Anionic Modification of the Cu-Tb Single-Molecule Magnets Based on the Compartmental Schiff-Base Ligand
JI Wen-Jie XIA Cheng-CaiZHANG Xin-Yu WANG Xin-Yi
(State Key Laboratory of Coordination Chemistry,School of Chemistry and Chemical Engineering,Nanjing University,Nanjing 210023,China)
Abstract:By using different anions,three Cu-Tb metal complexes,namely[Cu2(vanophen)2TbCl2(MeOH)2]Cl·3MeOH(1),[Cu2(vanophen)2TbCl2(MeOH)2](TCNQ)1.5·2MeOH(2),and[Cu2(vanophen)2Tb2(N3)6]·2MeOH(3),based on the compartmental Schiff-base ligand H2vanophen(H2vanophen=N,N′-bis(2-oxy-3-methoxybenzylidene)-1,2-phenylenediamine,TCNQ=7,7,8,8-tetracyanoquinodimethane)have been synthesized and characterized structurally and magnetically.Except for the different charge-balancing anions,complexes 1 and 2 have a very similar trinuclear[CuTbCu]structure,where the Cu(Ⅱ) ions are in the[N2O4]coordination pockets of the ligands,while the Tb(Ⅲ) ion is coordinated by all or some of the oxygen atoms from the[O4]pocket of the ligands.The charge-balancing anion is a Cl-ion in 1,while the positive charge is balanced by one TCNQ-0.5radical and a half of the TCNQ-radical in 2.As for complex 3,it has a tetranuclear[CuTb]2structure,where two[CuTb]units are bridged by end-end and end-on azides.Magnetic studies revealed that both 1 and 2 are field-induced SMMs while 3 is a zero-field SMM.The energy barriers of 1 and 3 were estimated to be(11.1±0.3)cm-1and(20.2±0.3)cm-1,respectively.As for complex 2,its energy barrier was lower than that of 1,which might be due to the weak magnetic interaction between the[CuTbCu]unit and the paramagnetic radical anions.CCDC:2165235,1;2165236,2;2165237,3.
Keywords:single-molecule magnet;compartmental Schiff-base ligand;anionic modification;3d-4f complexes
0 Introduction
Single-molecule magnets(SMMs)have attracted intense interest because of their potential applications in high-density information storage,quantum information processing,and spintronics[1].In this area,lanthanide-based SMMs(Ln-SMMs)have been proved to be very promising for the preparation of SMMs with high energy barriers(Ueff)and blocking temperatures(TB)[2].The ultrahigh magnetic anisotropy of these Ln-SMMs is a result of the large unquenched orbital angular momentum and spin-orbit coupling of lanthanide ions.By considering the coordination geometry and strength of the coordination bonds,the energy barriers and blocking temperatures have significantly increased in recent years.So far,the energy barriers have been increased to around 2 000 K[3]and the maximum blocking temperature has been pushed above the boiling temperature of liquid nitrogen[4].
In general,despite the strong magnetic anisotropy of the lanthanide centers,the performances of Ln-SMMs are in many circumstances hampered by the occurrence of other fast magnetic relaxation processes,such as the quantum tunneling of magnetization(QTM)process and Raman process.So far,tunning of the Raman process of the lanthanide complexes is rather difficult,and successful attempts have been carried out on several lanthanide organometallic compounds,including the one with very highTB[5-6].On the other hand,to suppress the QTM process,many strategies have been utilized,such as symmetry consideration of the lanthanide centers[7],magnetic dilution with diamagnetic La3+or Y3+ion[8],the introduction of strong magnetic interaction[9],external direct-current(dc)magnetic field[10].To introduce strong magnetic coupling involving the lanthanide centers,using radical bridging ligands[11-12]to connect the Ln3+ions and introducing 3dmetal ions to form the 3d-4fheteronuclear clusters[13]have been proved as two efficient strategies.
For the studies of 3d-4fcomplexes,a large number of complexes with dinuclear M-Ln,trinuclear M-Ln-M,tetranuclear[M-Ln]2or[M3-Ln],and multinuclear(such as[M4-Ln4])SMMs have been reported and many of them show very interesting SMM properties[13-19].In these compounds,the compartmental Schiff-base ligands with two coordination pockets selectively chelating 3dor 4fmetal ions are of special interest.The first 3d-4fSMM[Cu(L)Tb(hfac)2]was a compound with a compartmental Schiff-base ligand[18].In addition,many outstanding complexes,such as extended 0D clusters with SMM behavior[19],1D chain structures showing SCM(single chain magnet)behavior[20],and 2D layer compounds showing long-range magnetic ordering[21],have been constructed by connecting these[M-Ln]building blocks.In this regard,our group has also been working on this interesting family of complexes.We have reported the construction of a whole series of end-on azidobridged 3d-4fcomplexes,where the[CuTb]2complexes show SMM behaviors of considerably large energy barriers in the related complexes[22].Furthermore,the magnetic anisotropy axis of the Tb3+ion was determined and detailed magneto-structural relationships were studied to reveal the origin of the magnetic anisotropy of the Tb(Ⅲ)center[23].
As the continuous work on this subject,we utilized a new compartmental Schiff-base ligand,H2vanophen(N,N′-bis(2-oxy-3-methoxybenzylidene)-1,2-phenylenediamine),toconstructnew 3d-4fcomplexes.Different anions,such as Cl-,TCNQ-radical(TCNQ=7,7,8,8-tetracyanoquinodimethane),and N3-,were chosen for the structural modification of the structures and therefore the magnetic properties.A series of new complexes,namely[Cu2(vanophen)2TbCl2]Cl·3MeOH(1),[Cu2(vanophen)2TbCl2](TCNQ)1.5·2MeOH(2),and[Cu2(vanophen)2Tb2(N3)6]·2MeOH(3)were prepared and characterized structurally and magnetically.
1 Experimental
1.1 Physical measurements
Infrared spectra were obtained over a range of 4 000-400 cm-1on a Bruker TensorⅡFT-IR spectrometer.Elemental analyses were performed on an Elementar Vario ELⅢelemental analyzer.Powder X-ray diffraction(PXRD)patterns of all complexes were recorded on a Bruker D8 Advance diffractometer with a CuKαX-ray source(λ=0.154 06 nm,operated at 40 kV and 40 mA with a scanning range from 5°to 50°)at room temperatures.Magnetic measurements were performed with powder samples of ground single crystals on Quantum Design SQUID VSM magnetometers in a temperature range of 2 to 300 K with fields up to 7 T.Alternating-current(ac)susceptibility measurements were performed under a zero dc field(for complex 3)or applied external dc field(for complexes 1 and 2).All data were corrected for diamagnetism of the sample holder and the constituent atoms using Pascal′s constants[24].
1.2 Single-crystal X-ray diffraction
The X-ray data of 1-3 were collected on a Bruker APEXⅡ diffractometer with a CCD area detector.The APEXⅡ program was used to determine the unit cell parameters and for data collection.The data were integrated using SAINT[25]and SADABS[26].The structures for all complexes were solved by direct methods and refined by full-matrix least-squares based onF2using the SHELXL-2014[27]crystallographic software package.All the non-hydrogen atoms were refined anisotropically.Hydrogen atoms of the organic ligands were refined as riding on the corresponding non-hydrogen atoms.For 3,the disordered solvent molecules were squeezed.Related crystallographic information and partial bond lengths of complexes 1-3 are shown in Table 1 and 2.

Table 2 Selected bond lengths(nm)for complexes 1-3
CCDC:2165235,1;2165236,2;2165237,3.
1.3 Synthesis
All preparations and manipulations were performed under aerobic conditions except for special instructions.The starting material Cu(vanophen)·2MeOH·H2O was synthesized following a published method[28-29].Li(TCNQ)was synthesized according to the method reported in the literature[30].CAUTION!Azide salts are potentially explosive;such compounds should be synthesized and used in small quantities,and treated with the utmost care at all times.
1.3.1 Synthesis of complex 1
A mixture of 103 mg(0.2 mmol)Cu(vanophen)·2MeOH·H2O and 38 mg TbCl3·6H2O(0.1 mmol)was dissolved in 30 mL of methanol and stirred for several minutes.After filtration,the brown-yellow filtrate(10 mL each)was placed in three 20 mL test tubes.The test tubes were transferred to a bottle with ether atmosphere for solvent diffusion.After about a week,a large number of brown-yellow crystals were formed at the bottom of the test tubes.The crystals were obtained after filtration and washed with methanol three times.Yield:58 mg(44.8%).IR(KBr,cm-1):ν(C=N)1 620.Anal.Calcd.for C49H56Cl3Cu2N4O13Tb(%):C,45.20;H,4.30;N,4.30.Found(%):C,45.33;H,3.98;N,4.28.
1.3.2 Synthesis of complex 2
103 mg Cu(vanophen)·2MeOH·H2O(0.2 mmol)and 38 mg TbCl3·6H2O(0.1 mmol)were dissolved and stirred in a mixture of 30 mL dichloromethane and methanol(1∶1,V/V).After filtration,6 mL of the pale yellow filtrate was placed at the bottom of a 20 mL tube.Then 21.1 mg(0.1 mmol)Li(TCNQ)was dissolved in 30 mL methanol.After filtration,6 mL of the blue filtrate was slowly added to the upper layer of the test tube for diffusion.This diffusion experiment was set up in another four test tubes.About a week later,blue flake crystals suitable for single crystal diffraction appeared at the bottom and the wall of the test tubes.After carefully pouring out the mixed solution,methanol was added into the test tubes,and the crystals were scraped from the test tube wall with a scraping spoon.The solids were filtered,collected,and washed with methanol three times.Yield:29 mg(18.8%).IR(KBr,cm-1):ν(C=N),1 620;ν(C≡N),2 190,2 165.Anal.Calcd.for C66H58Cl2Cu2N10O12Tb(%):C,51.43;H,3.77;N,9.09.Found(%):C,51.30;H,3.89;N,9.11.
1.3.3 Synthesis of complex 3
This complex was synthesized using a very similar procedure as that for complex 2,using the following amounts of starting materials:50.2 mg Cu(vanophen)·2MeOH·H2O(0.1 mmol),38 mg TbCl3·6H2O(0.1 mmol),and 22.5 mg NaN3(0.3 mmol).Yield:81 mg(56%).IR(KBr,cm-1):ν(C=N),1 620;ν(N≡N),2 170.Anal.Calcd.for C46H44Cu2N22O10Tb2(%):C,36.56;H,2.91;N,20.40.Found(%):C,36.64;H,3.08;N,20.55.
2 Results and discussion
2.1 Syntheses and structures
Under aerobic conditions,we used Cu(vanophen)and TbCl3starting materials along with different counter-anions to construct a series of heteronuclear 3d-4fcomplexes.When the ratio of Cu(Ⅱ) to Tb(Ⅲ) was 1:1,the color of the solution was light brown,while the color became darker when the ratio was 2∶1.At the ratio of 2∶1 with no additional anions,brown crystals of complex 1 were obtained.Introducing the anions TCNQ-and N3-to the system,complexes 2 and 3 could be prepared.The purity of these complexes has been confirmed by the elemental analysis and PXRD patterns(Fig.S8-S10,Supporting information)of these complexes.
Complex 1 crystallizes in a triclinic space groupP.As shown in Fig.1a,it has a trinuclear[CuTbCu]structure where two Cu(vanophen)units are bridged by the Tb(Ⅲ) center.As anticipated,the Cu(Ⅱ) centers are in the[N2O2]pockets of the vanophen2-anions.Due to the presence of a coordinated Cl-anion,the two Cu(Ⅱ)centers are of different coordination geometries.While the Cu1 center has a five-coordinated square pyramid geometry with the[N2O2]pocket as the square basal plane,the Cu2 center is in a slightly distorted fourcoordinated square planar environment.As for the Tb(Ⅲ)center,it is nine-coordinated with the coordination atoms from four oxygen atoms of the[O4]pocket of the vanophen2-anion coordinated to Cu1,one phenoxide oxygen atom,and one methoxy atom from the vanophen2-anion coordinated to Cu2,two oxygen atoms from two coordinated methanol molecules,and one coordinated chloride ion(Fig.S1).The coordination polyhedron of the Tb(Ⅲ)center is close to the muffin conformation withCssymmetry with a CShM(continuous shape measure)value of 1.054 calculated using the SHAPE code(Table S2)[31].The Tb—O bond lengths are in a range of 0.235 3(2)-0.278 8(2)nm.The Cu1 and Tb1 atoms are bridged by two oxygen atoms(O1 and O2)with a Cu1…Tb1 distance of 0.344 1 nm,while Cu2 and Tb1 atoms are bridged by only one oxy-gen atom(O6)with a slightly longer Cu2…Tb1 distance of 0.385 1 nm.Another interesting feature of the structure worth mentioning is that the two vanophen2-ligands are different.While one of them uses all four oxygen atoms to coordinate with Tb(Ⅲ),the other ligand uses only two oxygen atoms.This is very unique among the reported Cu-Tb complexes with a compartmental Schiff base ligand[18-23].In addition,intramolecularπ-πinteractions are found between the two vanophen2-ligands with parallel-displaced distances in a range of 0.362 7 to 0.377 7 nm(Fig.1b).

Fig.1 (a)Molecular structure of complex 1;(b)Diagram showing the intramolecular π-π interactions between the two vanophen2-ligands
Complex 2 also crystallizes in a triclinic space groupPwith a[CuTbCu]structure(Fig.2a),which is the same as the trinuclear structure of complex 1.The coordination geometries of the metal centers are almost the same with slight differences in the bond lengths and bond angles(Table 2 and S1).The Tb(Ⅲ)center is still in the muffin geometry with a CShM value of 0.981(Table S2).The Tb—O bond lengths are in a range of 0.235 0(4)-0.281 2(5)nm.Different from complex 1 where one uncoordinated Cl-acts as the counter anion,the+1 charge of the[CuTbCu]cluster is balanced by one and a half TCNQ molecules in the asymmetric unit.As shown in Fig.2b,on average,two[CuTbCu]clusters share three TCNQ units,which are stacked together withπ-πinteraction with a distance of 0.351 6 nm.Although the radical anion TCNQ-was used during the reaction,some of them were oxidized during the crystal growth of the complex,probably by the oxygen in the solvent.As for the assignment of the charge of the TCNQ molecules,the parameterζwas calculated according to the Kistenmacher relationship from the bond lengths of the TCNQ molecules[32].The calculatedζvalues for the two TCNQ molecules close to the trinuclear clusters were 0.423,while it was 1.001 for the central TCNQ molecule.These values indicate that the TCNQ molecules close to the cluster have a-0.5 charge,while the one in the middle has a-1 charge.

Fig.2 (a)Molecular structure of complex 2;(b)Diagram of intermolecular interaction of complex 2 showing the π-π stacking interactions between TCNQ units and the vanophen2-ligands
As for complex 3 crystallized also in the triclinic space groupP,it has a[CuTb]2tetranuclear structure where the unique[CuTb]unit is bridged by four azido ions,two of which in the end-end-mode connect one Cu(Ⅱ) and one Tb(Ⅲ) ion while the other two in the endon-mode connect two Tb(Ⅲ)ions(Fig.3).This tetranuclear structure is very similar to that of our reported[CuTb]2compounds[23].The Cu(Ⅱ)ion lies in the[N2O2]coordination pocket of the ligand and has a coordination number of five with the additional nitrogen atom from the end-end azide.On the other hand,the Tb(Ⅲ)ion is eight-coordinated with four oxygen atoms from the[O4]pocket of the ligand and four nitrogen atoms from the azide anions(Fig.S2).The coordination polyhedron of the Tb(Ⅲ)center is close to the bi-augmented trigonal prismatic geometry with a CShM value of 2.76(Table S2).The Tb—O bond lengths are in a range of 0.235 0(5)-0.263 8(6)nm and the Tb—N bond lengths are in a range of 0.234 5(7)-0.244 8(8)nm(Table 2).These bond lengths and bond angles are all similar to the reported values.

Fig.3 Molecular structure of complex 3
2.2 Magnetic properties
The temperature-dependent magnetic susceptibility data(Hdc=1 000 Oe,powder samples,calculated according to their formula)of complexes 1-3 are shown in Fig.4a.At 300 K,theχMTvalues were 13.91,14.39,and 24.82 cm3·mol-1·K for 1-3,respectively.These values were close to the calculated values of 12.57,12.95,and 24.39 cm3·mol-1·K(Cu,S=1/2,g=2;Tb,J=6,g=3/2;the valueSof free radical anion TCNQ is considered to be 1/2).For complex 2,the higherχMTvalue at 300 K compared with 1 is due to the presence of TCNQ radicals.Upon cooling,theχMTvalues of complexes 1-3 first decreased to 13.69 cm3·mol-1·K(at 47 K),13.22 cm3·mol-1·K(at 18 K),and 24.38 cm3·mol-1·K(at 67 K)and then increased to 14.53,14.46,and 30.13 cm3·mol-1·K at 2 K.The decrease of theχMTvalues at high temperatures indicates the depopulation of the Stark levels of Tb(Ⅲ),while the increase at low temperatures suggests the ferromagnetic Cu(Ⅱ)-Tb(Ⅲ)interactions as observed in many related compounds[33-34].At 2 K,the field-dependent magnetizations of 1-3 have also been measured and shown in Fig.4b.Typical paramagnetic behaviors were observed for all of them.At the highest field of 70 kOe,the largestMvalues were 6.86μB,6.99μB,and 15.03μBfor 1-3,respectively.These values were all smaller than the calculated saturation values,indicating the magnetic anisotropy of these complexes.
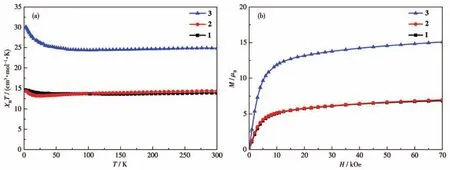
Fig.4 (a)Temperature-dependent χMT curves for complexes 1-3 measured at 1 kOe;(b)Field-dependent magnetization curves in a field range of 0-70 kOe at 2 K
To check if these complexes are SMMs,the ac susceptibilities of these complexes were measured.For complex 1,although no out-of-phase ac signal could be observed under a zero dc field,obvious frequency-and temperature-dependent ac signals were observed under a 1 000 Oe dc field,as shown in Fig.5a and S3.This indicates the field-induced SMM behavior of 1 as the applied dc field efficiently suppresses the QTM effect.The resulting semicircle Cole-Cole plots as depicted in Fig.S6 were analyzed using the generalized Debye model to give the relaxation timeτand its corresponding distribution parameterα(Table S3).Finally,from the linear fit of the high-temperature data in the Arrhenius plot by considering only the Orbach process of the magnetic relaxation,the energy barrier was estimated to beUeff=(11.1±0.3)cm-1(15.98 K)withτ0=6.80 μs(Fig.5b).

Fig.5 (a)In-phase(χ′)and out-of-phase(χ″)susceptibility vs frequency(ν)for complex 1 under a 1 000 Oe dc field;(b)Arrhenius plot of ln τ vs 1/T at 1000 Oe dc field for 1
As for complex 2,the ac measurements were also measured under a zero or 1 000 Oe dc field(Fig.6).We noticed that although an external dc field could partially suppress the QTM effect and lead to the observable out-of-phase ac data,the magnetic relaxation was still too fast to observe peaks in the ac curves in the measured temperature and frequency range.This indicates the lower energy barrier of complex 2 as compared with complex 1.As we can see from their structures,both complexes have a very similar[CuTbCu]trinuclear unit with very similar coordination bond lengths and bond angles(Table 2).In addition,the coordination geometries of the Tb(Ⅲ)ions in these complexes are of very similar muffin conformations withCssymmetry with CShM values of 1.054 and 0.981.Therefore,the different SMM behavior of complex 2 might originate from the existence of the TCNQ radical anions and the weak magnetic coupling between the radicals and the[CuTbCu]unit in 2.This result shows that the introduction of free radical anions as counter ions might not have a positive effect on SMM properties in this system.

Fig.6 χ′and χ″vs ν for complex 2 under a 1 000 Oe dc field
As for complex 3,typical zero-field SMM behavior was confirmed by the observation of obvious frequency dependent and temperature-dependent ac susceptibility curves(Fig.7a and S5).Analysis of these ac data gave the effective energy barrierUeff=(20.2±0.3)cm-1(29.1 K)andτ0=13.1 μs(Fig.7b).These behaviors were consistent with our reported results of similar azido-bridged[CuTb]2compounds[23].The introduction of the azide ligands to the[CuTb]system has a critical role in the structure and magnetic property of complex 3.On one hand,the azide ions change the coordination environments and thus the magnetic anisotropy of the Tb(Ⅲ)centers.On the other hand,they act as bridges between the Tb(Ⅲ)centers,which might lead to the magnetic coupling between the Tb(Ⅲ)centers and observation of the zero-field slow magnetic relaxation due to the suppression of the QTM.In addition,similar SMM behaviors and energy barriers of these complexes indicate that the substituent groups between the two C=N groups in the ligands have little effect on the SMM behaviors of the resulting azido-bridged[CuTb]2SMMs.

Fig.7 (a)χ′and χ″vs ν for complex 3 under a zero dc field;(b)Arrhenius plot of ln τ vs 1/T at a zero dc field for 3
3 Conclusions
Three heteronuclear 3d-4fcomplexes have been synthesized from a compartmental Schiff-base ligand and characterized structurally and magnetically.With different anions,the structures and hence the magnetic properties of these complexes were successfully modified.With the Cl-and TCNQ-anions,complexes of similar trinuclear[CuTbCu]clusters have been synthesized.However,using the N3-anion with bridging ability,an azido-bridged tetranuclear[CuTb]2complex has been prepared.Magnetic measurements on these complexes revealed the presence of the field-induced SMM behavior in the trinuclear[CuTbCu]complexes,while zero-field SMM behavior was observed in the azidobridged tetranuclear[CuTb]2complex.In addition,we noticed that the incorporation of the radical TCNQ molecules into the crystal lattice harms the SMM performance of the complex.
Supporting information is available at http://www.wjhxxb.cn

