Antioxidant, hepatoprotective and nephroprotective activities of Gazania rigens against carbon tetrachloride-induced hepatotoxicity and nephrotoxicity in rats
Alshymaa Abdel-Rhman Gomaa, Mamdouh Nabil Samy*, Eman Zekry Attia, Mina Ezzat Attya, Michael Atef Fawzy, Samar Yehia Desoukey, Mohamed Salah Kamel
1Department of Pharmacognosy,Faculty of Pharmacy,Minia University,Minia 61519,Egypt.2Department of Pathology,Faculty of Medicine,Minia University,Minia 61519,Egypt.3Department of Biochemistry,Faculty of Pharmacy,Minia University,Minia 61519,Egypt.
Abstract Objective: The current study investigated the efficacy of total ethanolic extract and various fractions of Gazania rigens to protect against carbon tetrachloride-induced liver and kidney damage in rats and evaluated their antioxidant activity. Methods: The antioxidant activity of the total extract and fractions (petroleum ether, ethyl acetate, and aqueous) of Gazania rigens was examined using 2,2’-diphenyl-1-picrylhydrazyl radical scavenging and phosphomolybdate assays, and their total phenolic and flavonoid contents were determined.The hepatoprotective and nephroprotective potentials were studied at a dose of 300 mg/kg for six consecutive days followed by induction of hepatorenal injury using carbon tetrachloride single dose (1.5 mL/kg,1:1 v/v in olive oil, intraperitoneal) the next day. Results: The results revealed the potent hepato- and nephroprotective effects of ethyl acetate and aqueous fractions by significantly (P<0.01 or P <0.001) reducing the serum levels of alanine aminotransferase, aspartate aminotransferase, total bilirubin, creatinine, urea, triglycerides, cholesterol, and lactate dehydrogenase, along with elevated serum albumin levels.The improvement of hepatic and renal antioxidant capacities and catalase activities, together with the prominent reduction of hepatic and renal malondialdehyde contents,were confirmed by histopathological examination.Conclusion: The potent hepatoprotective and nephroprotective effects of the total extract and different fractions of Gazania rigens may be attributed to the presence of highly phenolic and flavonoid compounds along with their complementary antioxidant scavenging properties.
Keywords:Asteraceae; Gazania rigens; antioxidant; hepatoprotective; nephroprotective
Background
The liver is the most important organ in the human body because it is responsible for metabolism, excretion, detoxification, and energy storage.Hepatic injury, including hepatitis, cirrhosis, and hepatocellular carcinoma, is caused by alcohol intake, drug abuse and misuse, and environmental pollutant exposure [1].Liver disease is a global problem, and although there are some conventional drugs capable of treating liver disease that can enhance liver function and provide hepatic protection or aid in hepatic cell regeneration, they have been shown to be hepatotoxic at specific doses[2, 3].Hence, the potential of novel herbal drugs derived from medicinal plants in preventing and treating hepatotoxic damage has become a major research focus[4].
The kidney plays an important role in the removal of toxic substances from the body through filtration and excretion.It is highly vulnerable to toxic injuries caused by drugs and toxins because of high blood flow and involved cellular transport systems that cause the accumulation of these compounds within nephron epithelial cells [5,6].The incidence of renal failure is currently increasing at an alarming rate, which is marked by loss of the kidney’s ability to excrete waste,collect urine,preserve electrolytes,and maintain fluid balance,as well as a high morbidity and mortality rate [7].Many synthetic drugs used to treat liver and kidney diseases are ineffective and can cause severe side effects.Considering these limitations, new drugs derived from natural sources are being studied to determine their safety and efficacy [1, 8, 9].
Gazania rigens(L.) Gaertn.(G.rigens, also calledGazania splendens,Gorteria rigensandOthonna rigens) is an herbaceous perennial belonging to theAsteraceaefamily and is commonly referred to as gazania and the treasure flower.It is native to South Africa and is widely considered one of the most beautiful greenhouse plants [10].Gazania is an evergreen plant with an extended flower life and strong resistance to dry sandy soils and hot weather;therefore,it is utilized in gardens for bedding, edging, and mass planting [11].Furthermore,plants of this genus are utilized in traditional medicine to prevent miscarriage and treat toothaches as well as to supplement purgative preparations with aloe [12].The available literature reveals that the plant contains several important phytochemical constituents, such as triterpenes, sterols, flavonoids, carotenoids, and phenolic acid derivatives [13-15].The plant has also been reported to exhibit antimicrobial, anti-inflammatory, and analgesic activities [12, 15],and few studies have investigated the hepatoprotective and antioxidant properties of the plant; however, this is the first report to evaluate its nephroprotective activity.Therefore, the current study focused on identifying and estimating the nephroprotective and hepatoprotective effects of the total extract and fractions of the plant against carbon tetrachloride (CCl4)-induced liver and renal injuries in rats, along with the evaluation of its antioxidant potential to correlate its significant nephroprotective and hepatoprotective effects with its phenolic and flavonoid contents and antioxidant properties.
Methods
Plant material
G.rigens(whole plants) were collected in December 2015 from the Nursery of the Faculty of Agriculture, Minia University, Egypt, and identified by Prof.Dr.Nasser Barakat (Professor of Botany, Faculty of Science,Minia University).The voucher sample(Mn-ph-Cog-025) was kept within the herbarium of the Pharmacognosy Department at the Faculty of Pharmacy of Minia University.
Chemicals
Ascorbic acid, rutin, gallic acid, aluminum chloride, Folin-Ciocalteu reagent, and 2,2’-diphenyl-1-picrylhydrazyl (DPPH) were obtained from Sigma Co.(St.Louis,MO,USA).Sulfuric acid,sodium carbonate,ammonium molybdate, sodium hydroxide, sodium nitrite, and disodium hydrogen phosphate were obtained from Wako (Osaka,Japan).Sodium dihydrogen phosphate and all solvents used(methanol, ethanol, petroleum ether, and ethyl acetate) were purchased from Merck Co.(Darmstadt, Germany).For the biological experiments, carboxymethylcellulose was obtained from El-Nasr Pharmaceutical and Chemical Co.(Cairo, Egypt).For hepatoxic induction, CCl4was obtained from Alpha Chemika (Mumbai,Maharashtra, India) and standard silymarin (legalon 140®) was purchased from MADAUS GmbH (Troisdorf, Germany).
Sample preparation
Samples of air-dried wholeG.rigensmaterial (4.5 kg) was extracted with 95% ethanol and concentrated under reduced pressure to yield 500 g of dried plant material.The concentrated ethanol extract ofG.rigenswas suspended in the least amount of distilled water,transferred to a separating funnel, and partitioned successively using petroleum ether and ethyl acetate.The fractions were concentrated under reduced pressure to obtain petroleum ether (153 g) and ethyl acetate fractions (19 g).The remaining mother liquor was concentrated to obtain an aqueous fraction (260 g).
Determination of total phenolic and flavonoid contents
The total phenolic content of the ethanolic extract and different solvent fractions ofG.rigenswas evaluated using the Folin-Ciocalteu method [16].The study was conducted by using 3.5 mL of deionized water,50 μL of the sample(extract or fractions)at 10 mg/mL,50 μL of 2N Folin-Ciocalteu reagent,and 300 μL of 10%sodium carbonate.The reaction was allowed to proceed for 30 minutes.The absorbance of the solution was recorded in triplicate at 730 nm against a blank.A standard curve was produced using gallic acid, and the total phenolic concentration was expressed as milligrams of gallic acid equivalent per gram of dried fraction.Total flavonoid content was calculated using the method described previously [16].Briefly, 0.3 mL of all samples (2.5 mg/mL for all fractions except the ethyl acetate fraction,1 mg/mL), 3.4 mL of 30% methanol, 0.15 mL of NaNO2(0.5 M), and 0.15 mL of AlCl3·6H2O (0.3 M) were mixed.After 5 min, 1 mL NaOH(1 M) was added.The solution was mixed well, and the absorbance was measured in triplicate at 506 nm against the blank.The standard curve for total flavonoids was determined using rutin, and the total flavonoid content was expressed as milligrams of rutin equivalents per gram of dried fraction.
Antioxidant activity screening
Each sample was dissolved in 95% methanol to yield a stock solution of 10 mg/mL for all extracts and fractions(excluding the ethyl acetate fraction, 2.5 mg/mL) and then diluted to prepare a series of concentrations for the two antioxidant assays.
DPPH radical scavenging assay.The free radical-scavenging activities of the extracts and fractions were measured using DPPH.Briefly, 200 μL of each sample was serially diluted and added to 2 mL of DPPH solution(0.1 mM).The reaction mixture was shaken well and incubated in the dark for 15 min at room temperature.The absorbance was measured in triplicate at 517 nm[16].
Phosphomolybdate assay.The total antioxidant capacity (TAC) of the fractions was determined by phosphomolybdate complex assay using ascorbic acid as a standard [16].Aliquots (0.3 mL of each sample solution were mixed with 3 mL of reagent solution (0.6 M sulphuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate).The tubes were capped and incubated in a water bath at 95 °C for 90 min.After the samples were cooled to room temperature,the absorbance of the mixture was measured in triplicate at six 95 nm against the blank.Ascorbic acid was used to generate a standard curve,and the reducing power was expressed relative to that of ascorbic acid.
Experimental animals
Male albino rats weighing 200±20 g were used for the animal model.The rats were housed in conventional cages under standardized environmental conditions at the animal house of the Faculty of Medicine, Pharmacology, and Toxicology Department, Minia University, Egypt, and fed a standard diet and water.The experimental protocol was approved by the Research Ethics Committee for Animal Experimentation, Department of Pharmacology and Toxicology, Faculty of Pharmacy, Minia University, Egypt (No.47/2019).The animals were acclimated to the laboratory environment for one week before conducting the experiment.
Hepatoprotective and nephroprotective activities
Experimental design.The total ethanolic extract and different fractions ofG.rigenswere examined to determine their hepatoprotective and nephroprotective activities using a CCl4-induced hepatorenal damage injury model in rats according to a well-established method [17, 18].The rats were divided into seven groups (four animals per group) before acclimatization to laboratory conditions for seven days.The total ethanolic extract and different fractions were administered as a single daily oral dose (300 mg/kg suspended in 0.05% carboxymethylcellulose for six consecutive days before a single CCl4injection (1.5 mL/kg, 1:1 v/v in olive oil,intraperitoneal) on the seventh day of the experiment.
Treatments were given to groups as follows.Group 1 served as the normal control group and received only 0.05%carboxymethylcellulose.Group 2 received only CCl4.Group 3 was administered a daily oral dose of 100 mg/kg of silymarin for six consecutive days.Group 4 was administered a daily oral dose of 300 mg/kg of total ethanolicG.rigensextract for six consecutive days.Group 5 was administered a daily oral dose of 300 mg/kg of petroleum etherG.rigensfraction for six consecutive days.Group 6 was administered a daily oral dose of 300 mg/kg of ethyl acetate fraction ofG.rigensgroup for six consecutive days.Group 7 received a daily oral dose of 300 mg/kg of aqueous fraction of whole G.rigensgroup for six consecutive days.
Estimation of biochemical parameters.The day after the last dose was administered, all rats were sacrificed under urethane anesthesia.Blood samples from each group were collected, and serum was separated by centrifugation at 3,000 rpm for 10 min and used to determine serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin, creatinine, urea, albumin,triglycerides, cholesterol, and lactate dehydrogenase (LDH), which were estimated by standard methods [19-25].Liver and kidney tissue samples were taken from each rat and separately homogenized with 0.1 M potassium phosphate buffer(pH 7.4).The resulting homogenate in each case was centrifuged, and the supernatant was used for the determination of malondialdehyde (MDA), catalase activity, and TAC of the liver and kidney according to specific methods [26-28].The remaining tissue was preserved in 10% buffered formalin for histopathological analysis.
Histopathological examination.The preserved liver and kidney tissues in 10% buffered formalin were embedded in paraffin, and the tissue sections were prepared using microtome and stained with alum-hematoxylin and eosin.Finally, the stained sections were observed under a photomicroscope (LEICA DM1000, Wetzlar,Germany) microscope with a digital camera (LEICA, EC3, Wetzlar,Germany) to observe histopathological changes.
Statistical analysis
The results were expressed as means ± standard error of mean.One-way analysis of variance followed by Dunnett’s test was used to determine significance when compared to the CCl4group.P-values of less than 0.05,0.01,and 0.001 were considered significant(*P<0.05,**P<0.01,***P<0.001).GraphPad Prism 5(GraphPad software, San Diego, CA, USA) was used for statistical calculation.
Results
Total phenolic and flavonoid contents
The ethyl acetate fraction ofG.rigenscontained the highest total phenolic and flavonoid contents (14.33 ± 0.53 mg gallic acid equivalent/g dried plant extract and 462.50 ± 0.82 mg rutin equivalent/g dried plant extract, respectively).In contrast, the petroleum ether fraction contained the lowest amounts (Figure 1 and Supplementary Table S1).
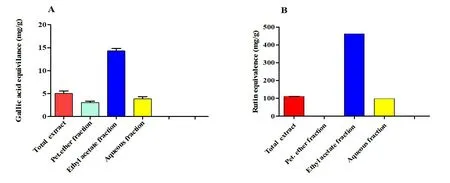
Figure 1 Total phenolic and flavonoid contents of total ethanolic extract and different fractions of Gazania rigens(whole plant).(A)Total phenolic content; (B) total flavonoid content.
Antioxidant activity
DPPH radical scavenging activity.All samples were tested for radical scavenging activity using the DPPH free radical scavenging assay (Figure 2 and Supplementary Table S2).The ethyl acetate fraction exhibited the highest activity with half maximal inhibitory concentration value of 0.18 ± 0.02 mg/mL (P< 0.001).The petroleum ether fraction exhibited the least scavenging activity.The antioxidant effects of the separated fractions evaluated by DPPH radical scavenging activity showed a direct correlation of 0.782 with phenolic and flavonoid contents.
Phosphomolybdate assay (total antioxidant activity).The ethyl acetate fraction had a higher antioxidant capacity than other tested extract and fractions (25.68 ± 0.73 mg ascorbic acid equivalent/g dried plant extract).Conversely, the least activity was shown by the aqueous fraction (5.53 ± 0.37 mg ascorbic acid equivalent/g dried plant extract).The ethyl acetate fraction exhibited the most potent antioxidant activity, which may be attributed to its high phenolic and flavonoid content (Figure 2 and Supplementary Table S2).
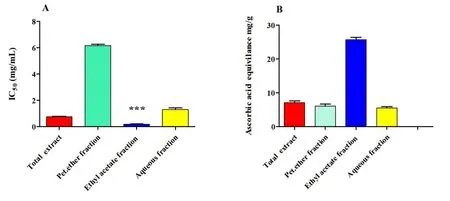
Figure 2 Antioxidant activity of the total ethanolic extract and different fractions of the whole plant of Gazania rigens using DPPH scavenging and phosphomolybdenum complex assays.(A)DPPH radical scavenging activity;(B)phosphomolybdenum complex assay.IC50,half maximal inhibitory concentration.DPPH, 2,2’-diphenyl-1-picrylhydrazyl.
Hepatoprotective activity
Effect of G.rigens on serum biochemical parameters.As indicated by the results (Figure 3 and Supplementary Table S3), injection of CCl4-induced hepatotoxicity caused elevation of serum levels of ALT,AST, total bilirubin, triglycerides, cholesterol, and LDH compared to those in the normal control group.However, pre-treated rats with the total extract and different fractions showed significantly lower hepatic injury and lipid profile markers(P<0.01 orP<0.001).Furthermore,among all testedG.rigensextracts and fractions, the ethyl acetate and aqueous fractions exhibited the maximum activity and promoted recovery toward a normal state in a manner comparable to silymarin treatment.
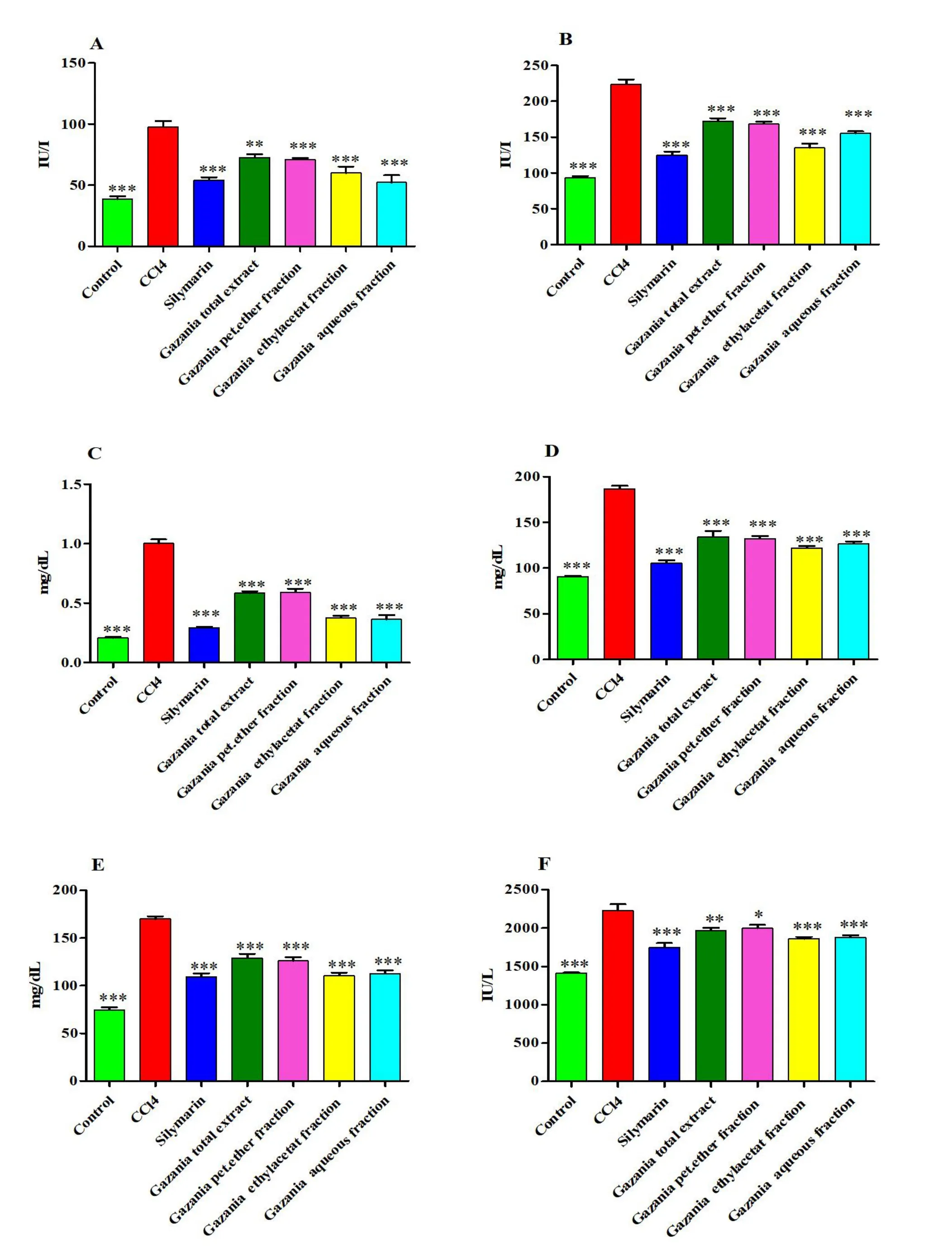
Figure 3 Effect of the total ethanolic extract and different fractions of Gazania rigens on serum biochemical parameters on CCl4 treated rats.*Significantly different from CCl4 group:*P <0.05,**P <0.01,***P <0.001.(A)Alanine aminotransferase;(B)aspartate aminotransferase;(C)total bilirubin; (D) triglhceides; (E) cholesterol; (F) lactate dehydrogenase.CCl4, carbon tetrachloride.
Effect of G.rigens on tissue biochemical parameters.As shown in Figure 4 and Supplementary Table S4, the CCl4treated group showed a noticeable decrease in hepatic antioxidant capacity and catalase activity together with an increase in MDA content compared to the control group.In contrast, rats pretreated with silymarin exhibited a significant reduction in elevated levels of MDA (P<0.001) and markedly increased levels of TAC and catalase activity in CCl4intoxicated rats.Similarly, all tested extracts and fractions yielded improved hepatic capacity and decreased MDA levels.Moreover, the ethyl acetate and aqueous fractions significantly restored the hepatic antioxidant capacity to normal levels and tended to return catalase comparable to the control values (P<0.001).
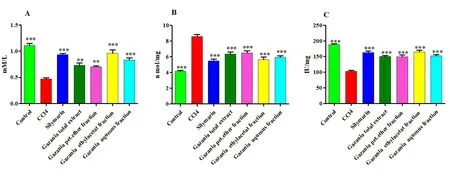
Figure 4 Effect of the total ethanolic extract and different fractions of Gazania rigens(whole plant)on liver tissue biochemical parameters on CCl4 treated rats.*Significantly different from CCl4 group:*P <0.05,**P <0.01,***P <0.001.(A) Total antioxidant capacity; (B)malondialdehyde; (C) catalase.CCl4, carbon tetrachloride.
Histopathological observation.Histopathological studies of liver sections of CCl4treated groups showed severe hepatic damage caused by CCl4administration, which was demonstrated by hepatic cell necrosis, bile duct proliferation, lymphocytic infiltration, and microvesicular fatty changes compared to normal controls (Figure 5),which showed normal architecture of hepatic lobules with a normal central vein surrounded by intact hepatocytes with defined cytoplasm and prominent nuclei.Pretreatment with silymarin almost completely restored the normal architecture of the liver,as illustrated in Figure 5.Furthermore, pretreatment with ethyl acetate and the aqueous fractions exhibited the most potent hepatoprotective effects.Pretreatment with these fractions showed recovery of hepatocytes from necrosis, indicating that these fractions preserved the structural integrity and architecture of liver cells damaged by CCl4administration, which was confirmed by previous biochemical analyses.Liver sections of rats treated with total extract showed a moderate degree of microvesicular fatty change.In addition, foci of hepatic necrosis and bile duct proliferation were detected in those treated with petroleum ether fractions.
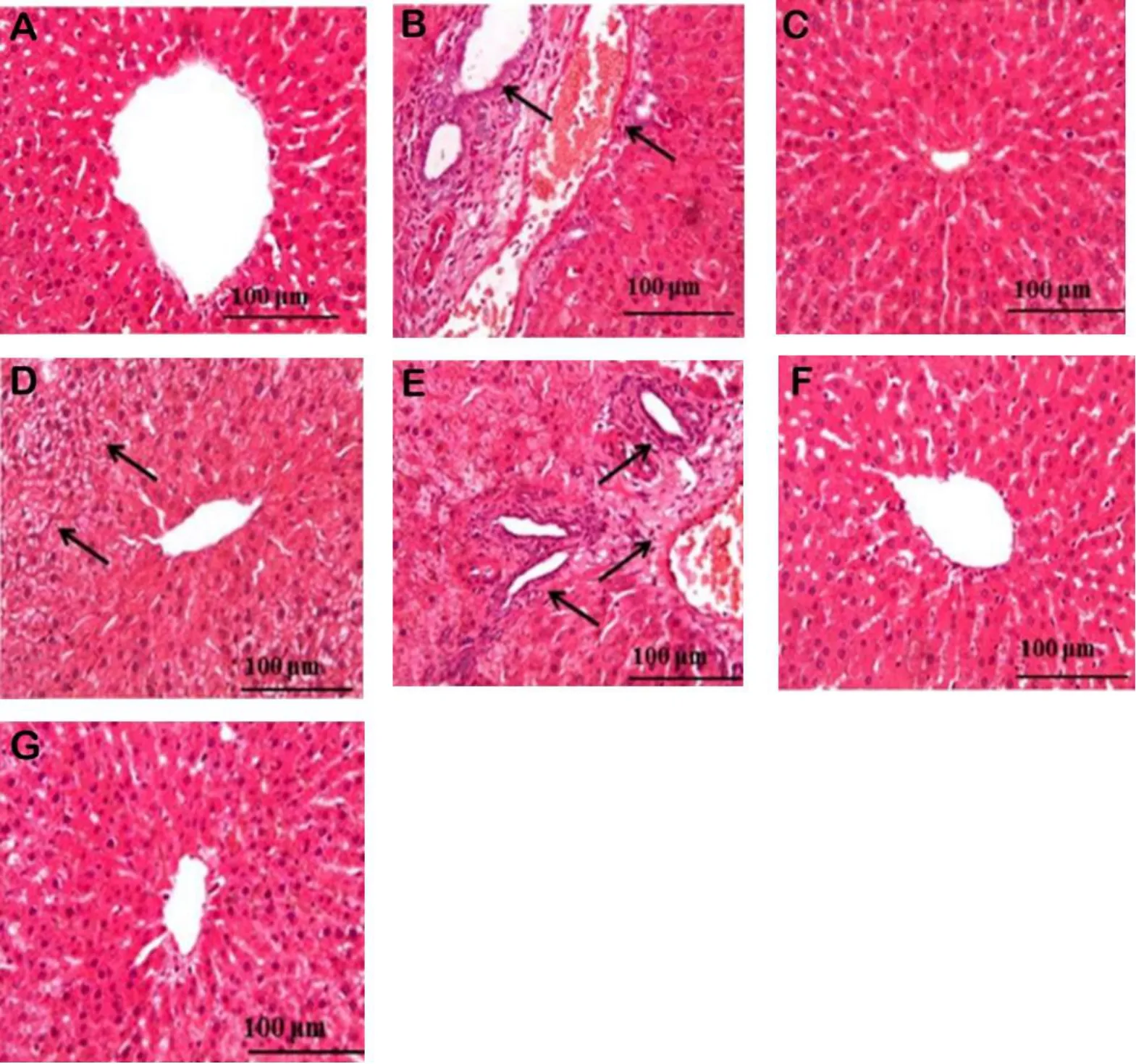
Figure 5 The hepatic histopathological analyses of silymarin and Gazania rigens extract and fractions on CCl4-induced acute liver injury in rats (100×).(A) Normal control group; (B) CCl4-treated group; (C) silymarin treated group; (D) total extract treated group showing moderate fatty change;(E)pet.ether fraction treated group showing bile duct proliferation;(F&G)ethyl acetate and aqueous fractions showing liver restoring to normalcy with little hepatic damage.CCl4, carbon tetrachloride.
Nephroprotective activity
Effect of G.rigens on biochemical parameters.Injection of CCl4-induced nephrotoxicity caused elevation of serum levels of creatinine and urea,along with a reduction in the serum albumin level,compared to the normal control group (Figure 6 and Supplementary Table S5).However,rats pre-treated with the total extract and various fractions showed significantly reduced renal injury (P<0.01 orP<0.001).Moreover, the ethyl acetate and aqueous fractions exhibited the highest activity and induced a recovery to normal in a manner comparable to silymarin treatment.
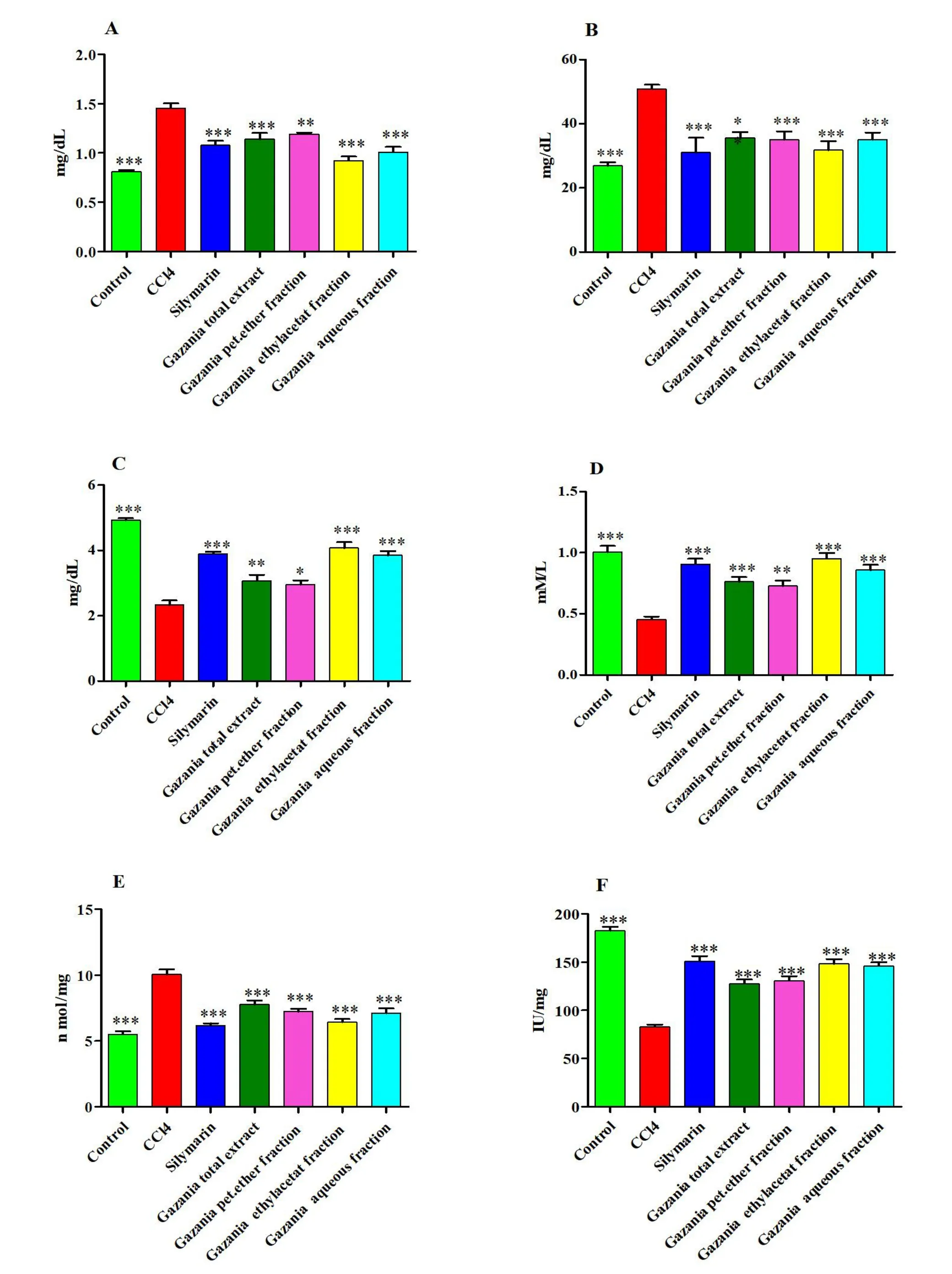
Figure 6 Effect of the total ethanolic extract and different fractions of Gazania rigens (whole plant) on renal biochemical parameters on CCl4 treated rats.*Significantly different from CCl4 group:*P <0.05,**P <0.01,***P <0.001.(A) Creatinine; (B) urea; (C) albumin; (D) total antioxidant capacity; (E) malondialdehyde; (F) catalase.CCl4,carbon tetrachloride.
Additionally, the CCl4treated group demonstrated a significant decrease in renal antioxidant capacity and catalase activity as well as an increase in MDA levels compared to the control group.However,pretreatment with silymarin resulted in a significant reduction in elevated MDA levels(P<0.001)and markedly improved the reduced TAC and catalase activity in CCl4-treated rats.Similarly, all tested extracts and fractions improved the reduced renal capacity and decreased the increased levels of MDA.Furthermore, the ethyl acetate and aqueous fractions significantly (P<0.001) restored the renal antioxidant capacity to normal levels and tended to return catalase levels to control values.
Histopathological observation.Histological examination of kidney sections from the normal control group showed normal glomeruli and tubules without any visible lesions, whereas CCl4renal intoxication was associated with severe glomerular and acute tubular necrosis, as illustrated in Figure 7,which was characterized by degeneration of the glomerular and tubular cells with complete obliteration of the tubular lumen compared to the normal control group.Furthermore,pretreatment with silymarin protected renal tissues against CCl4-induced damage, which prevented glomerular mass loss and cytoplasmic debris accumulation in tubule lumen.Moreover,administration ofG.rigensextract and fractions significantly reduced histopathological changes in the kidney sections, especially in rats treated with ethyl acetate and aqueous fractions, which induced a protective effect against degenerative injury caused by CCl4.These rats showed normal glomeruli, tubular dilatation, and no interstitial edema and capillary congestion.In addition, treatment with these fractions induced a marked recovery in kidney tissue similar to the silymarin treatment group (Figure 7); marked tubular degeneration,interstitial edema, and glomerular necrosis were detected in the petroleum ether fraction-treated groups.Pretreatment with the total extract of the same plant resulted in normal glomeruli and moderate cloudy swelling of the tubular epithelium with marked interstitial edema.

Figure 7 The renal histopathological examination of silymarin and Gazania rigens extract and fractions on CCl4-induced renal injury in rats (200×).(A) Normal control group; (B) CCl4 treated group; (C) silymarin treated group; (D) total extract treated group showing normal glomeruli and moderate tubular injury; (E) pet.ether fraction treated group showing necrotic glomeruli and injured tubules; (F&G) ethyl acetate and aqueous fractions showing normal glomeruli and mild tubular injury.CCl4, carbon tetrachloride.
Discussion
CCl4is among the most widely used hepato-renal toxic agent in experimental modeling to evaluate the hepatoprotective and nephroprotective activities of various medicinal plants.CCl4metabolism is activated by the cytochrome P450 system in liver microsomes to form a trichloromethyl radical that binds to cellular molecules (proteins, nucleic acids, and lipids), causing fatty degeneration (steatosis) and lipid metabolism impairment.The complex formed between this radical and DNA can initiate hepatic cancer.Furthermore,this radical is a highly reactive intermediate that converts into the trichloromethyl peroxy radical in the presence of oxygen [29, 30].This radical is a highly reactive species that initiates the chain reaction of lipid peroxidation and destroys polyunsaturated fatty acids in addition to affecting the permeability of the endoplasmic reticulum, mitochondria, and plasma membranes, resulting in hemostasis, cellular calcium loss, and ultimately cell damage.CCl4can also activate tumor necrosis factor-α and transforming growth factors-α and -β, which may lead to hepatocellular apoptosis and fibrosis[29, 31].
The elevation of serum marker enzymes(ALT and AST)is indicative of cellular leakage and functional integrity loss in the liver due to altered membrane permeability, which results in decreased levels of these enzymes in hepatocytes and increased serum levels [30, 32].An increased serum level of bilirubin is indicative of hepatic dysfunction as it is removed from the blood by the liver through conjugation and secreted in the bile; therefore, it is considered an index that can determine the extent of hepatic injury [1, 3, 32].
Likewise, CCl4administration results in changes to cholesterol and triglyceride serum levels, which may be attributed to accumulated triglyceride and inhibited bile acid synthesis from cholesterol, leading to increased cholesterol levels [33].Along with this change in the lipid profile, elevated LDH serum levels were observed in the CCl4treated group.LDH is a cellular cytoplasmic enzyme,and cell death or leakage of cellular membranes results in its elevation in serum;therefore, it reflects the extent of cellular damage[8].
Moreover, lipid peroxidation is considered one of the most important mechanisms of CCl4-induced nephrotoxicity that leads to elevated MDA levels together with the presence of abnormally high serum levels of urea, creatinine, and reduced serum albumin levels,resulting in severe damage to kidney tissues, as observed in histopathological examination [34, 35].The elevation of these serum markers is a possible indicator of renal injury, which may be attributed to changes in tubular re-absorption and glomerular infiltration rate.These changes can impair the kidney’s ability to excrete these products and lead to increased serum concentrations[34,36].
Silymarin is a flavonoid complex obtained from milk thistle that is widely used as a hepatoprotectant because of its radical-scavenging and antioxidant properties.Recently, silymarin has been reported to have various beneficial effects, including nephroprotective and anti-inflammatory properties.Therefore, it is considered a good candidate as a reference drug to evaluate the nephroprotective effects of many plant extracts[31, 37, 38].
In this study, the hepatoprotective and nephroprotective effects of the total extract and different fractions ofG.rigenswere evaluated using CCl4-induced hepatorenal toxicity rat model.The results revealed that CCl4-treated group exhibited severe hepatic and renal damage, which was confirmed by a marked elevation in ALT, AST,total bilirubin, creatinine, urea, triglycerides, cholesterol, and LDH serum levels compared with the control group.Elevated levels of these biochemical parameters are considered a direct reflection of changes in the hepatic and renal structural integrity.However, pretreated rats with the total extract and different fractions showed a significant hepato-renal protective potential, with the ethyl acetate and aqueous fractions exhibiting the highest activity among the tested fractions.Furthermore, the ethyl acetate and aqueous fractions ofG.rigensproduced a greater reduction in ALT,cholesterol,creatinine,and urea,together with a prominent decrease in total bilirubin and triglycerides in addition to a significant increase in serum albumin levels.
These results were confirmed by histopathological examination of the liver and kidney, as described previously, which revealed the potency of plant fractions in the amelioration of CCl4-induced hepato-renal damage.In addition, the remarkable decrease in the rat liver marker enzymes of groups treated with the total extract and different fractions in this study are in agreement with previous reports on the hepatoprotective potential of the total extract of the tested plant [39].
Oxidative stress plays an important role in CCl4-induced hepato-renal toxicity.Mainly, it occurs when cells lose their ability to protect themselves against the excess formed reactive oxygen species(ROS).Moreover, hydroxyl radicals and hydrogen peroxide are involved in oxidative stress, causing damage to cellular constituents,especially oxidative destruction of lipids and proteins [36].Oxidative injury can be monitored by detecting antioxidant enzymes including superoxide dismutase(SOD),catalase, and glutathione peroxidase[3].Catalase is a heme protein that activates the conversion of hydrogen peroxide to water and oxygen.Glutathione peroxidase catalyzes the reduction of hydrogen peroxide into non-toxic products, whereas SOD catalyzes the destruction of oxygen.The imbalance between antioxidant and oxidant systems (decrease in these antioxidant enzymes or increase in free radicals) could result in lipid peroxidation and oxidative stress.For this reason,these antioxidant enzymes play a critical role in protecting various organs from ROS, which may contribute to their free radical scavenging activity [36].CCl4administration induced lipid peroxidation and yielded a significant decrease in hepatic and renal catalase and SOD because ROS can easily block these antioxidant enzymes.MDA is the final product of the lipid peroxidation process; therefore, the increase in MDA can be used as a biomarker for evaluating hepatic and renal injury [3, 36].
TAC is a critical biomarker of oxidative damage and is considered a useful measurement tool for evaluating the cumulative action of enzymatic (SOD, glutathione peroxidase, and catalase) and non-enzymatic antioxidants (tocopherols, carotenoids, and uric acid).A significant correlation exists between CCl4intoxication and oxidative stress biomarker levels [39-41].This study demonstrated that levels of oxidative stress biomarkers were increased by CCl4administration, as illustrated by lowered TAC and elevated MDA content of the liver and kidney tissues compared to the control group.Pretreatment with the total extract and different fractions ofG.rigensproduced a significant elevation in the hepatic and renal TAC and decreased elevated MDA levels.The maximum effects were shown by the ethyl acetate and aqueous fractions, and the ethyl acetate fraction exhibited a more potent improvement in hepatic and renal TAC relative to the standard drug silymarin.
Based on the above-mentioned results, the present study illustrated the hepato-renal protective potential of the total extract and different fractions ofG.rigensagainst CCl4-induced hepatic and nephrotoxicity,specifically its ethyl acetate and aqueous fractions.Pretreatment with the extract and different fractions efficiently reduced the elevated ALT,AST, total bilirubin, creatinine, urea, triglycerides, cholesterol, and LDH, preventing CCl4damage.This hepatorenal protective effect of both plants was confirmed by decreasing the histological alterations in the liver and kidney.Furthermore, these fractions prevented the CCl4-induced decrease in catalase nd MDA activities and improved the hepatic and renal TAC, similar to silymarin, suggesting their maintenance of antioxidant levels to limit ROS generation and accumulation as well as to protect against CCl4intoxication.This finding is consistent with our study, which showed the potent antioxidant properties of the extract and different fractionsG.rigensusing DPPH radical scavenging activity and phosphomolybdenum complex assay.There was a positive linear correlation between the phenolic content and antioxidant capacity of medicinal plants [42].In addition, our previous work revealed that the metabolomic profiling of the total extract and different fractions ofG.rigensshowed the presence of coumarins, flavonoids, and phenolic acid derivatives [43]and that the antioxidant potential of the ethyl acetate and aqueous fractions of the same plant may be interpreted as having high phenolic and flavonoid contents.These high levels are associated with their hydroxyl groups, which play a critical role in absorbing and neutralizing free radical derivatives of CCl4, hence preserving the structural integrity of hepatic and renal tissues [31, 42].
Conclusion
The current study revealed thatG.rigenscould be considered a potential therapeutic substance due to its ability to prevent hepatic and renal injury and its antioxidant properties, which may be attributed to the presence of key complementary phytoconstituents.Therefore, this plant could be considered a prominent source for the development of new hepatoprotective, nephroprotective, and antioxidant properties because of its wide safety margin and relatively few side effects.However, further detailed studies are required to determine the exact mechanism of hepatorenal protection and antioxidant activity.
 Traditional Medicine Research2022年5期
Traditional Medicine Research2022年5期
- Traditional Medicine Research的其它文章
- G1-4A, an arabinogalactan polysaccharide derived from Tinospora cordifolia(Thunb.)Miers:a natural immunomodulator
- Pseudotargeted metabolomics for exploring the changes of neurotransmitters profile in aging rat brain and the potential neuroprotective effect of alkaloids from Uncaria rhynchophylla
- Traditional medicines and experimental analysis methods for Alzheimer’s disease
- Effect of Terminalia chebula Retz.extraction with water on Staphylococcus epidermidis activity and its biofilm formation
- Update on the preclinical and clinical assessment of Withania somnifera: from ancient Rasayana to modern perspectives
- Valerian(Valeriana officinalis)extract inhibits TNF-α and iNOS gene expression in mouse LPS-activated microglial cells
