Valerian(Valeriana officinalis)extract inhibits TNF-α and iNOS gene expression in mouse LPS-activated microglial cells
Hashem Marawne,Reza Mohammadhassan,Zahra Mohammadalipour*,Sedigheh Ahmadpour
1Amino Techno Gene Virtual Private Institute(NGO),Tehran 1495845882,Iran.
Abstract Background: Inflammation and damage to neurons and other cells in the nervous system can cause disorders of the central nervous system.Microglial cells are activated by pathogen infection and injury to release nitric oxide.Valerian (Valeriana officinalis) has been used as a sedative for the treatment of neurological diseases.This study evaluated inflammation of microglial cells and tumor necrosis factor α and induced nitric oxide synthetase gene expression influenced by valerian extract. Methods: Microglial cells were isolated from mice.Lipopolysaccharide (1 ng/mL) was used to induce inflammation and nitric oxide production in cells for an hour.The inflamed cells were then treated with different concentrations (0.1, 0.5, 2.5, 20, and 50 μL/mL) of valerian alcoholic extract for 1 and 24 h.nitric oxide production and tumor necrosis factor α and induced nitric oxide synthetase gene expression were determine by Griess assay and real-time polymerase chain reaction,respectively. Results: The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay showed no toxicity in several concentrations of the valerian extract.In addition,concentrations from 0.1 to 2.5 μL/mL significantly reduced inflammation and nitric oxide production in mouse microglial cells to levels observed in control samples.Furthermore,tumor necrosis factor α and induced nitric oxide synthetase gene expression decreased when 2.5 μL/mL extract was used. Conclusion: Based on these results, it can be concluded that 2.5 μL/mL valerian alcoholic extract is effective as a candidate alternative medicine for reducing inflammation and nitric oxide production and consequently, the inflammatory symptoms of neurodegeneration.
Keywords:microglial cells; nitric oxide; Valeriana officinalis;TNF-α; iNOS
Background
Disorders that involve nerve cell degradation are considered degenerations of the central nervous system (CNS) or neurodegenerative diseases, such as Parkinson's and Alzheimer's diseases, caused by degeneration and gradual death of neurons that lead to problems in memory, movement control, and perception [1,2].The CNS includes both neurons and neuroglial cells, and these neurons are protected by nonirritable neuroglial cells [3].Although these cells play a crucial role in neuronal protection, they are not considered as nerve cells.They are responsible for binding neurons together, called glue or glia.Neuroglial cells fill approximately half of the CNS because they are larger in number but smaller in size than neurons [4].Thier functions include surrounding and fixing neurons,feeding and providing oxygen, and separating neurons from each other.They are classified into four neuroprotector cells known as Schwann cells, namely microglia, astrocytes, oligodendrocytes, and ependymal cells [3].Microglial cells are among the brain's most effective and smallest protector cells, accounting for 20% of all non-neural cells [5].They play various roles, particularly in the same manner as macrophages, in temporally eliminating abnormal neurons.Hence, microglial cells are considered to have been derived from blood cells with a mesodermal origin [6].Generally, these cells can be observed as spindle shaped with glossy nuclei but can also be found in a circular shape when activated.Microglial cells branch under the influence of hormonal and plasma factors, proteins, and self-released substances.This means that the flexibility of these cells, as a remarkable feature, can be observed under different conditions [7].This characteristic allows them to control the extracellular parenchyma of the nervous system to react against microbial and inflammatory factors by releasing cytokines and antigens [8].During the development and growth of brain cells, microglial cells play many key roles, such as neurotrophic factor synthesis and regulation of synaptic messages to support synapse formation.Thus, these cells play vital roles in brain injury and neuro degenerative diseases [9].These diseases, including Alzheimer's disease, cerebral arteriosclerosis,Parkinson's disease, multiple sclerosis, and even brain cancer, can be considered as inflammation, which is defined as a tissue response to redundant and dangerous stimuli [2].
During inflammation, nitric oxide (NO) is biosynthesized by nitric oxide synthase (NOS) and released into the intercellular environment.NOS can be classified into three types, namely induced nitric oxide synthase (iNOS), endothelial NOS, and neuronal NOS [9].iNOS encodes a nondependent calcium/calmodulin enzyme that catalyzes NO production from L-arginine.iNOS expression has been observed in a broad range of cell types, particularly in the presence of cytokines and lipopolysaccharides (LPS).iNOS is generally expressed in glial cells, and specifically in microglial cells.Therefore, NO production can alter the function of proteins [10].Other key factors can also induce inflammation, including tumor necrosis factor-α (TNF-α),which is considered a cytokine and is generally produced and released by macrophages and microglial cells.This factor plays a role in the regulation of immune cell function.In addition, TNF-α induces inflammation, fever, septic shock caused by infection, and cachexia and inhibits tumor development[11].
Valerian(Valeriana officinalis) is conventionally used as a traditional herbal analgesic, anticonvulsant, antimigraine, and antiinflammatory medication.The anti-inflammatory effects of valerian extract on the CNS have been previously demonstrated [12, 13].Many secondary metabolites have been identified as the active ingredients in valerian extract, including valproate, isovalporate, didovalporate, valeric acid,valernal, valeron, isovaleric acid, and α- and β-pinene [14].Valeric acid has been shown to reduce neural nerve stimulation and neurogenic inflammation[12].In this study,we investigated the effect of valerian extract on NO production, cellular viability, and iNOS and TNF-α gene expression in microglial cells of the rat brain.
Materials and methods
Cell culture and separation
Microglial cells were split and cultured as described by McCarthy and Vellis [15].Small fragments of the brains from rats aged 1-4 d were transferred to a Petri dish containing 2 mL Dulbecco’s modified Eagle medium(DMEM).The suspension was gently pipetted,transferred to a T25 flask containing 20% DMEM and fetal bovine serum, and incubated at 37 °C.The medium was renewed every 3 d.After 10-14 d, the cells were spread to approximately 80% of the bottom of the flasks, allowing the glial cells to be completely recognized.Microglial cells are small, round, shiny cells [16].They were then separated using 25% trypsin and counted.Ethical approval for use of animals in research was obtained from the Amino Techno Gene Virtual Private Lab Animal Ethics Office (ethics numbers: ATG.VP.LAB.AEO.1398.01).All studies abided by and conformed to the requirements fromGuideline for the Care and Use of Laboratory Animals in Iran.
Valerian extract treatment
First, the microglial cells were transferred to media plates.Then, all samples were inflamed with 1 ng/mL LPS (Sigma-Aldrich Corp., St.Louis,MO,USA),an endotoxin derived from the wall of gram-negative bacteria [17].Subsequently, different concentrations (0.1, 0.5, 1.5,2.5, 20, 50, and 100 µL/mL) of valerian alcoholic extracts (Zardband Co., Tehran, Iran) were mixed with DMEM, and 10 µL of the mixtures was added to the media and incubated for 1 or 24 h.
NO measurement
Fifty microliters of each cell medium was transferred to each well of a new plate, to which 50 µL Griess reagent (Sigma-Aldrich) was added.The plates were then thoroughly covered for 15 min.NO was measured at 540 nm using an enzyme-linked immunosorbent assay reader.
Cell viability
Cell viability was e valuated using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay.Again, 50 µL of cell medium was transferred to the wells of a new 96-well plate.After adding 10µL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide solution (Sigma-Aldrich) to each well, the medium was incubated at 37 °C for 4 h, after which 100 µL of dimethyl sulfoxide was added to each well.All samples were analyzed at 580 nm using an enzyme-linked immunosorbent assay reader.
TNF-α and iNOS expression
After 4 h of incubation with valerian extract, total RNA was extracted using the RNX-Plus kit (SinaClon Co., Tehran, Iran).RNA quality was measured using A260/280 and A230/280 absorbance ratios.Subsequently, reverse transcription polymerase chain reaction (PCR)and real-time PCR were performed with the designed specific primers(Table 1) to analyze TNF-α and iNOS gene expression.
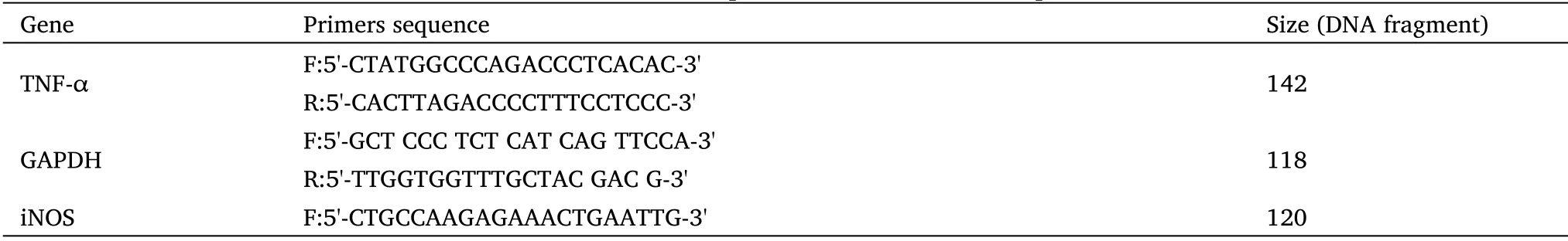
Table 1 Characteristics of primers in reverse transcription PCR
Statistical analysis
The data were statistically analyzed by one-way analysis of variance using SPSS software (version 24; SPSS Inc., Chicago, IL, USA).Means were compared using the least significant difference test (P>0.05).
Results
The cultured nerve cells covered the bottom of the flask.The morphology of the cells was observable on the third day, and the cells were observed via microscopy on the seventh day.Astrocytes and microglial cells showed star-shaped arms and shiny gibbous nuclei,respectively.Oligodendrocytes covered the bottom of the flask as mosaics.The microglial cells were separated from the other cells using trypsin (Figure 1).
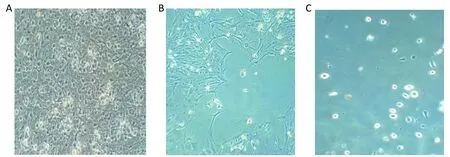
Figure 1 The morphology of the cultured nerve cells (X32).(A) Cell culture on the 14th day; (B) dispersed cells by trypsin; (C) separated microglial cells.
NO production
In general, all concentrations of the valerian extract significantly decreased NO production.Lower doses at 0.1, 0.5, and 2.5 µL/mL resulted in more significant changes in NO production.Meanwhile,higher concentrations increased NO production but not as much as the LPS-treated samples.These results were similar for both the 1 and 24 h treatments.The lowest and most significant changes were observed at 2.5 µL/mL of valerian extract (Figure 2).
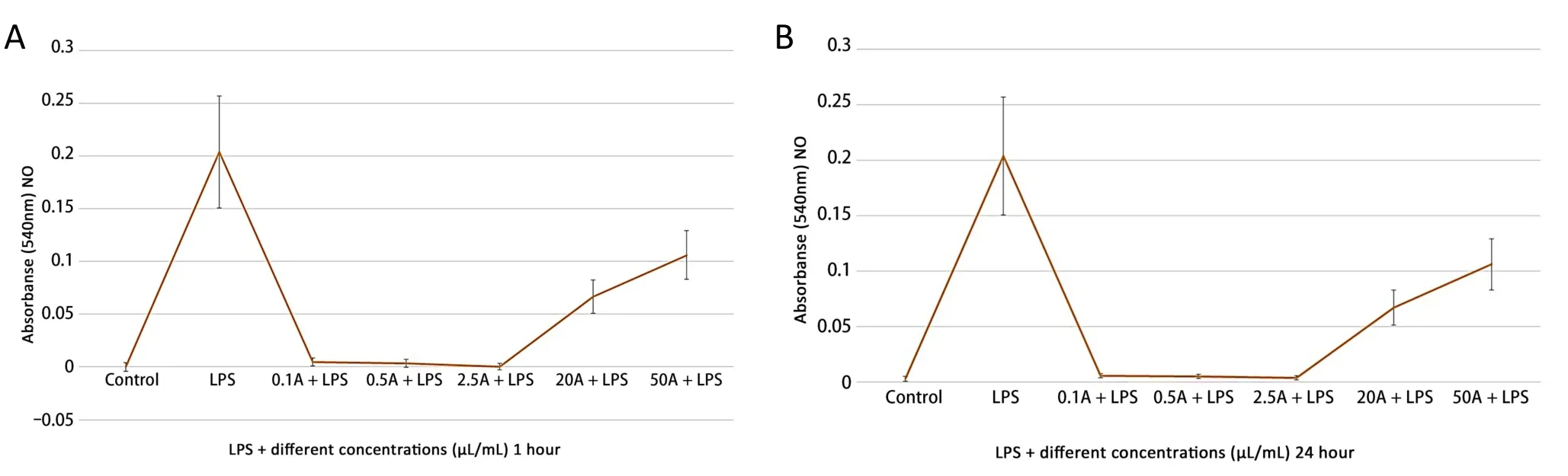
Figure 2 NO production in the treatment of several concentrations of valerian extracts.(A) 1 hour; (B) 24 hours.NO, nitric oxide; LPS,lipopolysaccharide.
Cell viability
No significant changes in cell survival were observed at lower concentrations of the valerian extract.In contrast, higher doses (20 and 50 µL/mL) significantly improved cell survival.The most significant change in cell survival was observed when 50 µL/mL of valerian extract was used.Furthermore, a similarity was observed between the 1 and 24 h treatment (Figure 3).
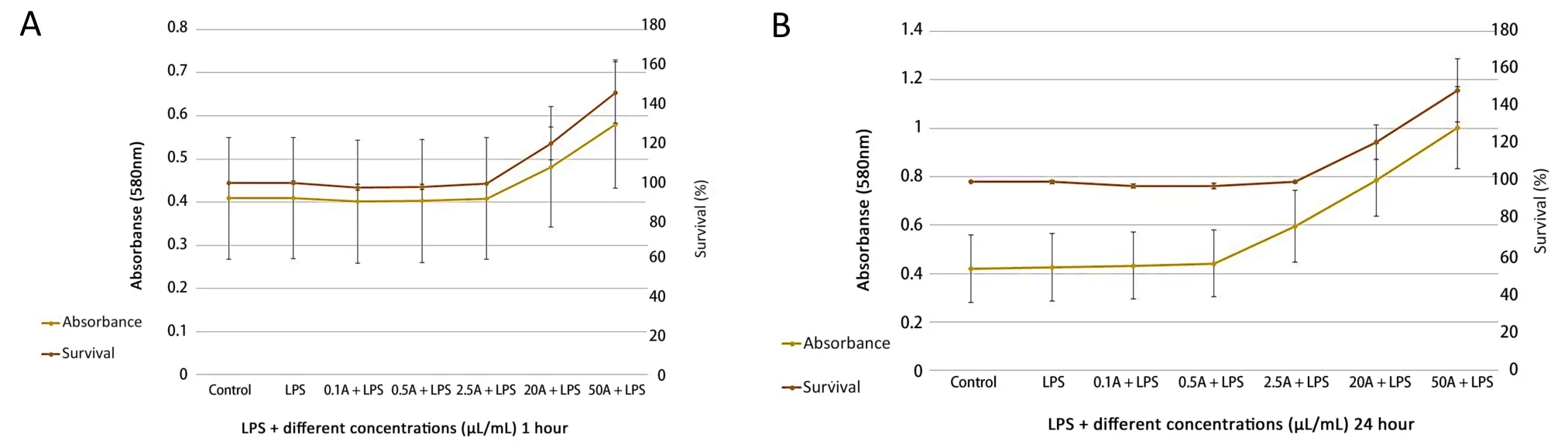
Figure 3 Cellular viability in the treatment of different doses of valerian extracts.(A) 1 hour; (B)24 hours.LPS, lipopolysaccharide.
TNF-α and iNOS expression
Although low doses, particularly 0.1 and 0.5 µL/mL, and higher doses of valerian extract, that is, 20 and 50 µL/mL, resulted in increased gene expression, no significant change in gene expression was observed as a result of higher and lower doses of valerian extract.However, the gene expression fluctuated.Meanwhile, significant changes in TNF-α and iNOS expression were observed when 2.5µL/mL valerian extract was used (Figure 4).
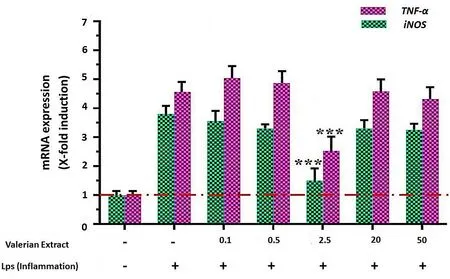
Figure 4 The effect of different doses of valerian extract on TNF-α and iNOS expression; stars indicate statistically significant data.*P <0.05;**P <0.01;***P <0.001.TNF-α, tumor necrosis factor α;iNOS, induced nitric oxide synthetase; LPS, lipopolysaccharide.
Discussion
The discovery of inflammatory mediators in neurodegenerative diseases supports the hypothesis that neuroinflammation may influence these conditions.Neuroinflammation is characterized by reactive morphology of glial cells, including astrocytes and microglia,in neurodegenerative disorders such as Alzheimer's disease, cerebral arteriosclerosis, Parkinson's disease, and multiple sclerosis [18].In neurodegenerative disorders, microglial activity has both positive and negative effects on neurodegeneration.Overactivated microglial cells contribute to neuroinflammation by synthesizing several neurotoxic substances, such as NO.Conversely, many studies have shown that activated microglia produce increasing NO [19, 20].
Medicinal plants can help patients with neurodegenerative disorders by suppressing neuroinflammation via various cellular and molecular pathways.Other neuroprotective mechanisms of traditional medicinal plants include reducing inflammatory responses, inhibiting the expression and activity of proinflammatory cytokines such as tumor necrosis factor and interleukins, and enhancing antioxidative function mediated by superoxide dismutase and catalase.Regulation of transcription, transduction, and cellular signalling with the regulatory function of promoters in inflammatory and oxidativestress-related mechanisms is significant in the ability of medicinal plants to prevent and treat neurodegenerative disorders [21-24].
Hence, it is possible that the discovery of effective anti-inflammatory substances that reduce microglial activation can lead to a potential therapeutic strategy for various neurodegenerative diseases.In this study, valerian alcoholic extract effectively reduced inflammatory activation of microglial cells in culture.
Moreover, NO concentrations were measured in activated microglial cells treated with valerian alcoholic extract.The results showed that 2.5 µL/mL valerian alcoholic extract significantly decreased NO production and the gene expression of TNF-α and iNOS involved in neuroinflammation.Meanwhile, 0.1 and 0.5 µL/mL concentrations reduced, although not significantly, the inflammation of LPS-activated microglial cells.In addition, higher doses(20, 50, and 100 µL/mL) did not result in significant changes in neuroinflammation.Although low concentrations of valerian extract decreased neuroinflammation and TNF-α and iNOS gene expression,cell survival was significantly increased by higher doses, particularly 20 and 50 µL/mL.
Many studies have demonstrated that valerian is utilized for the relaxing, sleep-inducing, and neuroprotective properties of its secondary metabolites.Valeric acid can positively influence gamma-aminobutyric acid, which is a neurotransmitter that regulates CNS function [25].In addition, valepotriates and valeric acid protect mRNAs in microglial cells against oxidants.Some studies have reported the antitoxic effects of valproic acid on the expression of the nuclear factor erythroid 2-related factor 2 gene as an emerging regulator of oxidant resistance in neural cells [26, 27].Valproic acid has been shown to significantly reduce nuclear factor erythroid 2-related factor 2 expression in central neural cells.Meanwhile,valerenic acid reduced neurodegeneration in a Parkinson's disease mouse model, which may protect dopaminergic neurons and recover motor function [28, 29].Valproate can reduce chronic constriction injury-induced thermal and mechanical pain responses in mice, and this is correlated with antineuroinflammatory effects, such as decreased proprioceptive neuromuscular facilitation β/iNOS/cyclooxygenase-2 expression and suppression of phosphorylated protein kinase B/pGlycogen synthase kinase 3-mediated neurodegeneration from peripheral nerve injury [30].It may be that valerian extract supports cell viability by antioxidant activities through flavonoids and polyphenols.Higher concentrations of the plant extract contained more antioxidants to improve oxidant resistance in cells; thus,they can tolerate inflammation and live longer[26, 27, 31, 32].
Conclusion
Based on these results, it can be concluded that high concentrations of valerian extract can increase both cell viability and NO production and consequently, inflammation.In addition, TNF-α and iNOS expression indicated that high concentrations of valerian extract could not prevent inflammation.In contrast, NO production was significantly decreased by low concentrations of valerian extract,particularly 0.5 and 2.5 µL/mL.In addition, real-time PCR results demonstrated that a concentration of 2.5 µL/mL inhibited TNF-α and iNOS expression in inflamed cells to levels comparable to those in normal cells.However, higher concentrations significantly affected cell viability.Therefore, 2.5 µL/mL may be the effective dose for valerian extract.
 Traditional Medicine Research2022年5期
Traditional Medicine Research2022年5期
- Traditional Medicine Research的其它文章
- G1-4A, an arabinogalactan polysaccharide derived from Tinospora cordifolia(Thunb.)Miers:a natural immunomodulator
- Pseudotargeted metabolomics for exploring the changes of neurotransmitters profile in aging rat brain and the potential neuroprotective effect of alkaloids from Uncaria rhynchophylla
- Antioxidant, hepatoprotective and nephroprotective activities of Gazania rigens against carbon tetrachloride-induced hepatotoxicity and nephrotoxicity in rats
- Traditional medicines and experimental analysis methods for Alzheimer’s disease
- Effect of Terminalia chebula Retz.extraction with water on Staphylococcus epidermidis activity and its biofilm formation
- Update on the preclinical and clinical assessment of Withania somnifera: from ancient Rasayana to modern perspectives
