Three Zn(Ⅱ)-MOFs Based on Imidazole Derivatives and 2,5-Dimethoxyterephthalic Acid:Syntheses,Crystal Structures,and Fluorescence Properties
SHI Ming-Feng GU Jiang-Hong WAN Yi XU Zhong-Xuan*,
(1School of Chemistry and Chemical Engineering,Zunyi Normal College,Zunyi,Guizhou 563002,China)
(2School of Chemistry and Environment,Yunnan Minzu University,Kunming 650504,China)
Abstract:In the presence of 2,5-dimethoxyterephthalic acid(H2DTA),three 3D metal-organic frameworks,namely{[Zn(DTA)(1,2,4,5-TIB)0.5]·1.5H2O}n(1),[Zn(DTA)(1,4-BMIB)0.5(H2O)]n(2),and{[Zn(DTA)(1,4-BMIN)]·H2O}n(3),have been synthesized by using imidazole derivatives 1,2,4,5-tetra(1H-imidazol-1-yl)benzene(1,2,4,5-TIB),1,4-bis(4-methyl-1H-imidazol-1-yl)benzene(1,4-BMIB),and 1,4-bis(4-methyl-1H-imidazol-1-yl)naphthalene(1,4-BMIN)as ligands to react to zinc ions,respectively.Their structures and fluorescent properties were studied.Single crystal X-ray diffraction analysis reveals that all complexes belong to the monoclinic crystal system.Among them,complex 1 based on four-coordinated 1,2,4,5-TIB is a four-connected framework with a point symbol of(62.84).Compared with complex 1,slim 1,4-BMIB and 1,4-BMIN ligands with low coordination numbers caused that complexes 2 and 3 are 2-fold interpenetration pcu-type and 3-fold interpenetration dia-type networks,respectively.Furthermore,the fluorescent properties of complexes 1-3 were also studied,and their emission spectra had a significant enhancement and blue shift compared to the emission peak of the ligands.CCDC:2129603,1;2129604,2;2129604,3.
Keywords:metal-organic framework;imidzaole derivationves ligands;2,5-dimethoxyterephthalic acid;interpenetration network;fluorescent property
0 Introduction
Metal-organic frameworks(MOFs)not only show exquisite microstructures and topology diversity[1-3]but also have many potential applications in gas storage and separation,catalysis,luminescence sensing,drug delivery,etc[4-7].Given these favorable characteristics,syntheses of MOFs have become a research hotspot in the fields of inorganic chemistry and materials science.Because MOFs are constructed by metal ions/clusters and organic ligands[8],the inherent properties of carefully designed/selected metal ions and organic ligands can endow MOFs with some unique functions[9-11].For example,the photoluminescent properties of MOFs can be adjusted by changing the metal centers and ligands in the hybrid system[12].
However,the final structures of MOFs are affected by many reaction factors,such as solvent system,ligand structure,temperature,pH,and metal ions species.Therefore,it is difficult to synthesize MOFs with excellent structure[13].Fortunately,organic ligands play a key role in the process of synthesizing MOFs.Among a variety of organic ligands,aromatic polycarboxylic acid and nitrogen heterocyclic ligands have a strong coordination ability with metal ions[14-15].So,it is a good choice to obtain target MOFs by using different nitrogen heterocyclic derivatives and aromatic carboxylic acids as ligands.
As one of the aromatic carboxylic acids,2,5-dimethoxyterephthalic acid(H2DTA)is an effective linker for its own advantages:(1)two rigid carboxylate groups indicate rich coordination sites;(2)two methoxy groups as electron-donating groups can increase the nucleophilicity of ligand[16-17].Based on aforementioned search backgrounds,H2DTA was selected to help midazole derivatives 1,2,4,5-tetra(1H-imidazol-1-yl)benzene(1,2,4,5-TIB),1,4-bis(4-methyl-1H-imidazol-1-yl)benzene(1,4-BMIB)and 1,4-bis(4-methyl-1H-imidazol-1-yl)naphthalene(1,4-BMIN)to assemble with Zn(Ⅱ)to build MOFs(Scheme 1).As a result,three 3D(three-dimensional)MOFs,namely{[Zn(DTA)(1,2,4,5-TIB)0.5]·1.5H2O}n(1),[Zn(DTA)(1,4-BMIB)0.5(H2O)]n(2),and{[Zn(DTA)(1,4-BMIN)]·H2O}n(3),were prepared under solvothermal condition.Herein,their syntheses,structures,and fluorescence properties are reported.

Scheme 1 Structures of H2DTA,1,3,4,5-TIB,1,4-BMIB,and 1,4-BMIN
1 Experimental
1.1 Reagents and instruments
All chemicals were commercially available and used without further purification.Powder X-ray diffraction(PXRD)patterns were performed on a Rigaku MiniFlex 600 diffractometer(Voltage:40 kV,Current:15 mA,CuKαradiation,λ=0.154 060 nm)in a range of 5.00°to 50.00°.Thermogravimetric analyses(TGA)data were collected by a NETSCHZ STA-F3 thermoanalyzer from room temperature to 800℃,respectively.IR spectra were measured as KBr pellets from 400 to 4 000 cm-1on a Tianjin Gangdong 7600 FT-IR spectrometer.
1.2 Synthesis of MOF 1
Zn(BF4)2·6H2O(36 mg,0.15 mmol),H2DTA(22 mg,0.10 mmol),and 1,2,4,5-TIB(34 mg,0.10 mmol)were dissolved in a mixed solvent of H2O(4 mL)andN,N-dimethylformamide(1 mL).Then,the resulting solution was stirred for 30 min and put into a 20 mL glass vial,and heated at 80℃for 3 d.The colorless block crystals were obtained and washed with ethanol and dried in the air with a yield of 35%(based on H2DTA).Anal.Calcd.for C19H18N4O7.5Zn(%):C,46.79;H,3.72;N,11.49.Found(%):C,45.83;H,3.58;N,11.62.IR(KBr pellet,cm-1):3 408m,3 107w,3 017w,1 592s,1530s,1488s,1460m,1403m,1257m,1348s,1232m,1 208s,1 068m,1 034m,985w,935w,859w,817w,796w,761w,650w,629w,530w.
1.3 Synthesis of MOF 2
Zn(NO3)2·6H2O(45 mg,0.15 mmol),H2DTA(22 mg,0.10 mmol),and 1,4-BMIB(23 mg,0.10 mmol)were placed in a 5 mL mixed solvent ofN,N-diethylformamide(DEF)and H2O(4∶1,V/V)and stirred for 10 min.Then,the resulting solution was heated at 100℃for 3 d.The colorless block crystals of 2 were obtained with a yield of 53%(based on H2DTA).Anal.Calcd.for C17H17N2O7Zn(%):C,47.85;H,4.02;N,6.57.Found(%):C,47.36;H,3.91;N,6.74.IR(solid KBr pellet,cm-1):3 491m,3 129w,2 947w,1 586s,1 522m,1 494m,1439m,1411s,1348s,1285m,1243m,1208s,1135w,1 027m,887w,845w,748w,650w,552w.
1.4 Synthesis of MOF 3
Zn(NO3)2·6H2O(45 mg,0.15 mmol),H2DTA(22 mg,0.10 mmol),and 1,4-BMIN(28 mg,0.10 mmol)were placed in a 4 mL mixed solvent of DEF,H2O,and methanol(2∶1∶1,V/V)and stirred for 30 min.The final suspending solution was put into a 20 mL glass vial and heated at 100℃for 3 d.The colorless block crystals of 3 were obtained with a yield of 61%(based on H2DTA).Anal.Calcd.for C28H26N4O7Zn(%):C,56.44;H,4.40;N,9.40.Found(%):C,57.56;H,4.23;N,9.45.IR(solid KBr pellet,cm-1):3 477w,3 101w,2 954w,2 921w,2 836w,1 600s,1 502w,1 439m,1 389m,1 341m,1 278s,1 208s,1 174w,1 124w,1 027m,935w,845w,775m,754m,664w,636w,440w.
1.5 Determination of the structure
The data of three single crystals with dimensions of 0.2 mm×0.1 mm×0.1 mm(1),0.20 mm× 0.20 mm×0.20 mm(2),and 0.30 mm×0.2 mm×0.20 mm(3)were obtained on a Rigaku 003 CCD diffractometer with a MoKαradiation(λ=0.071 073 nm)at room temperature,respectively.Their structures were solved via the SHELXT-2014 program and refined by SHELXL-2017 on Olex2-1.2 software[18-19].All non-hydrogen atoms were refined anisotropically.The H atoms from C atoms were positioned geometrically and disposed of by using a riding model,and the H atoms from H2O moieties were located by difference maps and constrained to ride on their parent O atoms.The crystal data and refinement parameters for 1,2,and 3 are summarized in Table 1,and some selected bond lengths and angles are listed in Table S1(Supporting information).
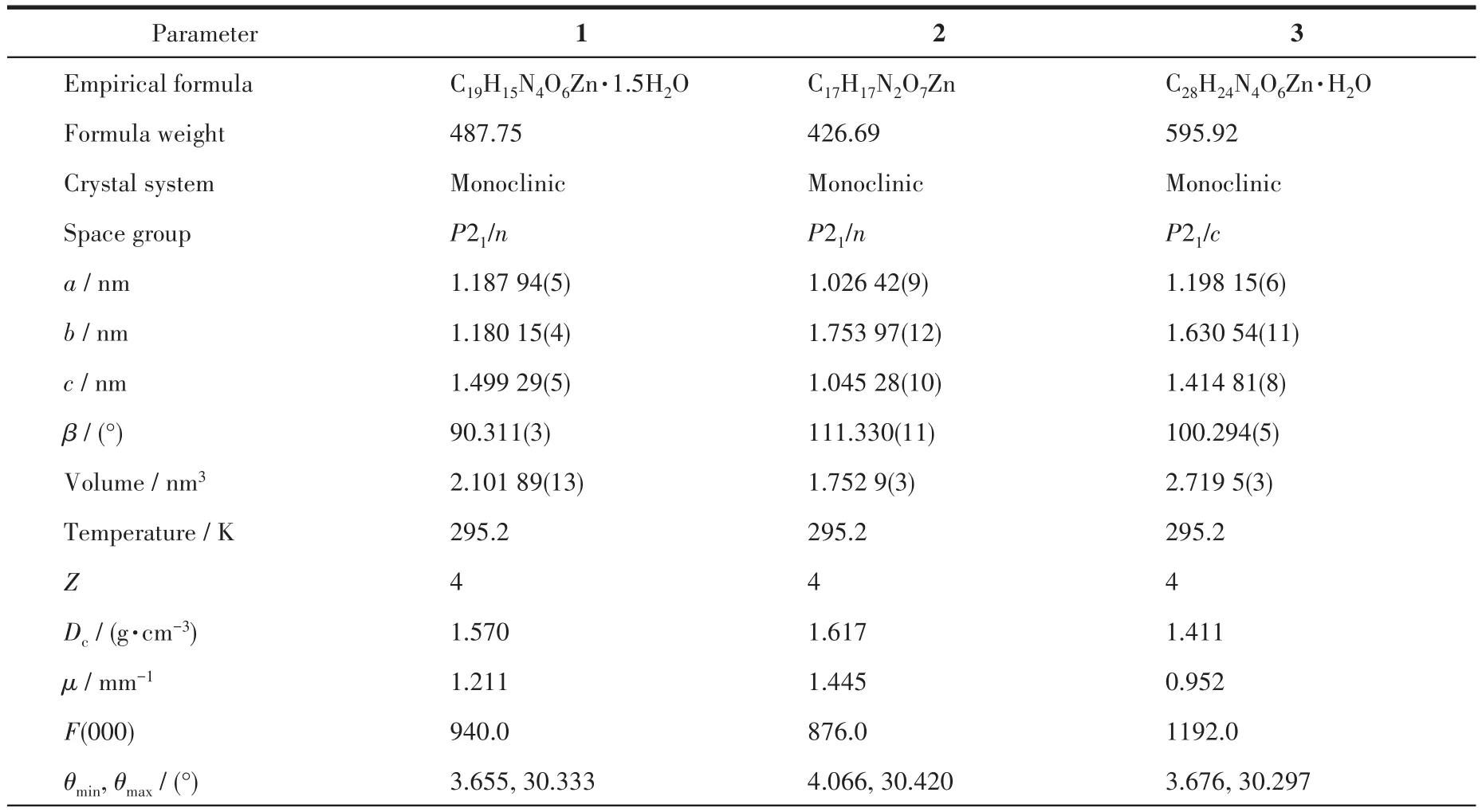
Table 1 Crystallographic data and refinement parameters for MOFs 1-3
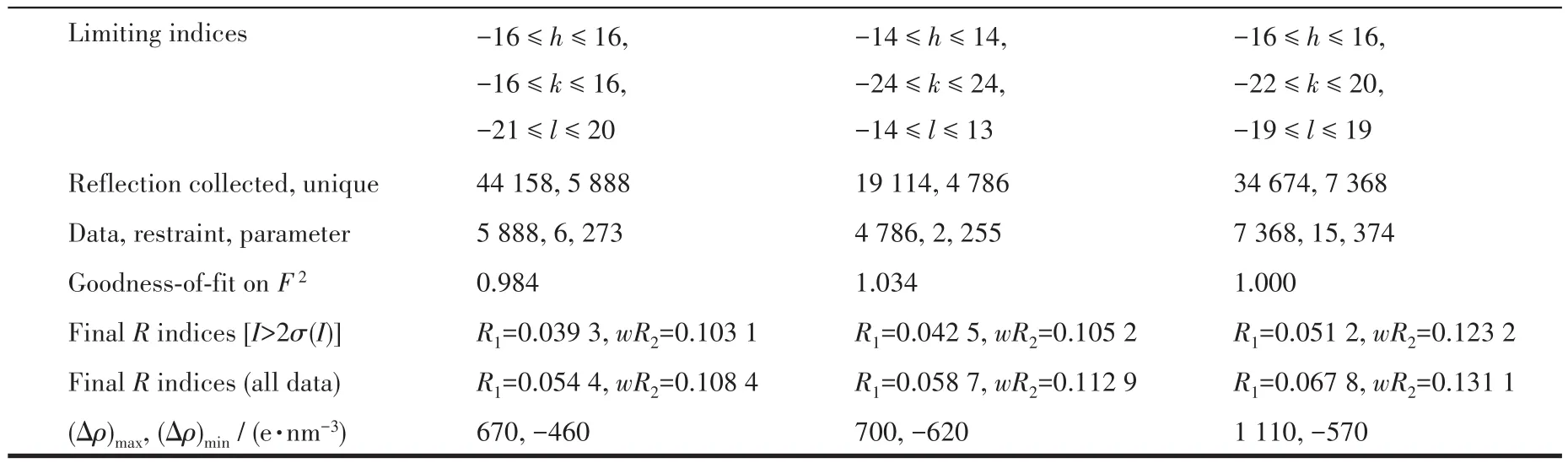
Continued Table 1
CCDC:2129603,1;2129604,2;2129604,3.
2 Results and discussion
2.1 Crystal structure of 1
Single-crystal X-ray diffraction reveals that complex 1 crystallizes in the monoclinic system with theP21/nspace group.The asymmetric unit of 1 contains one Zn(Ⅱ)ion,one DTA2-ion,and half a 1,2,4,5-TIB molecule.Additionally,some disordered guest molecules in the asymmetric unit could not be further identified,so their reflection data have to be subtracted from the corresponding single crystal structure by the SQUEEZE method[20].Even so,one and a half guest water molecules should exist in the asymmetric unit according to SQUEEZE information,elemental analysis result,and thermogravimetric data.The Zn(Ⅱ)ion center adopts a slightly distorted tetrahedral configuration to coordinate with two carboxyl oxygen atoms from two DTA2-ions and two nitrogen atoms from two imidazole groups of 1,2,4,5-TIB molecules(Fig.1a).If only 1,2,4,5-TIB molecules and Zn(Ⅱ)ions are considered,1,2,4,5-TIB molecules and Zn(Ⅱ)ion centers build a 2D layer parallel to the crystallographicbcplane(Fig.1b).Zn(Ⅱ)ion centers are further connected by DTA2-ions,forming a classical pillared-layer framework(Fig.1c).In the framework,the Zn(Ⅱ)center acts as a fourconnected node,while the DTA2-and 1,2,4,5-TIB ligands are only simple connectors.Therefore,the whole framework can be considered as a four-connected net with a point symbol of(62.84)from a topology view(Fig.1d)[21].
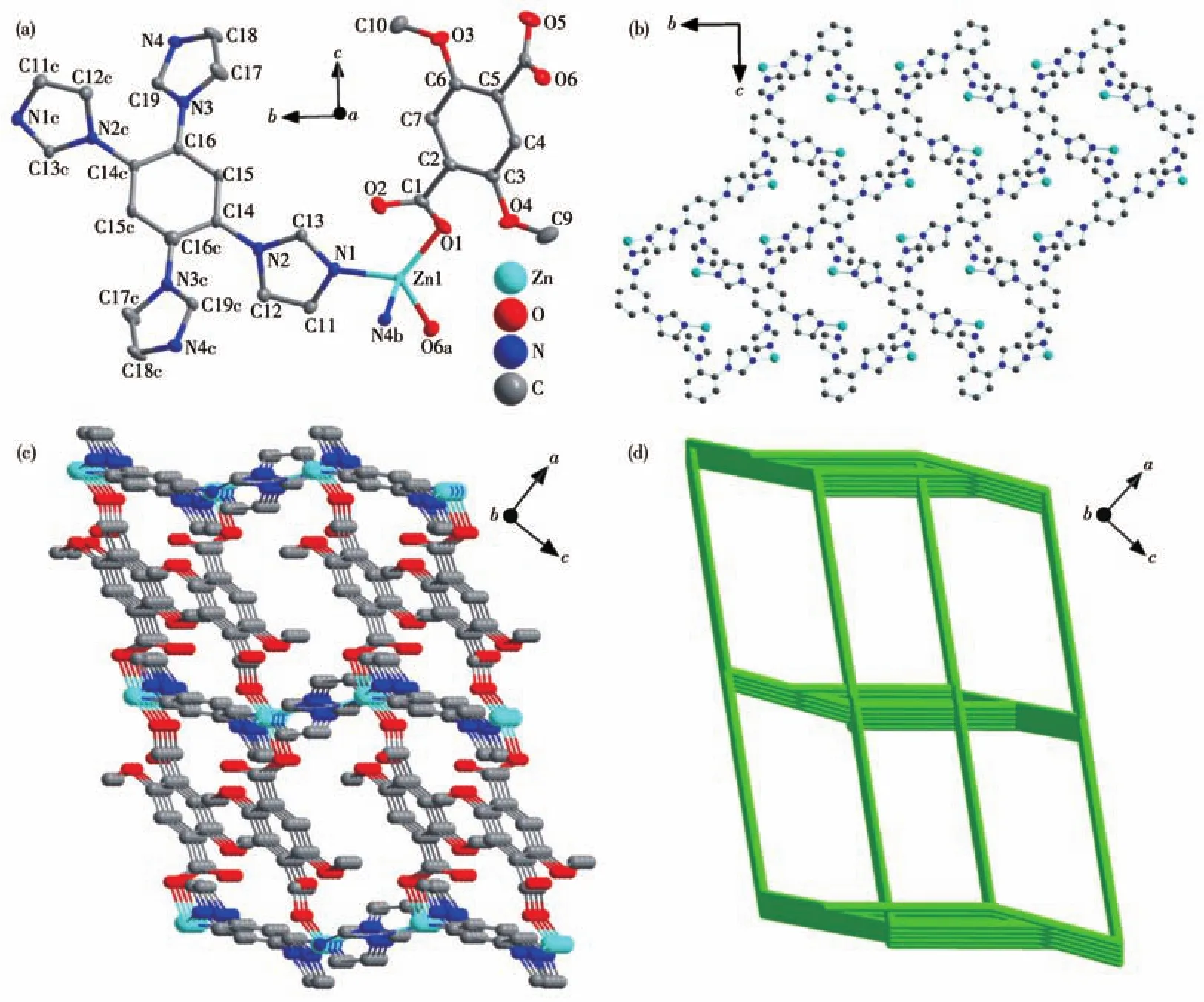
Fig.1 Crystal structure of 1:(a)coordination environment of Zn(Ⅱ);(b)perspective of 2D 1,2,4,5-TIB-Zn(Ⅱ)layer parallel to the bc plane;(c)3D pillared-layer framework;(d)4-connected net
2.2 Crystal structure of 2
Complex 2 also belongs to the monoclinic space groupP21/n.The asymmetric unit is comprised of a Zn(Ⅱ)ion,a DTA2-anion,half a 1,4-BMIB molecule,and a lattice water molecule.It′s important to note that two carboxyl groups from DTA2-indicate different coordination modes,where one carboxyl group is connected by a Zn(Ⅱ)ion and another carboxyl group is connected by two Zn(Ⅱ) ions.Hence,DTA2-is aμ3-linker connected by three Zn(Ⅱ)ions.For such coordination mode in DTA2-,a binuclear Zn(Zn1,Zn1a)unit Zn2(CO2)4is formed(Fig.2a).In the unit Zn2(CO2)4,Zn(Ⅱ)adopts a five-coordinate geometry of distorted trigonal bipyramid to coordinate with three carboxyl O atoms,an imidazole N atom,and a water molecule.Each Zn2(CO2)4unit is connected by four DTA2-ions,resulting in a 2D Zn-DTA layer parallel to the crystallographicabplane(Fig.2b).2D Zn-DTA layers are further connected together by 1,4-BMIB ligands to form a 3D framework(Fig.2c).Difference from 1,2,4,5-TIB,1,4-BMIB is a slim ligand with low coordination numbers.For this reason,structural interpenetration is difficult to avoid.In fact,the 3D framework of complex 2 is a 2-fold interpenetration structure(Fig.2d).In complex 2,each Zn2(CO2)4unit acts as a six-connected node,while DTA2-and 1,4-BMIB ligands are simple linkers.Therefore,the whole framework of complex 2 is a sixconnectedpcu-type net with a point symbol of(412.63)(Fig.S4)[22].
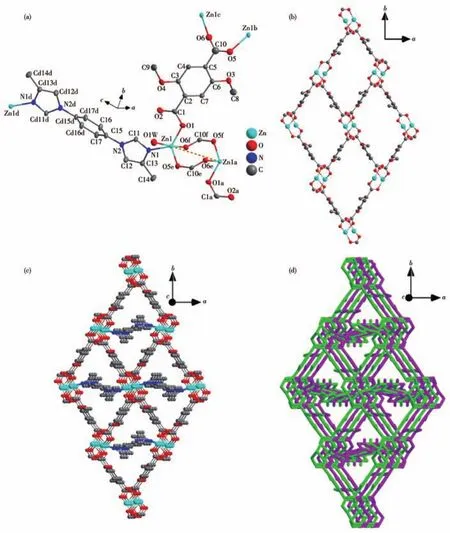
Fig.2 Crystal structure of 2:(a)coordination environment of binuclear unit Zn2(CO2)4;(b)perspective of 2D Zn-DTA layer parallel to ab plane;(c)3D framework constructed by Zn-DTA layers and 1,4-BMIB ligands;(d)2-fold-interpenetrating structure
2.3 Crystal structure of 3
Complex 3 crystallizes in the monoclinic system with space groupP21/c.The corresponding asymmetric unit is composed of one Zn2+ion,two-half DTA2-ions,and one 1,4-BMIN molecule.Some disordered guest molecules also exist in complex 3,which also have been disposed of by the SQUEEZE method[21].Based on the SQUEEZE information,elemental analysis result,and thermogravimetric data,each asymmetric unit in complex 3 should contain a disordered guest water molecule.Just as Zn(Ⅱ) ion in complex 1,the Zn(Ⅱ) ion is still a slightly distorted{ZnO2N2}tetrahedral sphere,surrounded by two carboxylate O atoms from two DTA2-anions and two N atoms from two 1,4-BMIN molecules(Fig.3a).DTA2-and 1,4-BMIN ligands connect the Zn(Ⅱ)ion centers together to obtain a 3D framework(Fig.3b),which is a triple-interleaveddia-type network with a point symbol of(66)(Fig.3c and 3d)[22].

Fig.3 Crystal structure of 3:(a)coordination environment of Zn(Ⅱ);(b)3D framework;(c)3-fold-interpenetrating structure;(d)4-connected dia net
2.4 PXRD patterns and TGA
The PXRD of 1-3 was performed at room temperature to check their phase purity.As shown in Fig.4a,the test PXRD patterns match well with the simulated ones,indicating the phase purity of complexes 1,2,and 3(Fig.4a).To further investigate their thermal stabilities,the TGA experiments for 1-3 were carried out from room temperature to 800℃under a nitrogen atmosphere(Fig.4b).The TGA curve of 1 indicated the first weight loss of 6.0% from room temperature to 110°C,which should be attributed to the release of one and a half disorder water molecules in each asymmetric unit(Calcd.5.6%).After 300℃,rapid weight losscontinued until the end of the experiment.Complex 2 indicated an about 4.5% weight loss from the beginning to 120℃,which mainly corresponds to the loss of coordination water molecules(Calcd.4.2%).The curve between 120 and 379℃was almost a straight line,revealing that complex 2 is very stable in this temperature range.After 379℃,the TGA curve droped sharply until the end of the experiment.Complex 3 displayed a 3.1% weight loss before 120℃,confirming that each asymmetric unit contains a guest water molecule(Calcd.3.0%).As the temperature further increased,the framework began to collapse from 320℃.

Fig.4 PXRD patterns(a)and TGA curves(b)of complexes 1-3
2.5 UV-Vis and luminescent properties
The solid UV-Vis absorption spectra of complexes 1-3 are shown in Fig.5a.In the UV region between 200 and 400 nm,all complexes had two maximum absorption peaks at 260 and 325 nm,respectively.These characteristic absorption peaks should be attributed to theπ-π*transition between the ligands and metal ions[23].
Consideringd10metal inorganic-organic hybrid coordination polymers with excellent luminescent properties,their fluorescence tests were performed(Fig.5b).Under the excitation light with a wavelength of 275 nm,H2DTA had a maximum emission peak at 410 nm.In contrast,the emission bands of the three complexes were stronger than that of the ligand,and the maximum emission peaks for complexes 1-3 were 398 nm(λex=340 nm),385 nm(λex=328 nm),and 393 nm(λex=281 nm),respectively.Compared to the emission peak of H2DTA,all complexes had a blue shift phenomenon,which may be caused by the intraligand(n-π*orπ-π*)transition.The enhanced luminescence of complexes 1-3 should be attributed to the combination of the ligand and the metal center,reducing the energy loss caused by non-radiation attenuation[24].
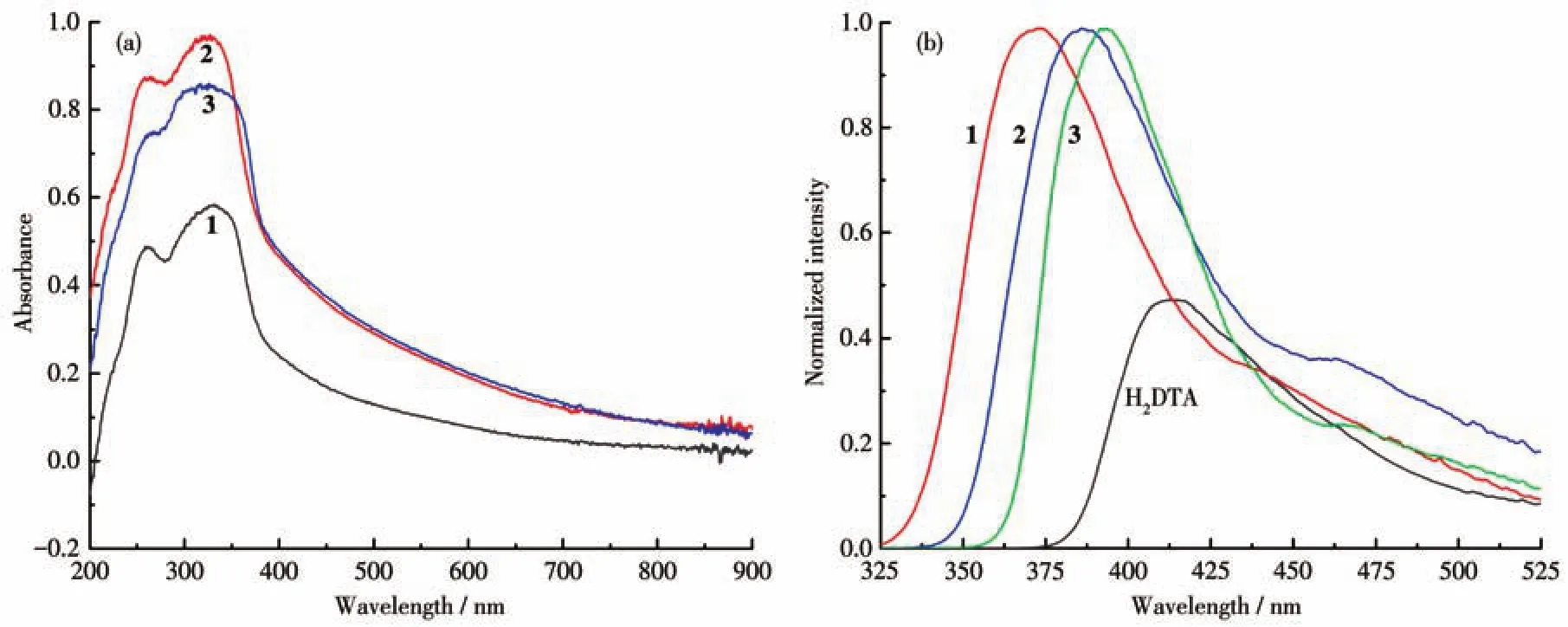
Fig.5 Solid UV-Vis absorption spectra of complexes 1-3(a)and fluorescent emission spectra(b)of 1-3 and H2DTA
3 Conclusions
In summary,different imidazole derivatives as linkers were introduced to assemble with Zn(Ⅱ)ions to obtain three 3D MOFs.Complexes 1,2,and 3 are a fourconnected net,a six-connectedpcu-type net with a 2-fold interpenetration structure,and a triple-interleaveddia-type network,respectively.The rigid imidazole derivatives play a key role in constructing complexes with different structures.Moreover,compared with the fluorescence of H2DTA,the fluorescence of the three complexes was enhanced and the blue shift occurred.
Supporting information is available at http://www.wjhxxb.cn

