Pseudotargeted metabolomics for exploring the changes of neurotransmitters profile in aging rat brain and the potential neuroprotective effect of alkaloids from Uncaria rhynchophylla
Shi-Wei Li,Yi-Song Wu,Shi-Fei Wu,Wen-Long Wei,Ming-Yang Qiu,Yun Li,De-An Guo*
1College of Pharmacy, Changchun University of Chinese Medicine, Changchun 130117, China.2Shanghai Research Center for Modernization of Traditional Chinese Medicine, National Engineering Research Center of TCM Standardization Technology, Shanghai Institute of Materia Medica, Chinese Academy of Sciences,Shanghai 201203,China.
Abstract Background: Aging is an essential risk factor for most neurodegenerative diseases, including Alzheimer’s disease and Parkinson’s disease.However, changes in the levels of neurotransmitters that are associated with aging are not well understood. Methods: Methods such as liquid-liquid extraction, protein precipitation, and solid-phase extraction, using 20 different extraction solvents, were evaluated to optimize the extraction of neurotransmitters.A pseudotargeted metabolomics approach was developed to detect neurotransmitters in brain tissues using ultra-high-performance liquid chromatography coupled with tandem triple quadrupole mass spectrometry.Alkaloids that crossed into the brain were used to evaluate the effect of glutamic acid-induced excitatory neurotoxicity in SH-SY5Y cells. Results: The overall extraction efficiency using protein precipitation was high.The changes in neurotransmitters’levels in the brain exhibited changes during the different growth cycles.The levels of seven neurotransmitters (aspartic acid, tyrosine, isoleucine, leucine, tryptophan, valine, and γ-aminobutyric acid) were significantly different.Meanwhile, alkaloids could reduce the excitatory neurotoxicity of glutamic acid-induced SH-SY5Y cells via suppression of oxidative stress.Conclusion:Significant differences were observed in neurotransmitter profiling between 1-and 8-month-old rats,and the discrepant neurotransmitters were associated with aging.Seven indole alkaloids from Uncaria rhynchophylla, which could cross the blood-brain barrier, were screened and used to explore their protective effects against aging. Uncaria rhynchophylla alkaloids exhibited a neuroprotective effect by inhibiting oxidative stress, indicating that the alkaloid could be a potential therapeutic candidate for neurological disorders caused by glutamic acid toxicity.
Keywords: neurotransmitters; aging; Uncaria rhynchophylla; indole alkaloids; neuroprotective effect; pseudotargeted metabolomics
Background
Aging is a universal physiological process,and changes in homeostasis are closely associated with neurodegenerative diseases, including senile dementia, Parkinson’s disease, and Alzheimer’s disease.Neurotransmitters, in the form of monoamine or acetylcholine compounds in the brain, are essential substances for neuromodulation[1].Neurodegenerative diseases are often accompanied by an imbalance in the neurotransmitters’ levels.Neurotransmitter homeostasis is unbalanced in the brains of patients with Alzheimer’s disease.Moreover, γ-aminobutyric acid (GABA) and 5-hydroxytryptamine metabolism are significantly different in the inferior temporal gyrus [2].Furthermore, the levels of homovanillic acid and 5-hydroxyindoleacetic acid are higher in patients with Alzheimer’s disease than those in controls, and the levels of homovanillic acid and 3,4-dihydroxyphenylacetic acid are lower in individuals with Parkinson’s disease [3, 4].Compared with those without depression, dopamine levels are remarkably increased in patients with Parkinson’s disease and depression [5].The levels of D-serine and aspartate in the cerebrospinal fluid and the level of D-alanine in the white and gray matter of patients with Alzheimer’s disease brains are higher than those of healthy controls [6].In addition, seven biomarker metabolites are associated with Parkinson’s disease, Alzheimer’s disease,and amyotrophic lateral sclerosis[7].
Metabolomics in the brain is confronted with some challenges: the main bottlenecks are the efficient extraction of brain tissue and the highly sensitive detection of multiple indices.At present, common extraction methods for neurotransmitters from biological samples include liquid-liquid extraction (LLE) [8, 9], protein precipitation(PPT) [10-15]and solid-phase extraction (SPE) [16-18].PPT and LLE are mainly used to extract neurotransmitters from brain tissue, and no more than 20 neurotransmitters have been simultaneously extracted[19-22].Therefore, different extraction methods (LLE, PPT, and SPE)should be systematically compared to screen for excellent conditions for the extraction of neurotransmitters.A multi-index and highly sensitive method should be developed for detecting neurotransmitters in the brain.
Uncaria rhynchophylla, a traditional Chinese herbal medicine, has been widely used to treat the internal stirring of liver wind, fright epilepsy, and convulsions (Chinese Pharmacopoeia, 2020 edition).The main chemical components ofUncaria rhynchophyllainclude indole alkaloids,triterpenoid acids,flavonoids,phenols, and coumarins[23].Alkaloids are the main active ingredients with a relatively high abundance [24].Additionally, indole alkaloids possess prominent anti-inflammatory, antihypertensive, and anti-neurodegenerative effects[25-28].
In this study, different extraction methods, including LLE, PPT, and SPE, were compared to evaluate the extraction efficiency of the neurotransmitters.Subsequently, a pseudotargeted metabolomics approach was developed for detecting neurotransmitters in brain tissues using ultra-high-performance liquid chromatography coupled with tandem triple quadrupole mass spectrometry (UHPLC-MS/MS).The alkaloids that could cross the brain were screened and used to evaluate their effect on glutamic acid (Glu)-induced excitatory neurotoxicity in SH-SY5Y cells.
Experimental section
Chemicals and reagents
5-hydroxytryptamine, epinephrine, dopamine, glutamine, aspartic,Glu, 2-phenylethylamine, tryptophan, GABA, 3-hydrpxyanthranilic acid, homovanillic acid, kynurenine, melatonin,5-hydroxyindoleacetic acid, xanthurenic acid, tryptophan-d8 were purchased from Sigma Aldrich (St.Louis, MO, USA).Kynurenic acid was purchased from Tag Control Information(Tokyo,Honshu,Japan).Tyrosine, phenylalanine, valine, isoleucine, leucine and adenosine were purchased from National Institutes for Food and Drug Control(Beijing,China).Norepinephrine was purchased from Shanghaiyuanye Bio-Technology Company (Shanghai, China), and glycine was bought from Meilunbio Company (Dalian, China).Acetycholine and choline were purchased from Sichuan Weikeqi Biological Technology Co.,Ltd.(Chengdu, China).Among of them, deuterated compound tryptophan-d8 was used as internal standard for neurotransmitters quantitative analysis.High-performance liquid chromatography-grade formic acid was purchased from Tag Control Information (Tokyo,Honshu, Japan).High-performance liquid chromatography-grade methanol was purchased from Merck (Darmstadt, Hesen, Germany).Ultrapure water was obtained from Milli-Q system(Boston,MA,USA).Other reagents were purchased from Sinopharm Chemical Reagent Co.,Ltd.(Shanghai, China).Human neuroblastoma SH-SY5Y cells were purchased from the Shanghai Cell Bank of Chinese Academy of Sciences (Shanghai, China).Glutathione (GSH) test kits, cell malondialdehyde (MDA) test kits, superoxide dismutase (SOD)activity test kits and reactive oxygen species (ROS) test kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing,China).Alkaloid reference standards including rhynchophylline,isorhynchophylline,corynoxeine,isocorynoxeine,hirsutine,hirsuteine and geissoschizine methyl ether(GSM)were isolated in our laboratory,and their purity ≥ 95% determined by ultraperformance liquid chromatography-ultraviolet method.The structural information for these standards is listed in Supplementary Figure S1.
Animals
The Sprague-Dawl ey rats,including one months and eight months old,were used for experiment.The animals were purchased from SLAC Lab Animal Center (Shanghai, China, approval number:SYXK-Shanghai-2020-0042).The rats were fed at a temperature of 25± 2 °C; relative humidity of 50 ± 15%; 12 h light/dark cycle and ad libitum available food and water for one week.Before the isolation of brain tissue, the rats were anesthetized and the blood was immediately collected in the heparin sodium tube and centrifuged at 4,000 rpm for 10 min.Then the whole brain was isolated on the ice.The animal protocol was approved by the Animal Management Committee of Shanghai Institute of Materia Medica(approved number:2020-10-GDA-75).
Sample preparation of neurotransmitters assay
The whole brain was homogenized with 0.1%formic acid-water(eight times volume) and sonicated on the ice bath, and a quality control sample was prepared through mixing the whole brain samples derived from three different rats.The quality control sample was used for methodological validation, and to evaluate method accuracy.
LLE sample preparation.An aliquot of 1 mL extractant(dichloromethane, trichloromethane, ether, ethyl acetate, methyl tert-butyl ether, n-butanol, isopropyl alcohol) was respectively added into a centrifuge tube which contained 100 μL homogenated tissues,then the mixture was vortexed for 3 min followed by centrifugation at 14,000 rpm for 10 min.The 800 μL supernatant was transferred into a centrifuge tube for dryness.The residue was redissolved with 200 μL 10%methanol-water containing internal standard(tryptophan-d8, 0.1 μg/mL), then vortexed for 3 min and followed by centrifugation at 4°C and 14,000 rpm for 10 min.
PPT method.Briefly, 100 μL homogenated tissues were transferred into a centrifuge tube, and then added 1 mL precipitation reagents(methanol, acetonitrile, methanol:acetonitrile (1:4, 1:1, 4:1)).The mixture was votexed for 3 min, and centrifuged at 4,000 rpm for 10 min at 4 °C.800 μL supernatant was removed into a new tube for drying.Subsequently, the residue was redissolved with 200 μL 10%methanol-water containing internal standard (tryptophan-d8, 0.1 μg/mL) for analysis.
SPE sample preparation.Eight different types SPE cartridges (Oasis WCX, Oasis MAX, Oasis MCX, Oasis WAX, Oasis PRiME HLB, Oasis HLB,Waters Sep-Pak Vac C18,Agilent Bond Elut Plexa)were selected,then 100 μL tissue sample was separately loaded, cleaned, eluted according to their manufacturer's instructions.Specific cleaning and elution reagents are shown in Supplementary Table S1.The cleaned and eluted liquids were collected separately in a new centrifuge tube and dried in a centrifugal dryer.Then redissolved with 200 μL solvent(10% methanol-water containing internal standard), and vortexed for 3 min,centrifuged at 4°C and 14,000 rpm for 10 min.The supernatant was taken for injection analysis.
Calibration curve preparation.1 mL methanol-acetonitrile(4:1)was added in centrifuge tube which contained 200 μL quality control sample for PPT.Then the linear mother liquor was obtained and proportionally diluted (1, 1/2, 1/4, 1/8, 1/16, 1/32, 1/64, 1/128)with 10% methanol-water containing internal standard(tryptophan-d8, 0.1 μg/mL).All the samples were prepared by PPT method with methanol-acetonitrile (4:1).
Determination of neurotransmitters by UHPLC-MS/MS
Liquid chromatography and mass spectrometry conditions.Quantitative analysis of neurotransmitters was performed on Agilent 1290 Infinity (Agilent, Palo Alto, CA, USA) and ABI-4000 QTRAP (AB Sciex,Toronto,Canada).LC/MS system equipped with an electrospray ionization.Chromatographic separation was performed by ACQUITY UPLC®HSS T3 column (2.1 mm×100 mm,1.8 μm,Waters,Milford,MA, USA) at 20 °C.The samples were maintained at 10 °C in a thermostatic autosampler.The mobile phase consists of A (0.2%formic acid-water) and solvent B (0.2% formic acid-methanol) was used, and a gradient elution was performed as follows: 0-5 min, 0%(B);5-12 min,0-30%(B);12-17 min,30%-90%(B);17-20 min,90%(B).The flow rate was 0.3 mL/min, and injection volume was 2 μL.
Mass spectra were acquired in positive mode (according to reference, the positive ion mode was mainly used for detection of neurotransmitters due to its excellent ionization efficiency [19-22]),the ion pairs of analytes and their parameters were listed in Supplementary Table S2.The other parameters: curtain gas, 30 psi;collision gas,medium;ion spray voltage,5,500 V,source temperature,500 °C; ion source gas 1, 60 psi;ion source gas 2, 40 psi.
Method validation.Methodological validation, including linearity,precision, and stability, was performed using quality control samples according to the pseudotargeted metabolomics requirements published inNature Protocols.
A standard curve containing eight concentration points was prepared,the preparation process was shown at 2.3.4.If >60%of the compounds were correlation coefficients (r2) >0.95, and 80% of the compounds werer2>0.8,the results were considered to be adequate.The quality control samples were injected 10 times for intra-day precision by analyzing the relative areas of compounds and injected on three consecutive days for inter-day precision.The relative standard deviation (RSD) values should be less than 30% and the result was considered sufficient.Stability was evaluated by comparing the change of quality control samples in 24 h and the requirement was the same with repeatability [29].
Statistical analysis.Data was acquired using Analyst V1.6.3 (AB Sciex, Toronto, Canada).The data were processed by the Soft Independent Modeling of Class Analogy (version 14.1, Umetrics,Sweden, Stockholm) for Principal component analysis and orthogonal partial least squares discrimination analysis(OPLS-DA).The quality of the models was evaluated with the relevantR2andQ2.Potential compounds biomarkers were selected on the basis of their contribution to the class separation and variation in the data set.The statistical significance was calculated using the one-way analysis of variance to compare the differences between two groups in SPSS(version 20, Chicago,Illinois, USA) software.Variables with aP-value<0.05 were considered statistically significant.
Alkaloids crossed the blood-brain barrier
Lyophilized powder ofUncaria rhynchophylladecoction preparation:Uncaria rhynchophylla(1 kg) were immersed in water (1:10, w/v) for 30 min and extracted twice by boiling at 100 °C for 30 min using a gallipot.The extracting solution was filtered and combined.Then the combined solution was concentrated and lyophilized using a freezing vacuum dryer.
According to the preliminary results, the rats had no obvious signs of toxicity when intragastricly administrated 2 g/kgUncaria rhynchophylla.Meanwhile, high dose administration of crude extract could screen more alkaloids (low content in extract) which crossed blood-brain barrier.The rats were intragastrically administrated with 2 g/kgUncaria rhynchophylladecoction, then the brain tissue was separated and smashed with normal saline (v/v, 1:3), followed by ultrasonic for 15 min.600 μL homogenated samples were extracted with 3 mL ethyl acetate, and the mixture was vortexed for 3 min followed by centrifuged at 4,000 rpm for 10 min.The 3 mL supernate was transferred to a clean centrifuge tube and dried through freeze-drying, then the residue was redissolved with 100 μL acetonitrile for analysis.
Waters Xevo G2-S QTof/MS was used for detection of alkaloids in brain.ACQUITY UPLC®BEH C18(2.1 mm×100 mm,1.7 μm;Waters,Milford, MA, USA) was used for separation.The column temperature was 35°C,flow rate was 0.4 mL/min.The mobile phase was composed of 0.1% formic acid-water (0.1% FA) and acetonitrile, and the elution gradient was as follows: 0-0.1 min, 10% (acetonitrile); 0.1-5 min,10-25%(acetonitrile);5-13 min,25%-40%(acetonitrile);13-15 min,40%-50% (acetonitrile); 15-19 min, 50%-95% (acetonitrile).The data were acquired in positive ion mode, the capillary voltage was 2 kV, cone voltage was 60 V, source temperature was 120 °C,desolvation temperature was 450 °C, cone gas flow was 30 L/h,desolvation gas flow was 800 L/h, collision energy was 20-50 V.
Analysis of neuroprotective effect of alkaloids at cellular level
Human hepatoma cell line SH-SY5Y cells were obtained from the Chinese Academy of Science (Shanghai, China).The freezing cells were thawed by immersing and whisking in a water bath at 37 °C.Then, the cells were cultured in Dulbecco’s Modified Eagle Medium(DMEM Sigma-Aldrich, St.Louis, MO, USA) containing 10% bovine fetal calf serum with penicillin (100 U/mL) and streptomycin (0.1 mg/mL).Cells were incubated at 37 °C with humidified air and 5%CO2, and the medium was replaced three times a week.Cells were frozen in liquid nitrogen until further analysis.
Cell viability assay.Stock solutions of all drugs were prepared in dimethyl sulfoxide and stored at-20°C.Cells were seeded in 96-well plates at a concentration of 5×103cells/well with a complete culture medium and allowed to adhere to the plate overnight.On the next day,cells were exposed to indicated alkaloids (20 µM) or 0.1% dimethyl sulfoxide(negative control)in the absence or presence of Glu(10 mM)for 24 h at 37 °C in 5% CO2.The cell survival assay was performed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide method.Briefly, cells seeded in 96-well microplates were treated with the drugs according to the indicated concentrations.After treatments, the medium was removed and 100 μL fresh medium with 10 µL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide solution (5 mg/mL) was added into each well, followed by a further incubation for 4 h.The formed formazan which represented the viability of cells was dissolved into 100 µL dimethyl sulfoxide for detection.The solution was subjected to the spectrophotometer and the colorimetric reading was determined at optical density (OD) 570 nm.The percentage of cell viability was calculated using the following formula:Cell viability(%)=Cells number(OD)treated/Cells number(OD) dimethyl sulfoxide control × 100%.Data were obtained from three independent experiments.
Determination of ROS,MDA,SOD,and GSH levels in cells.Briefly,cells seeded in 6-well microplates were treated with the drugs as the indicated concentrations.After treatment, the level of SOD and GSH were performed using the Commercial Kit II (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions, respectively.Protein concentrations were determined using a bicinchoninic acid assay kit with bovine serum albumin standards (Thermo Scientific, Waltham, MA, USA).For MDA assay, cellular MDA reacts with thiobarbituric acid at 90-100 °C and under acidic condition.The reaction yielded a pink MDA-thiobarbituric acid conjugate, which was measured at 532 nm using a Microplate Readers (BioTek, Vermont, USA).The intracellular ROS level was determined by 2,7-dichlorofuorescin diacetate probe according to the chemical assay kit instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Western blot.Cells were seeded at 4 × 105cells per 10 cm dish and treated with the drugs according to the indicated concentrations.Total proteins were extracted from the SH-SY5Y cells using RIPA lysis buffer with a 1%proteinase inhibitor cocktail(Sigma,St.Louis,MO,USA).A 20 µg portion of protein was separated on a polyacrylamide gel under denaturing conditions and transferred to a polyvinylidene fluoride membrane(Millipore, Bedford, MA, USA), and then blocked using 5%nonfat milk/phosphate-buffered saline with phosphate-buffered solution (Sangon Biotech, Shanghai, China).The membrane was incubated with primary antibody,p-p38/p38(Abcam,1:1,000),active caspase-3 (Abcam, cleaved-caspase-3, 1:1,000), phosphorylated extracellular receptor kinase (p-ERK)/extracellular receptor kinase(ERK) (Abcam, 1:1,000), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Abcam, 1:1,000), at 4 °C overnight and incubated with peroxidase-conjugated secondary antibody for 2 h.Proteins were visualized and quantified using the ImageJ software(version 1.8.0.112).In this study, the relative quantitation of target protein expression was carried out through western blot images of unsaturated blots and obtained by calculating the normalized band intensities to GAPDH using the ImageJ software.All the data were obtained from three independent experiments.
Cell experiment data analysis.All data were expressed as the(mean± standard error of the mean).GraphPad Prism (version 5.0) was used for a one-way analysis of variance.TheP-value less than 0.05 was considered to be statistically significant.
Results and discussion
Determination of neurotransmitters using UHPLC-MS/MS
Optimization of conditions for UHPLC-MS/MS method.In consideration of the polarity of the neurotransmitters, different RP-C18 columns (ACQUITY UPLC CSH C18, ACQUITY UPLC BEH Shield RP18, ACQUITY UPLC HSS T3) were compared and screened for their separation capacity.The results demonstrated that the separation capacity of the HSS T3 column was relatively high(Supplementary Figure S2).Different mobile phases were investigated,including formic acid and ammonium acetate in the aqueous phase and methanol and acetonitrile in the organic phase (Supplementary Figure S3).The separation capacity of the acidic mobile phase was excellent.When ammonium acetate was added to formic acid-water,the separation did not improve, and the overall response was low.Methanol performed better than acetonitrile for separation, and formic acid was added to the organic phase to maintain the pH balance of the system.Finally, 0.2% formic acid-water was selected as the mobile phase (A), and 0.2% formic acid-methanol was selected as the mobile phase(B).The separation capacity decreased as the column temperature increased; hence, 20 °C was selected as the column temperature (Supplementary Figure S4).The flow rate was set to 0.3 mL/min, and appropriate separation was achieved within 17 min.
The parameters of mass spectrometry were also optimized, and the ion source temperature was investigated at 450°C,500°C,and 550°C;spray gas pressure was set at 40 psi, 50 psi, 60 psi, and auxiliary gas pressure was set at 40 psi,50 psi,and 60 psi.No substantial difference in the response intensity was observed after optimizing the parameters.By comparing the signal-to-noise ratio, the appropriate conditions were selected as follows: ion source temperature, 500 °C; spray gas pressure, 60 psi; and auxiliary gas pressure, 40 psi.The multiple reaction monitoring mode was applied because of its high intensity and good selectivity in complex matrices [30].The UHPLC-MS/MS chromatogram of the neurotransmitters and the internal standard are shown in Supplementary Figure S5.
Optimization of extraction methods.Three extraction methods(LLE,PPT, and SPE) were investigated.For LLE, the extraction efficiencies of seven extractants with different polarities (dichloromethane,trichloromethane, ether, ethyl acetate, methyl tert-butyl ether,n-butanol, and isopropyl alcohol) were compared.For PPT, five types of precipitators (methanol, acetonitrile, and methanol: acetonitrile(1:4, 1:1, 4:1)) were screened.For SPE, eight different types of SPE cartridges obtained from different manufacturers were selected for comparison: Oasis MCX with cation exchange and inverting adsorption capacity; Oasis WAX with weak anion exchange and inverting adsorption capacity; Oasis WCX with weak cation exchange and inverting adsorption capacity; Oasis MAX with anion exchange and inverting adsorption capacity; Oasis PRiME HLB, Oasis HLB,Waters SEP-Pak Vac C18, and Agilent Bond Elut Plexa with hydrophilic, lipophilic, and inverting adsorption capacity.
The results showed that most neurotransmitters could be detected during the cleaning step (the eluent for this step is usually discarded)with SPE; therefore, it was not suitable for extracting polar components among the neurotransmitters.Owing to the principle of similar phase dissolution, fewer neurotransmitters were extracted from non-polar extractants using the LLE method.However, polar extraction agents (n-butanol and isopropyl alcohol) can extract the majority of the neurotransmitters.Protein precipitants (methanol,acetonitrile, and different proportions of methanol-acetonitrile) had a powerful capacity for extracting neurotransmitters,as shown in Figure 1.The PPT method with methanol-acetonitrile (4:1, v/v) exhibited superior extraction ability by comparing the relative chromatographic peak area corresponding to each neurotransmitter (Supplementary Figure S6).A total of 21 neurotransmitters were determined in brain homogenates (leucine, Glu, GABA, glutamine, tyrosine, isoleucine,aspartic, valine, tryptophan, phenylalanine, choline, adenosine,5-hydroxyindoleacetic acid, glycine, dopamine, norepinephrine,acetycholine, homovanillic acid, kynurenine, kynurenic acid, and 5-hydroxytryptamine).
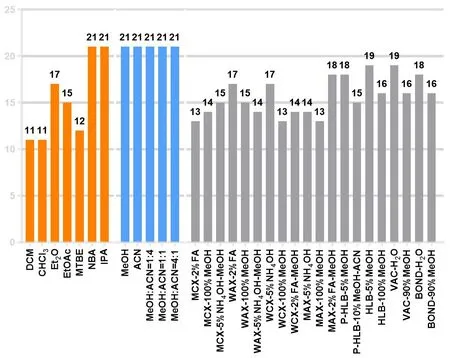
Figure 1 The number of neurotransmitters extracted by different extraction methods.DCM,dichloromethane;CHCl3,trichloromethane;Et2O,ether; EtOAc, ethyl acetate; MTBE, methyl tert-butyl ether; NBA, n-butanol; IPA, isopropyl alcohol; MeOH, methanol; ACN, acetonitrile.
Method validation.Linearity, precision, and stability experiments were performed, and the results met the requirements of pseudotargeted metabolomics (Table 1).The linearity of the method was satisfactory for each analyte in the brain homogenates (Figure 2).All ther2values were above 0.8 and exceeded 0.95 for 71% of the analytes.The RSD of intra-day precision of neurotransmitters was between 2.55% and 9.03%.The RSD of inter-day precision was between 2.02% and 12.55%, except for kynurenine in the brain(24.49%), where the chromatographic peak area corresponding to kynurenine in rat brains was lower than others.The intra- and inter-day precision of the neurotransmitters were acceptable.The RSD of stability ranged from 3.68% to 9.24%, indicating that the neurotransmitters had no evident variation within 24 h.
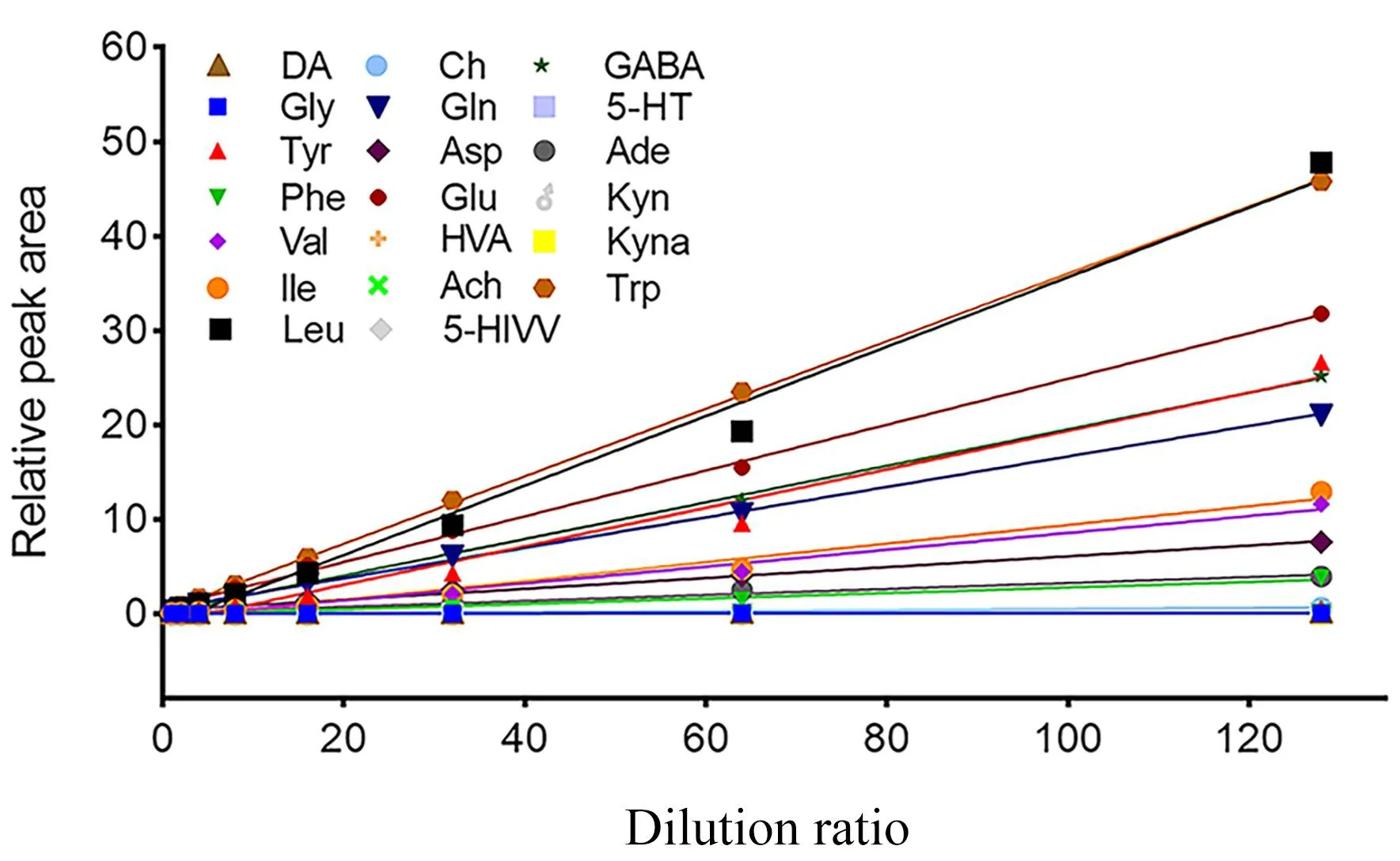
Figure 2 The linear curve of 21 neurotransmitters.DA, dopamine;Gly, glycine; Tyr, tyrosine; Phe, phenylalanine; Val, valine; Ile,isoleucine; Leu, leucine; Ch, choline; Gln, glutamine; Asp, aspartic;Glu, glutamic acid; HVA, homovanillic acid; Ach, acetycholine;5-HIAA, 5-hydroxy indole-3-acetic acid; GABA, γ-aminobutyric acid;5-HT, 5-hydroxytryptamine; Ade, adenosine; Kyn, kynurenine; Kyna,kynurenic acid; Trp, tryptophan.
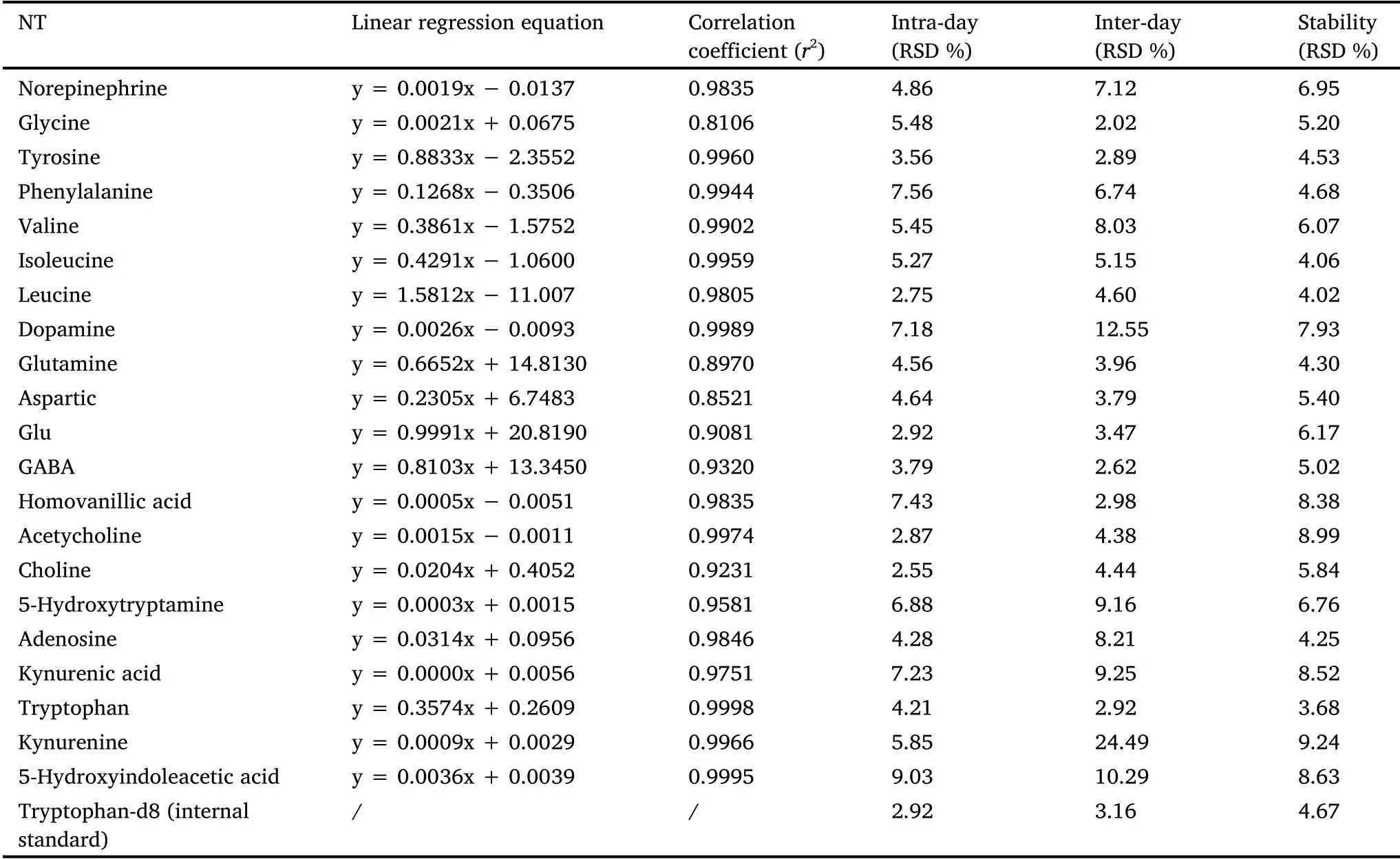
Table 1 Linearity,precision and stability for 21 analytes and internal standard in brain
Application for the detection of biological samples.Neurodegenerative diseases are chronic and frequently-occurring diseases that seriously threaten human health and quality of life.The number of patients with neurodegenerative diseases has been gradually increasing with increasing lifespan.Therefore, it is important to study endogenous substances in the brain during different growth cycles, which could provide a reference and basis for the prevention and treatment of diseases.In this study,we determined the profiles of neurotransmitters in brain tissue samples from 1-and 8-month-old rats.Unsupervised pattern recognition (principal component analysis) and supervised pattern recognition (OPLS-DA)were used for statistical analyses.The two groups of samples showed a tendency to separate.TheR2Y andQ2in the OPLS-DA model were 0.956 and 0.925, respectively, indicating that the model had an excellent fitting degree and predictive ability.
The 200 times permutations test of the OPLS-DA model revealed that the Y-axis slope was-0.63.The results obtained by the OPLS-DA model were stable and reliable (Supplementary Figure S7).The VIP values analyzed by Soft Independent Modeling of Class Analogy were verified with the results of the one-way analysis of variance using SPSS.Finally, seven differentiation markers were identified, namely GABA, leucine, aspartic, isoleucine, valine, tyrosine, and tryptophan(Supplementary Table S3), which increased with age.The degree of change in the levels of neurotransmitters in the rat brain is shown in Figure 3.
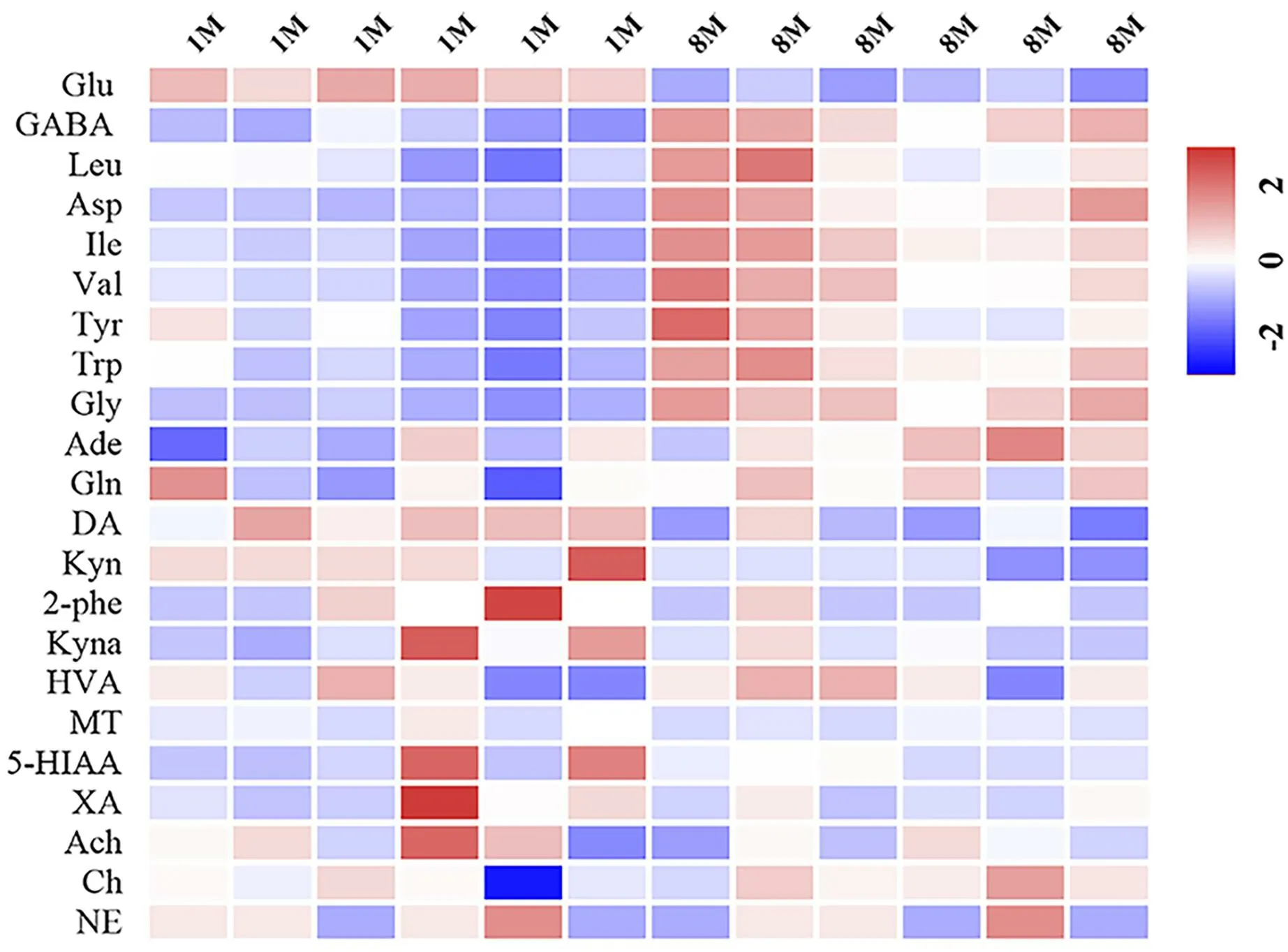
Figure 3 Heat maps of 21 analytes in two groups.Glu, glutamic acid; GABA, γ-aminobutyric acid; Leu, leucine; Asp, aspartic; Ile,isoleucine;Val,valine;Tyr,tyrosine;Gly,glycine;Ade,adenosine;Gln,glutamine; DA, dopamine; Kyn, kynurenine; 2-Phe,2-phenylethylamine; Kyna, kynurenic acid; Trp, tryptophan; HVA,homovanillic acid; MT, melatonin; 5-HIAA, 5-hydroxy indole-3-acetic acid; XA, xanthurenic acid; Ach, acetycholine; Ch, choline; NE,norepinephrine.
Oxidative stress is a primary characteristic of aging and is implicated in various age-related pathologies [31-33].Variations in neurotransmitters’ levels are closely related to oxidative stress.For example, the accumulation of L-tyrosine could cause oxidative stress in the brain of rats.Moreover, aberrant tryptophan metabolism in the brain of patients with Huntington’s disease could increase oxidative stress, and high levels of dietary valine result in increased oxidative stress.Taken together, the increase in the levels of tyrosine,tryptophan, GABA, valine, and aspartic could induce oxidative stress in the aging rat brain [34-39].
Alkaloids crossed the blood-brain barrier
Uncaria rhynchophylla,a traditional Chinese herbal medicine recorded in Mingyibielu during the Weijin Dynasty (220-450 C.E.), has potential value for treating neurodegenerative diseases [26-28].Indole alkaloids are the main active components ofUncaria rhynchophylla[24].To screen the chemical constituents that could cross the blood-brain barrier, the rats were intragastrically administrated 2 g/kgUncaria rhynchophylladecoction, and the presence of alkaloids in the brain tissue was analyzed.Using the mass spectral data of reference standards, rhynchophylline,isorhynchophylline,corynoxeine,isocorynoxeine,hirsutine,hirsuteine,and GSM were identified in the brain tissue.In conclusion, indole alkaloids can cross the blood-brain barrier(Supplementary Figure S8).
Alkaloids exhibited a neuroprotective effect
Effects of the seven alkaloids on the viability of Glu-exposed SH-SY5Y cells.The neuroprotective effects of alkaloids were evaluated in Glu-induced SH-SY5Y cells using a cell viability assay.As shown in Figure 4, cell viability was significantly decreased in the Glu-treated group (42.37% ± 1.48%) compared with that of the control group (relative cell activity of 100%), while hirsuteine(84.63% ± 2.25%), hirsutine (83.16% ± 3.26%), and GSM (92.06%± 3.60%) improved the viability of Glu-treated cells, which deserves further research and investigation.
Effects of the seven alkaloids on ROS, SOD, MDA, and GSH levels in Glu-exposed SH-SY5Y cells.Excessive extracellular Glu can specifically activate the N-methyl-D-aspartate receptors, resulting in neurotoxicity and triggering a burst of intracellular ROS and MDA levels, eventually leading to oxidative stress [38-40].Here, we examined the inhibitory effects of seven alkaloids on Glu-induced ROS production.The alkaloids showed moderate cytotoxic activity against SH-SY5Y cells (half maximal inhibitory concentration >200 μm).As shown in Figure 5A, treatment with Glu resulted in elevated ROS levels in SH-SY5Y cells, whereas hirsuteine, hirsutine, and GSM reduced ROS levels in Glu-exposed SH-SY5Y cells.
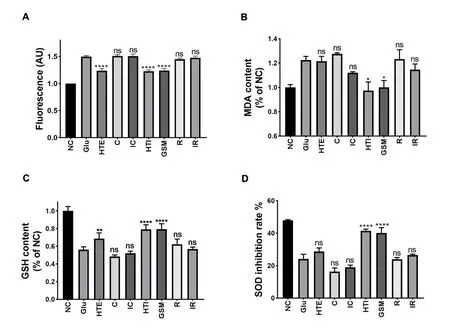
Figure 5 Effects of seven Uncaria alkaloids on ROS content, MDA, GSH levels, and SOD inhibition rate in Glu-treated SH-SY5Y cells.(A)ROS content;(B)MDA levels;(C)GSH levels;(D)SOD inhibition rate.Comepared with Glu-treated group,*P <0.05,**P <0.01,****P <0.0001;ns,not significant.NC, negative control; Glu, glutamic acid; HTE, hirsuteine; C, corynoxeine; IC, isocorynoxeine; HTI hirsutine; GSM, geissoschizine methyl ether; R, rhynchophylline; IR, isorhynchophylline.MDA, malondialdehyde; SOD, superoxide dismutase; GSH, glutathione.
Furthermore, the MDA, GSH, and SOD contents were determined to investigate whether hirsuteine, hirsutine, or GSM could alleviate Glu-induced neurotoxicity by inhibiting ROS production.As shown in Figure 5B-D, compared with those of the negative control, the intracellular GSH and SOD levels were substantially decreased after Glu exposure, whereas treatment with alkaloids showed different neuroprotective capacities against Glu-induced cell cytotoxicity.Compared with that of the negative control, the MDA content was significantly increased after Glu exposure, whereas the treatment with hirsuteine, hirsutine, and GSM decreased MDA content, which was consistent with the previous results of SOD and GSH.
Effects of the seven alkaloids on the protein expression of Glu-exposed SH-SY5Y cells.Oxidative stress is often triggered by ROS,such as hydroxyl radicals and superoxide anions,and is a typical activator of the p38 signaling pathway in Alzheimer’s disease.The involvement of major kinases such as mitogen-activated protein kinase (MAPK) and ERK is associated with the oxidative stress-mediated abnormal hyperphosphorylation of tau [41].Cellular stress and injury are closely related to the activation of JNK3-related pathways that initiate the expression and release of the apoptotic execution protein caspase-3 (the key protein for the cascade of apoptosis signals).
Glu-induced oxidative stress in SH-SY5Y cells can produce excessive ROS and result in apoptosis.Increased intracellular ROS levels mediate the activation of MAPK signaling and further activate p-ERK,which has been proven to be one of the signaling pathways activated by Glu.As shown in Figure 6, Glu substantially increased the expression levels of cleaved-caspase-3 compared with the negative control, while the alkaloids (hirsuteine, hirsutine, and GSM) reduced cleaved caspase-3 expression in Glu-treated SH-SY5 cells, indicating the protective effect of alkaloids on Glu-induced excitatory neurotoxicity.Additionally, these alkaloids decreased the protein expression of p-ERK and p-p38 in Glu-treated SH-SY5 cells.The results suggest that the alkaloids (hirsuteine, hirsutine, and GSM) effectively reduced Glu-induced cytotoxicity and oxidative injury via the ROS-MAPK/ERK signaling pathway.These results suggest that the alkaloids(hirsuteine,hirsutine,and GSM) exert antioxidant properties and represent promising therapeutic candidates for decreasing the ROS levels induced by Glu in SH-SY5Y cells.
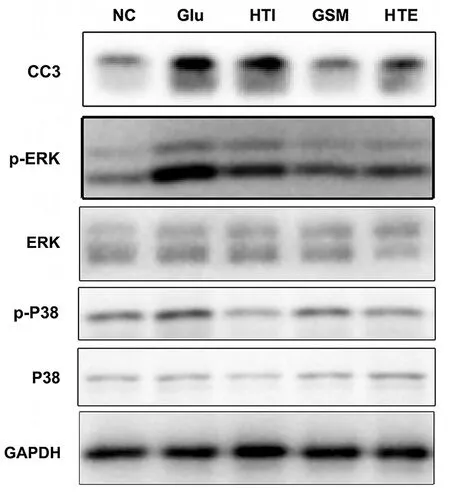
Figure 6 Effects of hirsutine, GSM, and hirsuteine on the expression of Cleaved-caspase-3, p-ERK/ERK, p-P38/p38 in Glu-treated SH-SY5Y cells.NC, negative control; Glu, glutamic acid;HTI hirsutine; GSM, geissoschizine methyl ether; HTE, hirsuteine;p-ERK, phosphorylated extracellular receptor kinase; ERK,extracellular receptor kinase; CC3, cleaved-caspase-3; p-P38,phosphorylated p38 mitogen-activated protein kinase; P38, P38 mitogen-activated protein kinase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Neurotransmitters are chemical substances that deliver messages between neurons or effector cells.Changes in neurotransmitters’levels could provide indications of some diseases and symptoms to a certain extent during aging.In this study, different extraction methods for neurotransmitters were investigated to screen for an excellent method.A pseudotargeted metabolomics approach was developed to detect the homeostasis of neurotransmitters in the brain during aging.The results showed that the variation in the levels of neurotransmitters(tyrosine, tryptophan, GABA, valine, aspartic) was closely related to oxidative stress during aging.Uncaria rhynchophyllais a traditional Chinese herbal medicine that has long been used to treat the internal stirring of liver wind, fright epilepsy, and convulsions.In addition,Uncaria rhynchophyllahas potential therapeutic effects on neurodegenerative diseases.Hence, we screened the micromolecules that could cross the blood-brain barrier and verified their therapeutic effect on oxidative stress in aging.Among these, hirsuteine, hirsutine,and GSM crossed the blood-brain barrier and exhibited strong antioxidant activity, making them potential drug candidates for the treatment of neurodegenerative diseases associated with oxidative stress.
Conclusion
In this study, the extraction methods for neurotransmitters were systematically evaluated; the PPT method using methanol-acetonitrile(4:1, v/v) exhibited superior extraction ability.Subsequently, an analytical method was established for the simultaneous detection of 26 neurotransmitters (including amino acids, monoamines, and purines) and was applied for analyzing neurotransmitters in different growth cycles.A total of 21 neurotransmitters were detected in the brain, and seven amino acid neurotransmitters (aspartic, tyrosine,isoleucine, leucine, tryptophan, valine, and GABA) showed significant changes between 1-and 8-month-old rats.The increase in tyrosine,tryptophan, GABA, valine, and aspartic levels could induce oxidative stress in the aging rat brain.Subsequently, the brain tissue was analyzed to determine whether the alkaloids inUncaria rhynchophyllacrossed the blood-brain barrier, and seven alkaloids were detected(rhynchophylline, isorhynchophylline, corynoxeine, isocorynoxeine,hirsutine, hirsuteine, and GSM).The protective effect of the main alkaloids was evaluated by detecting the level of molecular factors related to oxidative stress (ROS, GSH, MDA, and SOD) in Glu-treated SH-SY5 cells.The results showed that alkaloids fromUncaria rhynchophyllacould reduce excitatory neurotoxicity in Glu-induced SH-SY5Y cells by inhibiting oxidative stress and apoptotic pathways,providing reliable evidence thatUncaria rhynchophyllacan be used to treat aging-related diseases.
 Traditional Medicine Research2022年5期
Traditional Medicine Research2022年5期
- Traditional Medicine Research的其它文章
- G1-4A, an arabinogalactan polysaccharide derived from Tinospora cordifolia(Thunb.)Miers:a natural immunomodulator
- Antioxidant, hepatoprotective and nephroprotective activities of Gazania rigens against carbon tetrachloride-induced hepatotoxicity and nephrotoxicity in rats
- Traditional medicines and experimental analysis methods for Alzheimer’s disease
- Effect of Terminalia chebula Retz.extraction with water on Staphylococcus epidermidis activity and its biofilm formation
- Update on the preclinical and clinical assessment of Withania somnifera: from ancient Rasayana to modern perspectives
- Valerian(Valeriana officinalis)extract inhibits TNF-α and iNOS gene expression in mouse LPS-activated microglial cells
