPT, zinc pyrithione; MIC, minimal inhibitory concentration.
Time(h) Control group TRW-1/2MIC TRW-MIC TRW-2MIC ZPT-1/2MIC ZPT-MIC ZPT-2MIC 0 4.92 ± 0.04 4.94 ± 0.07 4.82 ± 0.02 4.76 ± 0.01 4.53 ±0.06 4.28 ± 0.02 3.14 ± 0.01d 2 5.63 ± 0.06 5.62 ± 0.08 4.62 ± 0.04b## 4.59 ± 0.08b 3.88 ±0.25d 3.76 ± 0.06d 2.90 ± 0.14d 4 6.34 ± 0.23 6.11 ± 0.55 4.29 ± 0.12d# 4.28 ± 0.01d 4.01 ±0.28d 3.56 ± 0.08d 0 6 7.04 ± 0.14 6.72 ± 0.59 4.02 ± 0.13d 3.84 ± 0.23d 4.12 ±0.26d 3.45 ± 0.00d 0 8 7.90 ± 0.04 7.63 ± 0.35 3.85 ± 0.08d 3.74 ± 0.22d 4.12 ±0.27d 3.35 ± 0.19d 0 10 8.30 ± 0.09 7.95 ± 0.40 3.20 ± 0.50d* 2.95 ± 0.79d** 4.09 ±0.30d 3.30 ± 0.30d 0 12 8.52 ± 0.02 8.25 ± 0.40 2.82 ± 0.47d*** 2.15 ± 0.35d***# 4.44 ±0.19d 3.08 ± 0.33d 0 24 9.07 ± 0.05 8.56 ± 0.66 2.58 ± 0.24d*** 2.33 ± 0.33d*** 4.67 ±0.18d 1.76 ± 0.11d 0
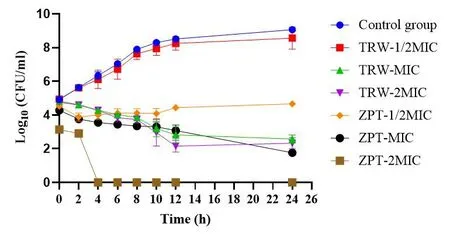
Figure 1 Time-kill curves assay of tested drugs against SE11(±SD,n=6).TRW,Terminalia chebula Retz.extraction with water; ZPT,zinc pyrithione; CFU, colony-forming unit; MIC, minimal inhibitory concentration; SE11, Staphylococcus epidermidis 11; ± SD, mean ±standard deviation.
In the control group, SE11 entered the logarithmic growth stage(2-12 h) and grew rapidly; however, after 12 h, gradual growth was observed.Compared with the control, TRW (MIC and 2MIC)significantly inhibited SE11 growth (P<0.0001) from 4 h to 24 h.
Compared with ZPT (1/2MIC), TRW (MIC) significantly inhibited SE11 growth at 10 h (P<0.05),12 h (P<0.0001), and 24 h (P<0.0001).Compared with ZPT (1/2MIC), TRW (2MIC) significantly inhibited SE11 growth at 10 h(P<0.01), 12 h(P<0.0001), and 24 h(P<0.0001).
Compared with ZPT (MIC), TRW (MIC) significantly inhibited SE11 growth at 2 h (P<0.01) and 4 h (P<0.05).Compared with ZPT(MIC), TRW (2MIC) significantly inhibited SE11 growth at 12 h (P<0.05).
Compared to the control group, TRW had inhibitory effects on the growth cycle of SE11 in a dose- and time-dependent manners.Compared to ZPT (1/2MIC), TRW (MIC and 2MIC) displayed superior antibacterial activity during the stable growth stage (12-24 h).Compared to ZPT (MIC), TRW (MIC) displayed superior antibacterial activity during the logarithmic growth stage(2-12 h).
Staphylococcus epidermidis biofilm model in vitro
Biofilm growth dynamic curve of Staphylococcus epidermidis.The biofilm growth dynamic curve ofStaphylococcus epidermiswas measured using the XTT-test; the results are displayed in Supplementary Materials Table S1 and Figure S1.
Biofilm total amount growth curve of Staphylococcus epidermidis.The biofilm total amount growth curve ofStaphylococcus epidermiswas measured using a semi-quantitative crystal violet experiment; the results of this experiment are shown in Supplementary Materials Table S2 and Figure S2.
Biofilm microstructure of Staphylococcus epidermidis based on SEM.The microstructure ofStaphylococcus epidermisbiofilm is shown in Supplementary Materials Figure S3.
Effect of drugs on the total amount of biofilm
The results are shown in Figure 2.Compared with the control group,TRW (MIC) significantly inhibited the total amount of biofilm in the adhesion (P<0.05) and aggregation stages (P<0.05).Further,compared with the control, TRW (2MIC) significantly inhibited the total amount of biofilm in the adhesion (P<0.01) and aggregation stages(P<0.05).
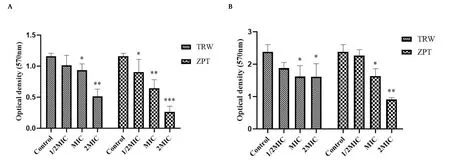
Figure 2 Effect of drugs on the total amount of Staphylococcus epidermidis biofilm at the adhesion and aggregation stages based on the semi-quantitative crystal violet experiment(±SD,n=6).(A) Biofilm in the adhesion stage.(B)Biofilm in the aggregation stage.Compared with the control group,*P <0.05,**P <0.01,***P <0.001.± SD, mean ± standard deviation; TRW, Terminalia chebula Retz.extraction with water; ZPT, zinc pyrithione; MIC, minimal inhibitory concentration.
There was no significant difference in the effect of TRW (1/2MIC)and ZPT (1/2MIC) on the total amount of biofilm inhibition in the adhesion (P>0.05) and aggregation stages (P>0.05).Similarly,there was no significant difference in the effect of TRW(MIC)and ZPT(MIC) on the total amount of biofilm inhibition in the aggregation stage (P>0.05).TRW (2MIC) and ZPT (2MIC) had no significant effect on the total amount of biofilm inhibition in the adhesion stage(P>0.05).
Effect of drugs on the number of biofilm viable bacteria
The results are shown in Figure 3.Compared with the control, TRW(MIC) resulted in a significantly reduced number of viable biofilm bacteria in the adhesion stage(P<0.05).Compared with the control,TRW (2MIC) led to a significantly reduced number of viable biofilm bacteria in the adhesion stage(P<0.05) and aggregation stage (P<0.05).Compared with the control, TRW (1/2MIC) (P>0.05), (MIC)(P>0.05), and (2MIC) (P>0.05) had little effect on biofilms at the mature stage.
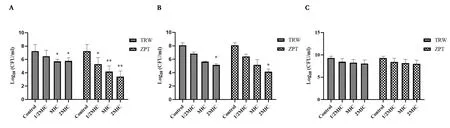
Figure 3 Influence of the drugs on the number of viable bacteria in biofilm at the stages of adhesion, aggregation,and maturity based on the viable count method(±SD,n=6).(A)Biofilm in the adhesion stage;(B)biofilm in the aggregation stage;(C)biofilm in the mature stage.Compared with the control group,*P <0.05,**P <0.01.± SD, mean ± standard deviation; TRW, Terminalia chebula Retz.extraction with water; ZPT, zinc pyrithione; MIC, minimal inhibitory concentration;CFU, colony-forming unit.
There was no significant difference in the effect of TRW (1/2MIC)and ZPT (1/2MIC) on the number of viable biofilm bacteria in the adhesion(P>0.05)and aggregation stages(P>0.05).Similarly,the effect of TRW (MIC) and ZPT (MIC) on the number of viable biofilm bacteria in the adhesion(P>0.05)and aggregation stages(P>0.05)was not found to significantly differ.
Effect of drugs on biofilm microstructure
The results are shown in Figures 4 and 5, respectively.The biofilm microstructure of the control group was intact in the adhesion (6 h),aggregation (12 h), and maturation (24 h)stages.
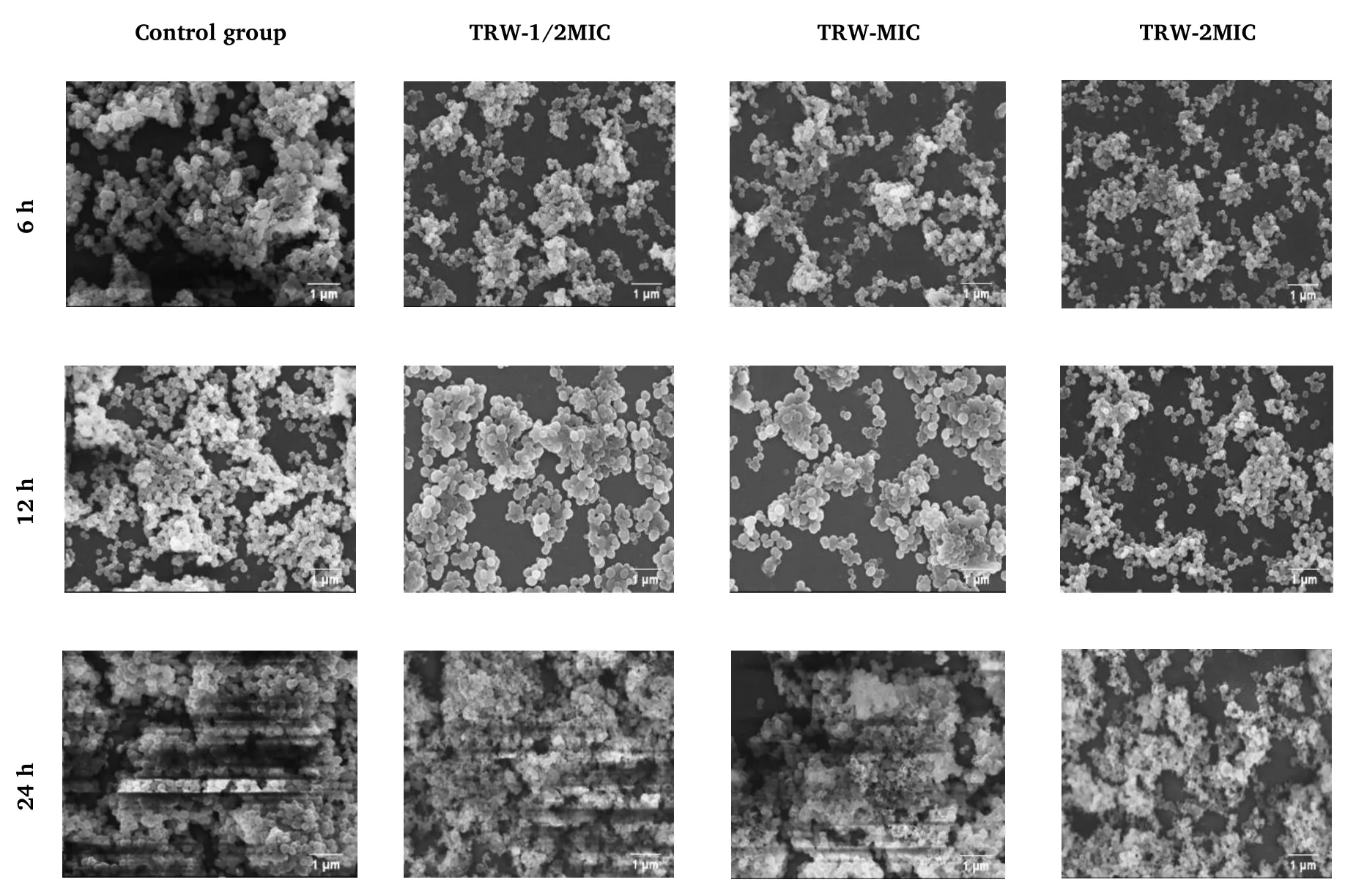
Figure 4 Effect of TRW on Staphylococcus epidermidis biofilm based on SEM (3,000×).6 h, adhesion stage; 12 h, aggregation stage; 24 h,maturation stage.TRW,Terminalia chebula Retz.extraction with water;MIC,minimal inhibitory concentration;SEM,scanning electron microscopy.
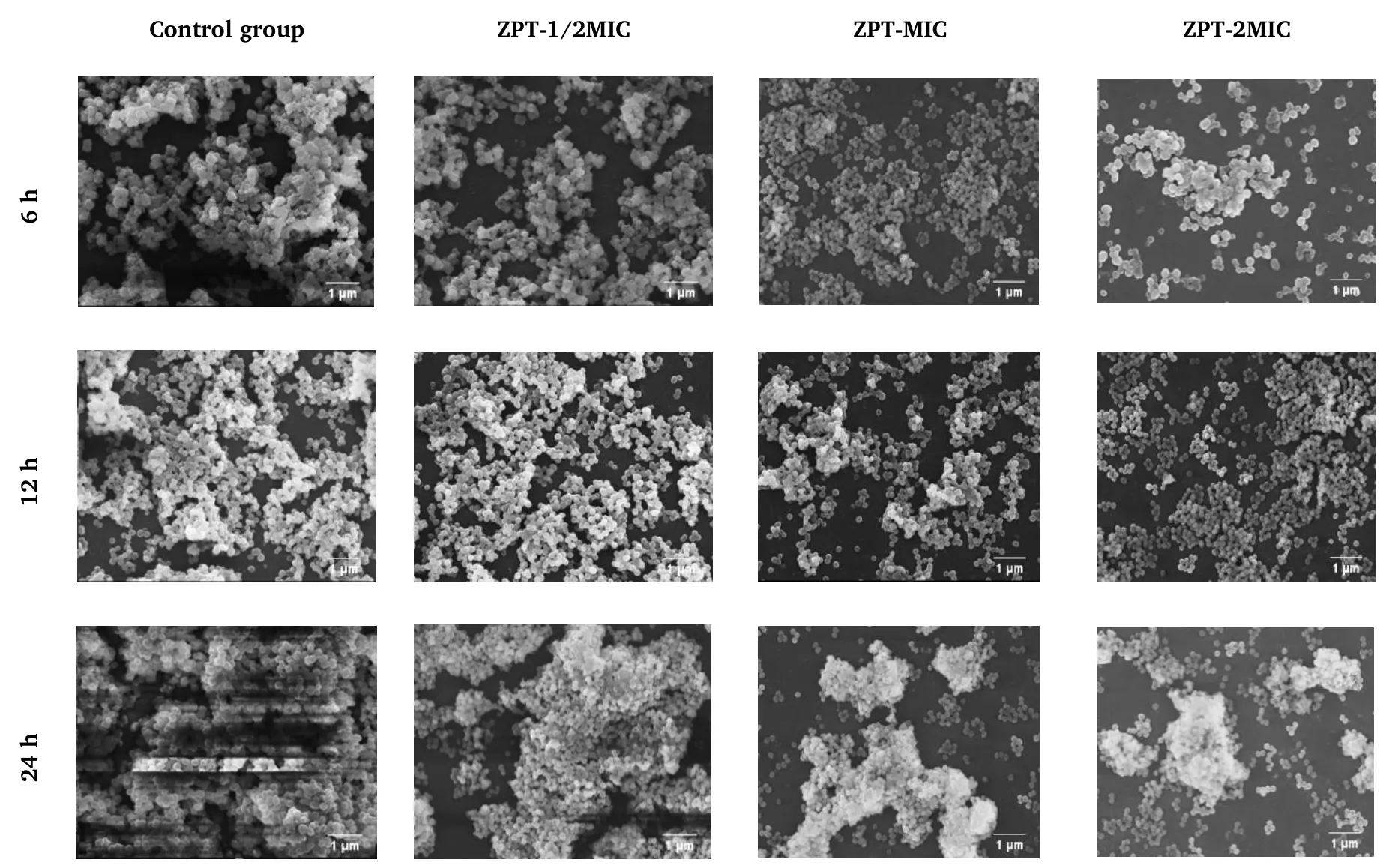
Figure 5 Effect of ZPT on Staphylococcus epidermidis biofilm based on SEM (3,000×).6 h, adhesion stage; 12 h, aggregation stage; 24 h,maturation stage.ZPT, zinc pyrithione; MIC, minimal inhibitory concentration; SEM, scanning electron microscopy.
The effects of TRW and ZPT on the microstructure of the biofilm in the adhesion and aggregation stages were found to be similar.After treatment with TRW (1/2MIC, MIC, and 2MIC) or ZPT (1/2MIC, MIC,and 2MIC),the biofilm in the adhesion and aggregation stages became loose,the secretion of extracellular polymers decreased,and some cell morphology shrunk and deformed.Some of the cells in the group administered TRW(2MIC)were found to rupture at the adhesion(6 h)and aggregation (12 h)stages.
Effects of drugs on Staphylococcus epidermidis cell membrane integrity
Effects of drugs on the electrical conductivity of Staphylococcus epidermidis suspension.The results are presented in Table 3 and Figure 6.Changes in the electrical conductivity of the bacterial suspension can indirectly reflect the integrity of the bacterial cell membrane.In the control group, the conductivity of the bacterial suspension was stable and low, indicating good integrity of the cell membrane.Compared with the control group, the groups administered TRW (1/2MIC, MIC, and 2MIC) displayed significantly increased electrical conductivity of the bacterial suspension (P<0.0001) from 0 h to 8 h.Compared with the group administered ZPT(1/2MIC), the group administered TRW (1/2MIC) showed significantly increased electrical conductivity of the bacterial suspension (P<0.001) at 0 h.Compared with ZPT (1/2MIC), TRW(1/2MIC) resulted in significantly increased electrical conductivity of the bacterial suspension(P<0.0001)from 1 h to 8 h.Compared with ZPT (2MIC), TRW (2MIC) led to significantly increased electrical conductivity of the bacterial suspension (P<0.0001) at 6 h.TRW(2MIC) caused significantly increased electrical conductivity of the bacterial suspension (P<0.0001) at 8 h.
Table 3 Changes of electrical conductivity (μs/cm) (± SD,n =6)
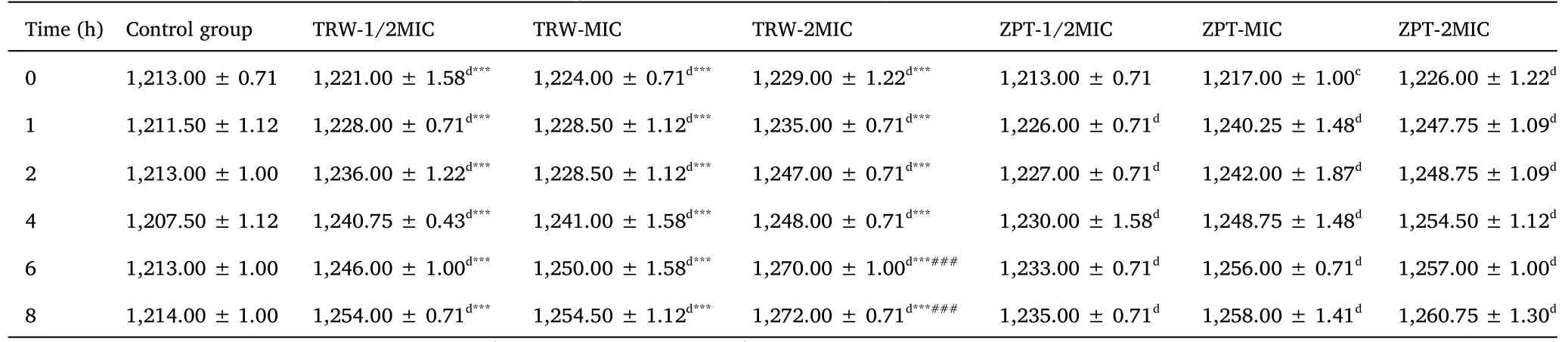
Table 3 Changes of electrical conductivity (μs/cm) (± SD,n =6)
Compared with the control group,aP <0.05,bP <0.01,cP <0.001,dP <0.0001.Compared with the ZPT group(1/2MIC),*P <0.05,**P <0.01,***P <0.0001.Compared with the ZPT group (2MIC),###P <0.0001.± SD, mean ± standard deviation; TRW, Terminalia chebula Retz.extraction with water; ZPT, zinc pyrithione; MIC, minimal inhibitory concentration.
?
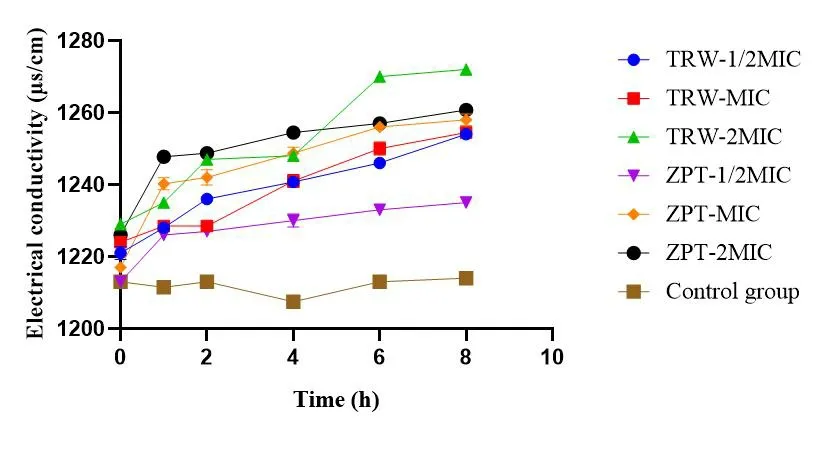
Figure 6 Changes in electrical conductivity (μs/cm) (± SD, n=6).TRW, Terminalia chebula Retz.extraction with water; ZPT, zinc pyrithione; MIC, minimal inhibitory concentration; ±SD, mean ±standard deviation.
The effect of TRW (1/2MIC) on the leakage of soluble substances was more obvious than that of ZPT(1/2MIC) from 0 h to 8 h.Further,the effect of TRW (2MIC) on the leakage of soluble substances was more obvious than that of ZPT (2MIC) from 6 h to 8 h.These results indicate that TRW can destroy the cell membrane ofStaphylococcus epidermidisand result in the leakage of soluble substances.
Effect of drugs on the soluble protein content of Staphylococcus epidermidis.The results are presented in Table 4 and Figure 7.Changes in the soluble protein content can indirectly reflect the integrity of the bacterial cell membrane.In the groups administered TRW (1/2MIC, MIC, and 2MIC), the soluble protein content of SE11 rapidly increased within 0-2 h, and gradually leveled off.
Table 4 Soluble protein content of Staphylococcus epidermidis(mg/mL) (±SD, n =6)
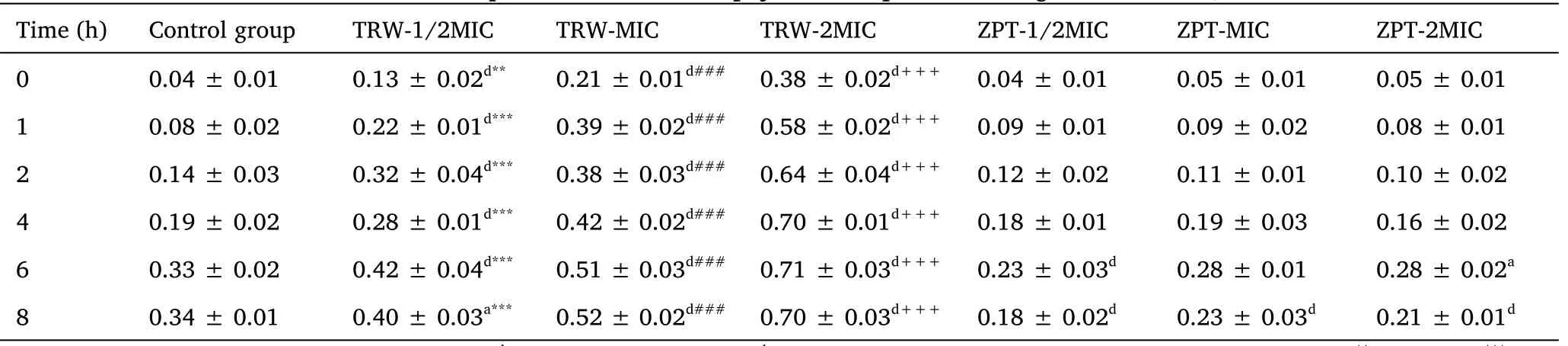
Table 4 Soluble protein content of Staphylococcus epidermidis(mg/mL) (±SD, n =6)
Compared with the control group,aP <0.05,bP <0.01,cP <0.001,dP <0.0001.Compared with the ZPT group (1/2MIC),**P <0.01,***P <0.0001.Compared with the ZPT group (MIC),###P <0.0001.Compared with the ZPT group (2MIC),+++P <0.0001.±SD, mean ±standard deviation; TRW, Terminalia chebula Retz.extraction with water; ZPT, zinc pyrithione; MIC, minimal inhibitory concentration.
?
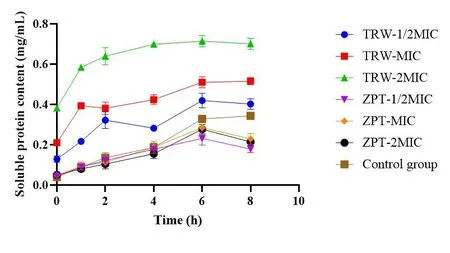
Figure 7 Soluble protein content of Staphylococcus epidermidis(± SD, n =6).TRW, Terminalia chebula Retz.extraction with water;ZPT,zinc pyrithione;MIC, minimal inhibitory concentration;±SD,mean ± standard deviation.
Compared with the control group, the group administered TRW(1/2MIC) had significantly increased soluble protein content (P<0.0001)from 0 h to 6 h.Further,compared with the control group,the groups administered TRW (MIC and 2MIC) showed significantly improved soluble protein content (P<0.0001) from 0 h to 8 h.
Compared with ZPT (1/2MIC), TRW (1/2MIC) resulted in significantly improved soluble protein content at 0 h(P<0.001),and 1 h to 8 h (P<0.0001).Compared with ZPT (MIC), TRW (MIC)caused significant improvements in the soluble protein content (P<0.0001)from 0 h to 8 h.Compared with ZPT(2MIC), TRW(2MIC)led to significantly improved soluble protein content(P<0.0001)from 0 h to 8 h.
Altogether, TRW may increase the permeability of the cell membrane, leading to the leakage of intracellular soluble proteins.
Discussion
Staphylococcus epidermidisis an important pathogen of scalp seborrheic dermatitis [39, 40].Based on the findings of this study,TRW inhibits the activity ofStaphylococcus epidermidisand its biofilm formation, thereby providing strong support for TRW as an antimicrobial additive for the treatment of scalp seborrheic dermatitis.On one hand, TRW was found to have good antibacterial activity againstStaphylococcus epidermidis.According to an antibacterial activity study of TRW,the MIC values of TRW toStaphylococcus aureusandEscherichia coliwere 3.9 mg/mL and 7.825 mg/mL, respectively[24, 41].In this study, the MIC of TRW againstStaphylococcus epidermidis(Staphylococcus epidermidis1-15) ranged from 0.75-5.99 mg/mL, which indicates that TRW had good antibacterial activity againstStaphylococcus epidermidis1-15.On the other hand, TRW was found to inhibitStaphylococcus epidermidisbiofilm formation during the adhesion and aggregation stages.The effect of TRW and ZPT on the total biofilm amount and biofilm viable bacteria inhibition was not found to significantly differ in the adhesion and aggregation stages.
Based on the antibacterial effect of TRW and the treatment status of scalp seborrheic dermatitis, additional studies can be performed to determine whether TRW affects polysaccharide intercellular adhesion,extracellular polymeric substances,and fibrinogen binding proteins in the biofilm adhesion and aggregation stages to provide a reference for TRW as an antibacterial additive for the treatment of scalp seborrheic dermatitis.The formation of Staphylococcus epidermidis biofilms is a continuous dynamic process that mainly includes three stages:adhesion, aggregation, and maturation.The adhesion stage is mainly affected by fibrinogen binding proteins,which promote Staphylococcus epidermidis binding to fibrinogen on the surface of the biomaterial.The aggregation stage is mainly affected by polysaccharide intercellular adhesion and extracellular polymeric substances [42].Polysaccharide intercellular adhesion can promote the aggregation and connection of individual Staphylococcus epidermidis to form a biofilm to resist the host’s immune response.Extracellular polymeric substances can promote Staphylococcus epidermidis to adhere to and form microcolonies, ultimately thickening the biofilm.
Due to the particularity of traditional Chinese medicine extracts,this study may serve as a reference for antibacterial research using traditional Chinese medicine extracts,which would help to expand the antibacterial spectrum of traditional Chinese medicine extracts and promote the development of traditional Chinese medicine extracts as antibacterial additives.The specific reference significance is outlined below.
The color of traditional Chinese medicine extracts affects the interpretation of the XTT-test results.Therefore, the viable count method is more suitable for detecting the influence of traditional Chinese medicine extracts on biofilms than the XTT-test.
When the content of the mature stage biofilm was detected using semi-quantitative crystal violet experiments, the sample optical density values could not be detected by the microplate reader; this is mainly due to the sample optical density values exceeding the detection limit of the microplate reader owing to the substantial total amount of mature biofilm.Therefore, only the influence of TRW onStaphylococcus epidermidisbiofilm adhesion and aggregation stage was detected through a semi-quantitative crystal violet experiment in this study.As a result, the content of mature stage biofilms was not detected.
Conclusion
This study indicates that TRW had good inhibition activity againstStaphylococcus epidermidisand its biofilm.There was no significant difference in the effect of TRW and ZPT on the total amount of biofilm inhibition and the number of viable biofilm bacteria in the adhesion and aggregation stages, which provides support for TRW to replace ZPT as an antimicrobial additive for the treatment of scalp seborrheic dermatitis.
 Traditional Medicine Research2022年5期
Traditional Medicine Research2022年5期
- Traditional Medicine Research的其它文章
- G1-4A, an arabinogalactan polysaccharide derived from Tinospora cordifolia(Thunb.)Miers:a natural immunomodulator
- Pseudotargeted metabolomics for exploring the changes of neurotransmitters profile in aging rat brain and the potential neuroprotective effect of alkaloids from Uncaria rhynchophylla
- Antioxidant, hepatoprotective and nephroprotective activities of Gazania rigens against carbon tetrachloride-induced hepatotoxicity and nephrotoxicity in rats
- Traditional medicines and experimental analysis methods for Alzheimer’s disease
- Update on the preclinical and clinical assessment of Withania somnifera: from ancient Rasayana to modern perspectives
- Valerian(Valeriana officinalis)extract inhibits TNF-α and iNOS gene expression in mouse LPS-activated microglial cells
