Traditional medicines and experimental analysis methods for Alzheimer’s disease
Hassan Zubeir Kombo,Sunil Kumar,Gurfateh Singh*
1University Institute of Pharma Sciences,Chandigarh University,Gharuan-Mohali,Punjab 140413,India.2UIAHS Medical Lab Technology,Chandigarh University,Gharuan-Mohali,Punjab 140413,India.
Abstract Alzheimer’s disease is a complex neurodegenerative ailment accompanied by cognitive problems and progressive memory loss.The main causes of the disease are indistinguishable even though aging is said to be the key risk factor, along with the hypotheses such as β-amyloid plaques,tau-proteins, neuroinflammation, and oxidative stress.Currently, Alzheimer’s disease is not curable, and the available medicines are only used to provide symptom relief.Scientists and researchers have developed numerous models to study Alzheimer’s disease, such as in silico methods, in vitro models of cultured rat cortical neurons, in vivo rodent models involving mice and rats, and non-rodent models as well.This review provides an insight into the modality of Alzheimer’s disease treatment using medicinal plants that are natural sources with abundant bioactive substances, and traditional formulations (such as an extract from Notoginseng Radix et Rhizoma, Chinese herbal formulations (i.e., traditional Chinese medicine kidney-nourishing formula, supplements such as coenzyme Q10, alpha-lipoic acid, Ginkgo Biloba, omega-3’s, and acetyl-L-carnitine).In addition, we have discussed the evaluation parameters for experimental Alzheimer’s disease, which are used to assess the mechanism of memory and pharmacological effects of molecules for drug discovery and development.The common experimental analysis methods for Alzheimer’s disease include behavioral, biochemical, and histological studies.This work will allow researchers to access an informative reference of traditional medicines along with experimental analysis methods in one place for Alzheimer’s disease research.
Keywords:Alzheimer; animal model; behavioral; biochemical; traditional medicine
Background
Alzheimer’s disease (AD) is a complex neurodegenerative ailment accompanied by cognitive problems along with progressive memory loss.Theories that explain the pathological pathways of the disease include the hypotheses of β-amyloid plaques, tau proteins,neuroinflammation, and oxidative stress [1, 2].Aging is a key risk factor for this disease.Although preclinical and clinical studies have been performed using natural, semi-synthetic, and synthetic molecules, AD is currently not curable, and the only available medicines are only used to provide symptom relief [3-6].
For AD studies, the assessment of memory mechanisms and the pharmacological effects of molecules is a vital task and is mandatory.The common experimental analysis methods for AD include behavioral studies (e.g., Morris water maze tests, Y-shaped maze test,novel-object cognitive tests, passive-avoidance test, and open-field maze test) and biochemical tests (e.g., sample collection techniques,preparation of cells or tissues samples, analysis of enzyme levels,oxidative stress, and other biomarkers that represent health conditions).Histological studies have also been used to study the structural features of cells or tissues using microscopy.All data obtained using any of the above-mentioned methods were analyzed statistically to draw a conclusion and answer the research questions.
Traditional medicines for AD
The management of the disease mainly involves traditional or allopathic medicines.Supportive therapies for AD approved by the Food and Drug Administration, which are used for mild to moderate symptoms, are agents that inhibit cholinesterase enzymes (e.g.,donepezil,rivastigmine,and galantamine),and agents that antagonize N-methyl-d-aspartate (e.g., memantine) used for moderate to severe symptoms.In addition, doctors also recommend supplements such as coenzyme Q10, alpha-lipoic acid,Ginkgo biloba, omega-3, and acetyl-L-carnitine [7].
Traditional formulations such as coenzyme Q10 are promising for AD therapy, and they enhance cognition through neuroprotective and antioxidant activities, stabilizing mitochondrial functions and reducing Aβ plaques [8].Alpha-lipoic acid supplementation is beneficial against AD and acts as an enzymatic cofactor in the regulation of energy production and metabolism, as well as mitochondrial performance.Additionally, it has antioxidant effects,which reduce the production of free radicals and affect epigenetic mechanisms associated with inflammatory mediators [9].Ginkgo Bilobais considered to have therapeutic potential in memory impairment through multiple mechanisms, including the apoptotic pathway and stabilizing mitochondrial activities [10].Formulation of omega-3 fatty acids is said to be helpful in AD because of their ability to reduce oxidative stress, inflammatory events in the brain, and control functions of cholinergic neurons [11].The use of acetyl-l-carnitine supplements has been suggested in patients with AD because of their contribution to acetylcholine biosynthesis,facilitation of cholinergic neurotransmission, and inhibition of β-amyloid phosphorylation [12].
Traditional natural medicines, from the time of existence of human beings until today, have been trusted for treating many diseases.Several studies have reported that traditional medicine may be effective in the treatment of AD [13-15].For instance, a study has shown that extract fromNotoginseng RadixetRhizomahas the ability to diminish the aggregation of amyloid proteins along with neuroprotective activity [14].Another study showed the effectiveness of a Chinese traditional agent (traditional Chinese medicine kidney-nourishing formula in comparison with donepezil for treating AD [15].Apart from that, there is a collection of some traditionally suggested medicinal herbs that may be used in AD;however,there is a knowledge gap due to a lack of in-depth scientific studies [7].However, some medicinal plants such asMelissa Officinalis,Crocus Sativus,andNigella Sativahave shown to be able to help cognition and treat AD in clinical trials [16].
Progress of research on natural products for AD
In recent years, several traditional compounds have been isolated and tested for safety and efficacy against AD.The classes of compounds that showed a greater therapeutic potential through different targets and mechanisms are as follows [17]: flavonoids (such as ginko flavonoids, soy flavonoids, apigenin), alkaloids (such as huperzine A,arecoline, and securinine),phenylpropanoids (such as salvianolic acid B, curcumin, and osthole), triterpenoid saponins (such asPanax Notoginsengsaponins, ginsenoside, and gypenosides), and polysaccharides (such asRehmanniae Radixoligosaccharides) [17].
However, “substantial studies suggest that berberine which is a natural isoquinoline alkaloid extracted fromCoptidis Rhizoma, may be beneficial to AD by limiting the pathogenesis of extracellular amyloid plaques and intracellular neurofibrillary tangles.Studies in recent years have also found that berberine can additionally treat AD by affecting neurotransmitter, anti-oxidative stress, metabolism and other multi-target pathways[18].”Several studies on medicinal plants have been conducted in animal models and some have been assessed in clinical trials in AD.Hence, additional experiments and clinical studies are needed to assess the usefulness of plants in cognition and AD as well as to define their principal ingredients [16].This list is presented in Table 1[19-35].
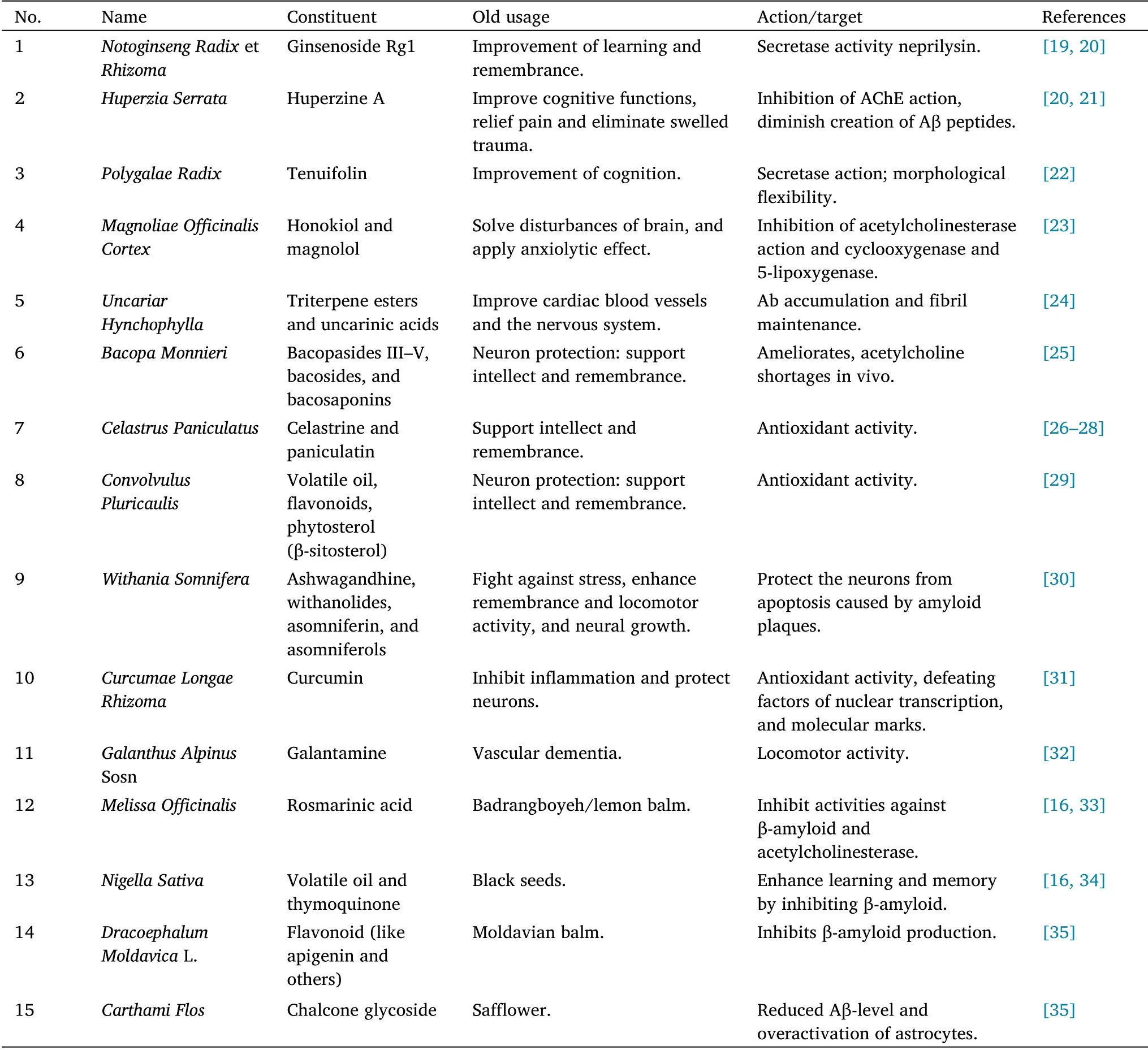
Table 1 List of traditional medicines for the management of Alzheimer’s disease
Experimental models
Computerized techniques (in silico), labware usage experiments (in vitro), and studies performed on biological systems (in vivo) have been developed to study AD.In silico experiments were conducted through computer technology simulations to speed up the rate of drug discovery and reduce the cost of laboratory and clinical trials.Several compounds screened in silico showed the possibility of a potential treatment for AD, such as synthetic antipsychotics checked for repurposing, as well as medicinal plants such as the leaf extract ofCarissa Carandas[36-38].
In vitro models are not performed in a biological context.There are different studies of AD conducted using this type of model; for example, ezeprogind (AZP-2006) and quinacrines might treat AD due to the results found in an in vitro model [39, 40].Additionally,“human induced pluripotent stem cell-derived brain cells in vitro and co-culture platforms technologies have changed the face of preclinical research” [41].
In vivo models are another valuable tool, which can be used to study AD.For example,studies have assessed the relationship between biomarkers,aspects of early or late onset of cognitive impairment,and therapeutic approaches using transgenic animals (mice or rats) [40,42-45].The model used for the early onset of AD is as follows:3xTg-AD transgenic mouse line, with mouse Thy1.2, amyloid precursor protein & Tau protein) and endogenous, presenilin-1 as the promoters, which are involved in transgenic mutations of amyloid precursor protein Swedish + presenilin-1 M146V + Tau P301L.These mutations yield 3-6 months depositions of amyloid plaque and 12 months of hyperphosphorylated Tau, as well as 12 months of neurofibrillary tangles [46, 47].Moreover, there are non-rodent models that use fruit flies (Drosophilastrains) and zebrafish [48-50].
Scopolamine, which can be used to induce memory deficits in rodents, can be dissolved in distilled water or normal saline solution(0.9% NaCl) and administered after pre-treatment with the test drug(i.e., scopolamine post-treatment) at a dose of 1 mg/kg by intraperitoneal route daily for 2 weeks [51-53].It can also be administered as a single dose of 16 mg/kg via intraperitoneal injection[54, 55].After 30 min to 1 h from the time of administration,behavioral assessments can be performed.
Another model uses aluminum chloride, administered as a 100 mg/kg intraperitoneal injection for 60 days, or animals can receive aluminum chloride dissolved in drinking water (10 mg/kg body weight per day) [56, 57].Scopolamine and aluminum trichloride is used to induce disease, as well as other chemicals (e.g., heavy metals such as aluminum, copper, zinc, lead, ethanol, colchicine,streptozotocin, lipopolysaccharide, and okadaic acid) have been studied in developing AD models based on their mode of action and ability to induce cognitive impairment [56, 58, 59].
Evaluation parameters of AD
Behavioral, biochemical, histological, and statistical analyses have been used to assess the mechanism of memory in rodents.For behavioral analysis, the basic methods used included fear or avoidance conditioning, simple maze learning, spatial learning,rotating-rod performance for coordination and motor learning,nociception, and light-dark exploration for vision testing.For biochemical analysis, the main methods used involve techniques that focus on biological markers associated with disease, such as enzyme levels, by-products produced via biochemical processes, oxidative stress, and antioxidants.For the histological analysis, the evaluations are based on the structural features of the cells and tissues.Each of these experimental methods is performed under specific conditions and principles, as explained in this review [3, 60, 61].
The Morris water maze
The Morris water maze method has been employed to evaluate if the given drug model (such as scopolamine) was able to induce an AD phenotype in rats, along with examination of the consequence of the tested drug on memory and spatial learning.Briefly, the equipment is made of a large circular pool (130-136 cm in diameter and 35-50 cm in depth) containing water, which maintains a temperature of 25 ± 2°C, and a submerged translucent platform (a circle-shaped platform with a diameter of 10 cm or a rectangle-shaped platform of 8×6 cm2area) maintained approximately 2 cm beneath the water surface(figure 1) [62].Approximately 150 g of powdered milk or 1 L of milk is added to the water to make it opaque, thereby preventing the visibility of the platform.The rim of the tank has predetermined spots north, south, east, and west, making four hypothetical quarterly sections(northwest, northeast, southeast, and southwest)[3, 63, 64].
The rat held with its front facing the wall of the water tank at any one of four predetermined locations (north, east, west, or south) is gently released into the pool (do not drop animal) at the start of each experiment.In the first 5 days,the rats were skilled in identifying and escaping toward the top of the platform.Every day the training encompassed four sessions with each one consisting of four trials (i.e.,rats trained for 16 trials).The time the animal took to get out of the water and onto the hidden platform was measured (maximum trial duration, 90 s).If the animal failed to discover the platform for a duration of 90 s, it was directly placed onto the platform and permitted to rest on it for 20 s.The location of the platform remained constant during the training period.The trial interval for using the same animal is 15-20 min,which allows animals to dry, which means that towels and an appropriate heat source are required to warm the animals (but the animals should not be overheated) [56, 63, 65].
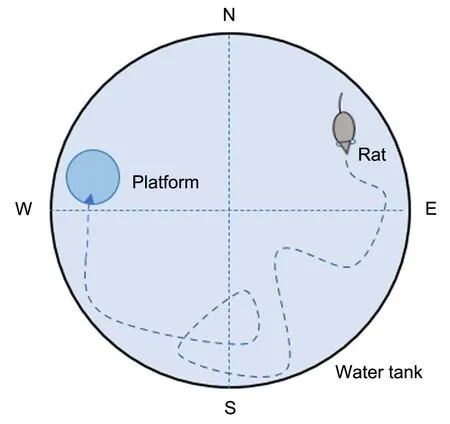
Figure 1 Scheme of Morris water maze.N, north; S, south; E, east;W,west.
On day 6, the platform is removed from the water for 120 s, as part of the probe test.The behavior of the rats is recorded using an Etho-Vision tracking system above the water maze to film their activities, including the duration, distance, and speed, to locate the submerged platform, as well as the time is taken by the subject to stay in the quadrant selected in the probe trial [56, 61].
Y-shape maze test
The method examines short-term spatial learning together with memory by observing the willingness of animals to explore a new surrounding environment (spontaneous alteration, i.e., willingness to change the environment unforced).Typically,rodents have a tendency to enter new arms of the maze rather than the one that was recently visited because of brain function involving the hippocampus,prefrontal cortex, basal forebrain, and septum, which have the capability to remember a previous environment [66, 67].
Spontaneous alteration testing occurs in a Y-shaped maze, which is mainly constructed using three identical well-painted wooden arms or polyvinyl chloride opaque plastic arms(Figure 2)[68,69].Three arms(A, B, and C) were designated to be at 120° equal angles from one another with a dimension of 40 cm length × 35 cm height × 12 cm wide.One animal is placed at the center and permitted to explore the maze throughout a session of 5-8 min.The score is assigned when all four limbs of the subject are completely placed in a particular arm.Ethanol (70%) is then used to clean the maze arms and remove any residual odors after every trial.Spontaneous alternation is well-defined as successive entries in three changed arms on successive choices(i.e., ABC,CAB,or BCA)by excluding repetitions such as BAB,CBC, and ABA, but the same arm returns were also noted.Calculation of the percentage of spontaneous alternation is found with the help of the following equation after completion of trials and collection of data(i.e., total counts of entries and number of alternations).The total number of entries represents the signal of locomotor activity.Hence,the cognitive deficit in rodents and the effects caused by novel chemical entities can be quantified using the Y-shaped maze test[2,46].
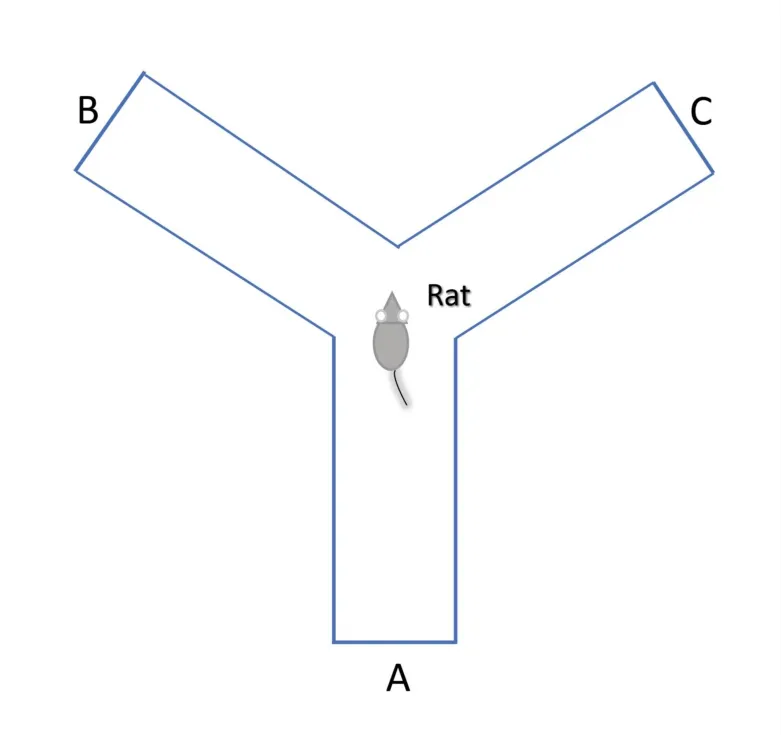
Figure 2 Scheme of Y-shape maze test
The percentage of spontaneous alteration is calculated as follows the formula:

Novel object recognition test
The novel object recognition test can be used for the analysis of nonspatial and short-term memory in rodents.The apparatus box can be made of plastic or gray-painted wood, with different dimensions,such as 100×100×100 cm3or in a rectangular form 40×40×35 cm3(Figure 3) [69, 70].The experiment is conducted in three phases(i.e.,habituation phase,in which the animal are exposed to the box for 10 min on the 1st day for acclimatization; training/familiarization phase,in which the animals are allowed for 5 min on the 2ndday to get familiarized with two identical objects (object 1 and object 2)fixed in parallel and are unmovable in opposite corners of the box; and the probe test sessions/retention phase, in which the test session is performed 1 h after the familiarization phase or on the 3rdday).The test session observes the spontaneous behaviors of the animal for 5 min once the familiar object was substituted by the novel object.The time taken by the animal to explore each object during the experimental work was recorded using a video tracking system for analysis [64, 71].
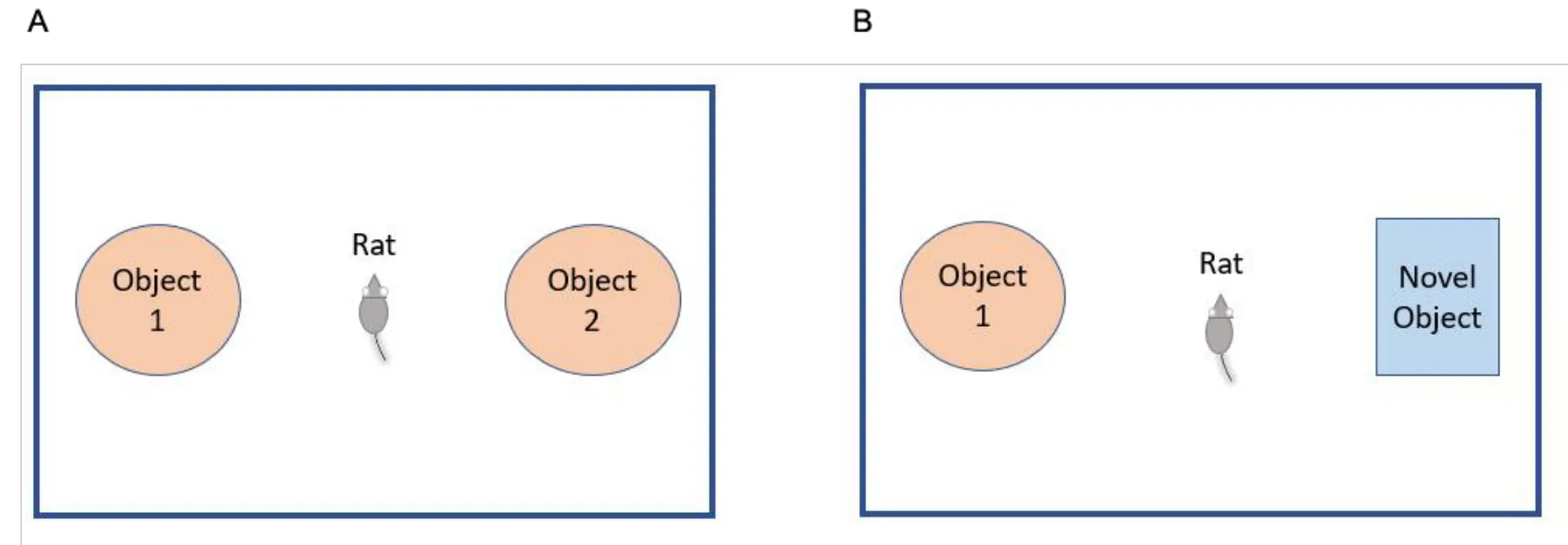
Figure 3 Scheme of novel object recognition test.(A) Training day;(B) testing day.
The percentage of exploration time of a distinguished novel object is calculated using the following formula:

In this test method of behavior, the total time of exploration (time of novel + time of familiar) should be greater than 10 s in the probe test; if it became less than <10 s, it means that animals were not included in the analysis [2].
Passive avoidance test
This assessment relies on the normal wish of the animal to stay in a dark place and avoid compartments that have an unpleasant stimulus.The apparatus used to assess fear learning and emotional memory is a step-through light-dark or cliff/step-down apparatus.The step-through apparatus is constructed with two compartments, each with dimensions of 25 cm×20 cm× 17 cm,separated by a movable automatic closure when the animal passes across another compartment [72, 73].Figure 4 shows an overview of the equipment[73].
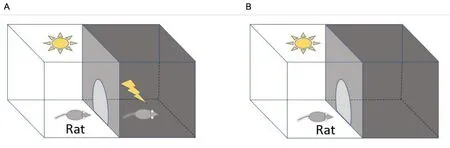
Figure 4 Scheme of passive avoidance test.(A) Training day; (B) testing day.
On the first day of the experiment, the animals are trained by placing individual animals in a particular area and permitted 60 s for exploration, but then the light is turned off and the gate is closed.After that time, the light is switched on and the gate is unlocked to allow free movement of the animal.The time taken by the animal to enter the dark area is measured.As soon as the animal entered the dark area, the gate is automatically locked and a foot electric shock(0.5 mA) is transmitted via the grid floor for 3 s.After completion of the shock, the animals are kept there for approximately another 15 s to sense the environment of the unpleasant stimulus.If the animal did not cross toward the darken area after turning on the light for 300 s,it was excluded from the study.The next 24 h after training, every successful animal was subjected to a retention trial.The experiment is conducted in a manner similar to the training trial, except that when the animal enters the dark area, there is no foot electric shock.The duration is taken to enter the darken area was noted, and if any animal did not enter the area within the maximum duration of 300 s,it is returned to its cage and a 300 s time was noted as a latency [72].
Open-field maze test
Animals are acclimatized to the location where the test is to be conducted at least 30 min prior to the experiment.The subject is placed at the center of a chamber and allowed to move uninterrupted for 10 min.The video tracking system is simultaneously started to film the activities of the animal.After each test and before cleaning with 95% ethanol, observation and counting of the fecal boli pellets in the maze should be performed and noted by hand for more assessments;more fecal matter is said to be an indicator of increased anxiety levels in the animals.The whole distance moved and duration taken in the central zones (apart from the peripheral zone) is used to support the locomotor activity to anxiety-related emotional behaviors[66, 75].
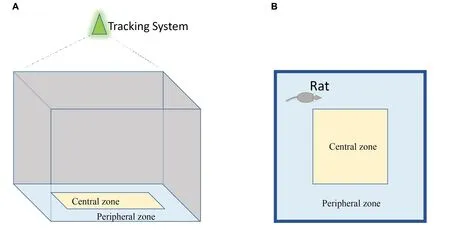
Figure 5 Scheme of open-field maze test.(A) Side view; (B) bird view.
Biochemical analysis
Experimental work on AD requires evaluation of biological markers,which means that the initial important steps are the collection of blood and preparation of cells and/or tissue samples from the brains of sacrificed animals,prior to analysis of parameters such as the status of acetylcholinesterase (AChE) enzymes, nitrite level, malondialdehyde(MDA), glutathione (GSH), and others, as discussed in the following subsections.
Collection of blood sample
After the completion of behavioral studies,a specific method for blood collection is set according to the type of animal used,amount of blood sample, site, and method of blood collection, as well as requirements for blood sample collection,as highlighted:(1)requirements for blood collection from the retro-orbital flexus include anesthetics,microcapillaries, blood collecting tubes, cotton, and water; (2) the requirements for blood collection from the tail vein include cotton,70% ethanol, syringes, needles, and blood collecting tubes; (3) the requirements for blood collection from the sublingual area include forceps, sharp needles, blood collecting tubes, cotton, and water; (4)requirements for blood collection by cardiac puncture include anesthetics, syringes, needles, and blood collection tubes[76, 77].
The drawn blood sample is centrifuged at 3,000 rpm for 10 min to separate the serum(supernatant liquid fraction) from the whole blood and stored at -20°C for further biochemical analysis [60].
Preparation of brain homogenates
When the behavioral studies are completed, animals were anesthetized and sacrificed using euthanasia techniques,and the brain is carefully removed from the skull and washed with normal saline[78, 79].The hippocampal tissue separated from the whole brain is kept at -80 °C until the day of performing the biochemical studies.On the day of the experiment, the hippocampal brain sample is thawed at 4 °C, and then, homogenized in cold normal saline, cold 1.15% potassium chloride (3 mL/g), or ice-cold phosphate buffer (0.1 M, pH 7.4).Brain homogenates are centrifuged at 1,000×gfor 10 min, and the supernatants are used for further biochemical studies [3,60].
Analysis of AChE level
AChE and butyrylcholinesterase (pseudocholinesterase) are the two main cholinesterases prevalent in the nervous system and other tissues.Elliman’s technique is used to evaluate the level of AChE in the supernatant.The analysis is conducted by mixing 0.05 and 0.1 mL of the supernatant and Elliman’s reagent, 5,5’-dithiobis-(2-nitrobenzoic acid),respectively,followed by 0.1 mL of acetylthiocholine iodide and 3 mL of sodium phosphate buffer (pH 8).The absorbance changes of the mixture are read at 412 nm using a spectrophotometer at 30 s interval for 2 min.Based on this hypothesis, AD is associated with a decrease in acetylcholine neurotransmitters, which might be caused by an increase in the activity of the AChE enzyme,which breaks down acetylcholine into choline and acetic acid.Therefore, AChE levels can be used as an indicator of AD [64, 80-82].
Analysis of nitrite level
The quantity of nitrite in the hippocampus is measured using a colorimetric assay with the Griess reagent (0.1% N-(1-napththyl)ethylenediamine dihydrochloride, 1% sulfanilamide, and 2.5%phosphoric acid).Equal quantities of the supernatant and Griess reagent are combined and incubated at room temperature in the dark for 10 min.The absorbance of the supernatant is assessed spectrophotometrically at 540 nm.Nitrite levels are evaluated using a sodium nitrite standard curve and are expressed as µmol/mg protein[64].
Analysis of MDA level
The measurement of MDA, a byproduct of the lipid peroxidation process, is considered a marker of oxidative damage in AD[83].In an acidic medium, MDA reacts with thiobarbituric acid to form a pink chromogen when heated.Butanol is used to extract the chromogen,and its absorbance is determined using a spectrophotometer at 532 nm[60, 61, 64].
Analysis of GSH level
GSH is an antioxidant; there could be several reasons for its depletion,including the presence of chronic diseases such as AD.Ellman’s approach is used to assess the diminished levels of GSH in the hippocampus.In the colorimetric procedure, equal parts of the tissue homogenate and 10% trichloroacetic acid are combined and centrifuged for 15 min at 3000 rpm.Next,they are combined with 0.5 mL of 5,5’-dithiobis-(2-nitrobenzoic acid), 2 mL of phosphate buffer(pH 7.4), and 0.4 mL of double-distilled water.The absorbance of the samples is determined at 412 nm within 15 min of adding 5,5’-dithiobis-(2-nitrobenzoic acid) [64].
I must be more tired than I thought, he said to himself, and, after telling them to be sure to wake him next morning at eight o clock, he went to bed
Histopathological studies
Apart from behavioral and biochemical studies, histological studies show the necessity to study whether there are any structural changes to the tested subject related to the given compounds and disease pathogenesis.For instance, in this review, techniques such as hematoxylin-eosin staining, Nissl staining, and thioflavin S staining are discussed to provide a brief understanding of histopathological studies [61].
Hematoxylin-eosin staining
Animals are sacrificed, and the whole brain is removed and stored overnight in 10% formalin for histopathological analysis.The brain tissue samples (hippocampus and cortex) are fixed in 10% formalin and then embedded in paraffin wax for 4 h.Paraffin blocks are cut into sections of 5 μm thickness by using a section cutter.Sections are mounted on silane-coated slides, deparaffinized by washing in xylene,and then a rehydration process is followed in graded ethanol, and finally, the samples are stained with hematoxylin-eosin.Morphological alterations are examined at 400×magnification under a light microscope[64, 84].
Nissl staining
After tissue sample preparation, the samples are fixed in paraformaldehyde and implanted in paraffin.Tissue slices of 7 μm thick are made from a selected part of the hippocampus, carbonic anhydrase-1.Then, 0.1% cresyl violet acetate is used to stain the sections, and histological analysis is carried out under 200×magnification with the help of computer software [61].
Thioflavin S staining.
After sacrifice, the animals are sacrificed, and tissue preparation processes are completed, thioflavin S staining is used to examine the aggregation status of Aβ in hippocampal tissues.Briefly,deparaffinized sections are positioned on slides coated with polylysine, followed by incubation with 0.05% thioflavin S in 50%ethanol for 30 min in the dark.The sections are then washed twice with 50% ethanol and once with tap water prior to mounting.Aggregated Aβ is assessed in the stained sections using a fluorescence microscope[61].
Statistical analysis
Data analysis is important in biomedical research because it leads to data projection and the ability to draw conclusions.Common methods of statistics are implied in the analysis of data, either parametric/descriptive or nonparametric/inferential.In parametric statistical methods, data are stated in relation to mean, median value,and standard deviations, whereas in nonparametric statistical methods, data are stated in ways other than the mean and can be summarized with the help of computer systems, involving statistical techniques such as Student’st-test and analysis of variance test.A suitable statistical method is selected based on three key factors:study objectives, type of data with its distribution, and nature of data observation (either paired or unpaired) [85].
Data collected in each experimental trial of memory performance in relation to particular testing methods, including the Morris water maze test, involved escaping latency, total distance traveled by the subject,swimming speed,and time of staying in a selected section and other parameters of histology study and changes associated with oxidative stress were evaluated as per the aim and objective of the experiment.Ap-value of 0.05 or less is regarded as statistically significant [2, 61].
Conclusion
AD is a big challenge because it is not curable, and the available medications are used as supportive therapy to ease the signs and symptoms or to silence the progression of the disease.Experimental analysis methods, including behavioral studies, biochemicals, and histological and statistical analysis methods,have opened a new arena for drug discovery and development, especially for non-curable diseases, such as AD.
This review provides an insight into the modality of AD treatment using medicinal plants that are natural sources with abundant bioactive substances and traditional formulations (such as an extract fromNotoginseng RadixetRhizoma, Chinese herbal formulations (i.e.,traditional Chinese medicine kidney-nourishing formula),supplements such as coenzyme Q10, alpha-lipoic acid,Ginkgo Biloba,omega-3’s, and acetyl-L-carnitine).In addition, this work discusses preliminary studies including in silico, in vitro, and in vivo, chemical and disease models,experimental evaluation methods,and the studied parameters.This review provides researchers with access to an informative reference in one place for AD research work and enables them to perform evaluation in a rational manner and achieve the desired outcomes.
 Traditional Medicine Research2022年5期
Traditional Medicine Research2022年5期
- Traditional Medicine Research的其它文章
- G1-4A, an arabinogalactan polysaccharide derived from Tinospora cordifolia(Thunb.)Miers:a natural immunomodulator
- Pseudotargeted metabolomics for exploring the changes of neurotransmitters profile in aging rat brain and the potential neuroprotective effect of alkaloids from Uncaria rhynchophylla
- Antioxidant, hepatoprotective and nephroprotective activities of Gazania rigens against carbon tetrachloride-induced hepatotoxicity and nephrotoxicity in rats
- Effect of Terminalia chebula Retz.extraction with water on Staphylococcus epidermidis activity and its biofilm formation
- Update on the preclinical and clinical assessment of Withania somnifera: from ancient Rasayana to modern perspectives
- Valerian(Valeriana officinalis)extract inhibits TNF-α and iNOS gene expression in mouse LPS-activated microglial cells
