Organometallic Gels Based on Metal Ion Exchange for the Detection of Antibiotics and Nitroaromatic Compounds
YUAN Yi-Zhen YANG Yun-Shang ZHAO Yu-Chen ZHANG Ying-Peng
(School of Petrochemical Engineering,Lanzhou University of Technology,Lanzhou 730050,China)
Abstract:Rapid detection of organic compounds in wastewater has always been an important issue.Fluorescent Tb-based metal-organic gel MOG(Tb)was prepared by the metal ion exchange method.The studies have shown that trace amounts of furazolidone(FZD),metronidazole(MDZ),2,4-dinitrotoluene(2,4-DNT),and 4-nitrophenol(4-NP)could effectively quench the fluorescence emission of MOG(Tb)even in the presence of other analytes,demonstrating that the MOG(Tb)xerogels could effectively detect antibiotics(FZD,MDZ)and nitroaromatic compounds(2,4-DNT,4-NP).However,penicillin G potassium salt(PCLP)could enhance the fluorescence of MOG(Tb).In addition,the recyclability and water stability tests of the MOG(Tb)xerogels were also carried out,and satisfactory results were obtained.
Keywords:metal-organic gel;antibiotics;nitroaromatic compounds;fluorescence quenching
0 Introduction
There are many methods to detect antibiotics and organic explosives,including electrochemical methods[20],infrared spectroscopy[19],and liquid chromatography[21].However,these methods are limited by complex operations and costs[22].Therefore,there is an urgent need to explore a simple and effective strategy for antibiotic detection[23].Fluorescence quenching-based detection methods[24-25]are a very promising method[28]with the advantages of high efficiency[26],low cost[27],and short reaction time.
Although metal-organic frameworks(MOFs)are often used to detect a variety of substances[29],metalorganic gels(MOGs)are very rare as a new type of material[30].Compared to MOFs,MOGs have a smaller density,lower crystallinity,and larger surface area[31-34],which would be very beneficial for the detection of antibiotics and nitroaromatic compounds (NACs)[35-36].However,the development of MOGs has only just begun and much work[38]remains to be done on MOGs due to uncertainties such as their gelation process and structure[37].
In this work,inspired by the metal replacement method,Al-based MOG(MOG(Al))achieved irreversible partial metal ion exchange.With this post-treatment,the Al ion-based gel was converted into a metal-organic gel containing rare-earth ions(MOG(Tb)).Unexpectedly,due to its intense fluorescence and excellent water stability,it showed high selectivity and sensitive detection of antibiotics and NACs.Moreover,the method here is very convenient and feasible for the large-scale synthesis of MOG agglomerates.This will open up a new avenue for the application of MOG materials.
1 Experimental
1.1 Materials and general methods
According to the reference[39],4,4,4-tricarboxylic acid triphenylamine(H3TCA)was synthesized by Friedel-Crafts acylation reaction(Scheme 1).All other chemical reagents were directly purchased through merchants,and the purity was sufficient,no further processing was required.The powder X-ray diffraction(PXRD)pattern was gained on a Rigaku Ultima Ⅳ polycrystalline powder X-ray diffractometer,where the radiation source was CuKαtarget,λ=0.154 06 nm,U=40 kV,I=30 mA,scanning range 2θ=5°-90°,scanning speed was 5(°)·min-1.The UV-Vis spectrophotometer model was UV-2700,from Varin,USA.The fluorescence spectrophotometer was F-7000 from Varin,USA.The thermogravimetric(TG)curve was recorded in a Q5000IR thermal analyzer,and the heating rate was 10 ℃ ·min-1in nitrogen.The nitrogen adsorptiondesorption was carried out in Mike ASAP2020 HD.
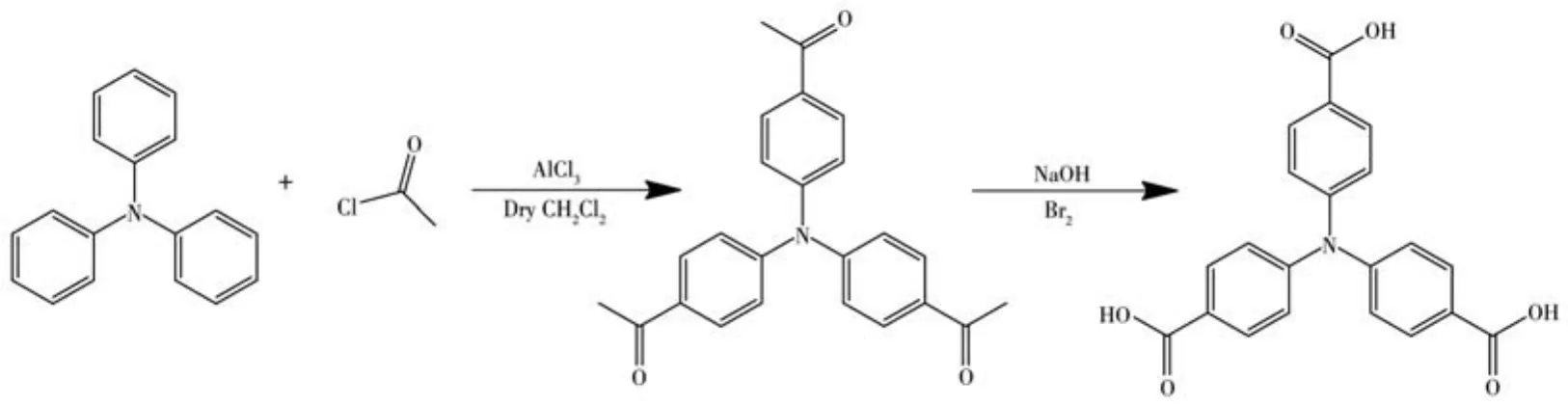
Scheme 1 Synthetic route of H3TCA
1.2 Synthesis of MOG(Tb)
MOG(Al)was prepared by hydrothermal synthesis.Al(NO3)3·9H2O(0.375 1 g,1 mmol)and H3TCA(0.565 5 g,1.5 mmol)were put into 20 mL ethanol and stirred well,then the solution was transferred to a steel autoclave lined with tetrafluoroethylene,heated at 140℃for 8 h.After cooling to room temperature,the ocher gel was obtained,which was washed three times with ethanol.For the synthesis of homogeneous MOG(Al)gel,the molar ratio between the metal source and the ligand was 2∶3.
The xerogel of MOG(Al)was soaked in an ethanol solution containing Tb(NO3)3·6H2O(30 mL,3 mmol)for 4 d to obtain MOG(Tb).After soaking,the gel was washed with ethanol to remove excess Tb3+ions,centrifuged to remove the supernatant,and then dried at 70℃for 12 h to obtain a xerogel of MOG(Tb).
1.3 Water stability test
To test the stability of MOG(Tb)in water,the powder sample of the xerogel was soaked in water with different pH values(2,3,5,7,9,11,and 12)for 48 h,after drying all samples were tested by PXRD.
Vor der Kasernevor dem grossen Torstand eine Laterneund steht sie noch davor…Vor der Kasernevor dem grossen Torstand eine Laterneund steht sie noch davor…
1.4 Fluorescence experiment
The ultraviolet absorption spectrum of MOG(Tb)was measured,then the solution was diluted so that the absorbance was between 0.06 and 0.08.The excitation wavelength was 355 nm,and the slit width was 8.5 nm for excitation and emission.The absorbance at the fluorescence emission wavelength and the absorption maximum wavelength in a range of 355-540 nm were measured.
Using the fluorescence quantum yield of quinine sulfate in 0.1 mol·L-1sulfuric acid solution as the benchmark(Φr=0.58),the fluorescence quantum yields of MOG(Al)and MOG(Tb)were calculated by the following formula:

wherenis the refractive index of the solution,Ais the absorbance of the compound at the excitation wavelength,r represents the reference compound,Iis the fluorescence intensity of the compound,g represents the gel compound sample,andΦis the fluorescence quantum yield of the sample.
To effectively detect antibiotics and NACs,4 mg of the MOG(Tb)xerogel powder was put into a 15 mL aqueous solution of antibiotics and organic explosives(c=0.4 mmol·L-1)at room temperature.All the mixtures were sonicated for 50 min to become stable suspensions.
Selective detection was then performed:(1)4 mg of MOG(Tb)xerogel powder was added to furazolidone(FZD)and metronidazole(MDZ)aqueous solutions(0.4 mmol·L-1),respectively,and other antibiotics were added;(2)4 mg of MOG(Tb)xerogel powder was put into an aqueous solution (0.4 mmol·L-1)of 2,4-dinitrotoluene(2,4-DNT)and 4-nitrophenol(4-NP),and other NACs were added.All the mixtures were also sonicated for 40 min.
1.5 Cytotoxicity test
To examine the biocompatibility of MOG(Tb),the MTS(3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulopheny)-2H-tetraolium,inner salt)method was used to detect the toxicity of MOG(Tb)to pig kidney cells(PK cells)and baby hamster Syrian kidney cells(BHK cells).First,PK and BHK cells were cultured using Dulbecco′s modified Eagle medium(DMEM)containing 10% fetal bovine serum(FBS)in a humid air environment with a temperature of 37℃and a 5% volume fraction of CO2.Then DMEM was used to prepare the dried sample into a homogeneous solution,and the solutions with concentrations of 10,20,100,150,200 μg·mL-1were added to the cells,making six copies,and leaving a set of blanks as a comparison.After the cells were incubated for 24 h,10 μL of MTS solution was added to the cells and incubated for 3 h.A microplate spectrophotometer was used to measure the optical density(OD)value of each well at a wavelength of 490 nm.
2 Results and discussion
2.1 Characterization of MOG(Al)and MOG(Tb)
Compared with conventional MOFs,MOGs have lower crystallinity and only a few broad peaks as seen byobservation of PXRD.Similar characterization results have also been reported for xerogels.After metal ion exchange,the PXRD pattern of MOG(Tb)was almost identical to that of MOG(Al)(Fig.1a).Nitrogen adsorption-desorption tests were then performed to observe the porosity of MOG(Tb).As shown in Fig.1b,it was aⅠ-type isotherm,which was similar to the Langmuir-type adsorption isotherm,reflecting the micropore filling phenomenon,and indicating that MOG(Tb)has a certain microporous structure,and the saturated adsorption value is equal to the micropore.By NLDFT(nonlocal density functional theory)analy-sis of nitrogen adsorption-desorption isotherms,the pore size distribution of MOG(Tb)was about 1.1 nm,and the BET(Brunauer-Emmett-Teller)specific surface area was about 168 m2·g-1.
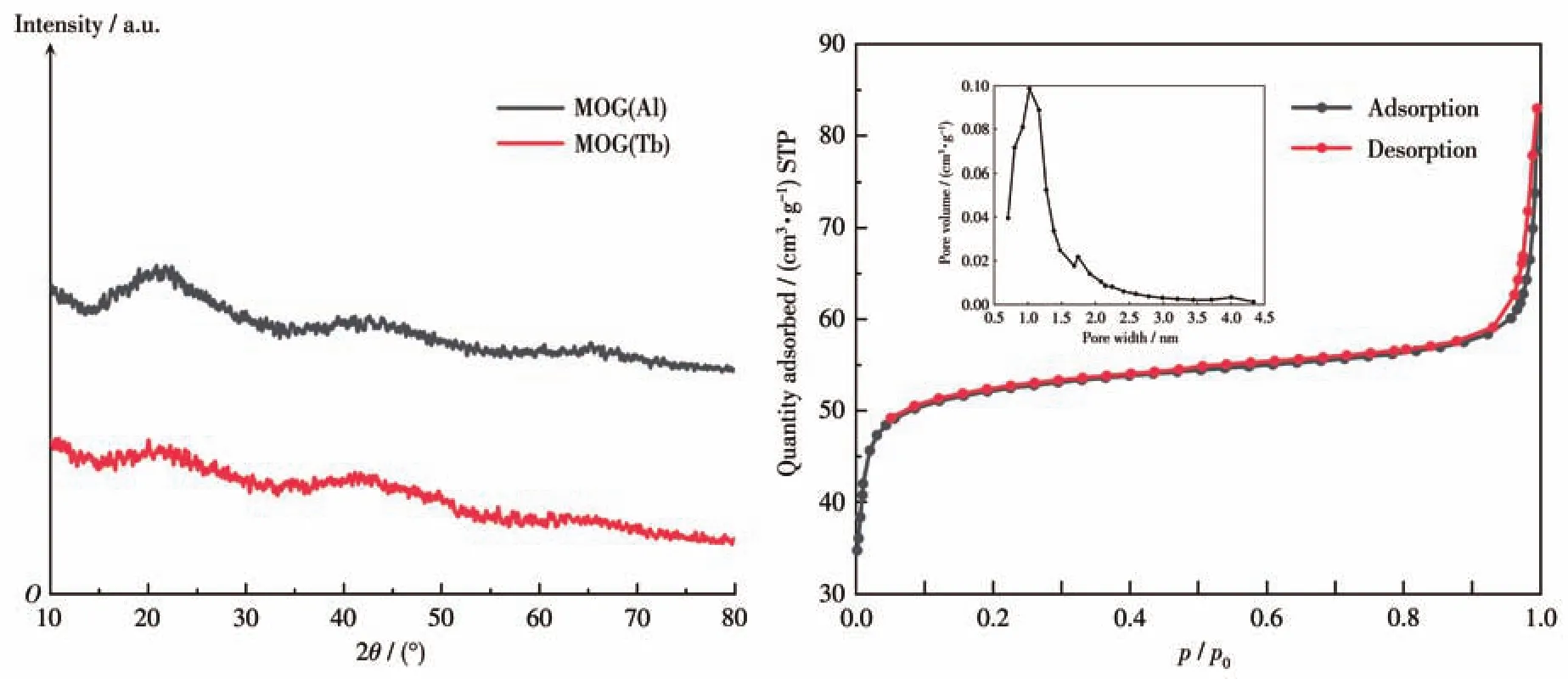
Fig.1 (a)PXRD patterns of MOG(Al)and MOG(Tb);(b)N2adsorption-desorption isotherms of MOG(Tb)
As can be seen from the TG curves(Fig.S1,Supporting information),MOG(Al)and MOG(Tb)have similar thermal stability.There were two stages of weight loss from room temperature to 700℃for MOG(Al).The first weight loss from room temperature to 300℃is probably due to the solvent removal,removal of structural water,and volatilization of small molecular components.The subsequent weight loss can be attributed to the thermal effects of organic ligand decomposition and burning of the functional group.For MOG(Tb),the weight of the sample slowly decreased with increasing temperature.The weight of the sample decreased rapidly until 400℃.After the unstable groups in the sample have been decomposed,the weight of the sample did not change when the temperature was above 700℃,indicating that the organic components of the sample are completely oxidized to carbon dioxide and other relatively stable small molecular gaseous products.The difference in thermal stability of the two MOG compounds may be due to the fact that MOG(Al)is formed at high temperatures,whereas MOG(Tb)only undergoes ion exchange at room temperature,although the central ion has a larger radius and can be accommodated at the periphery.The more ligands there are,the larger the ionic radius of Tb and the less attractive it is to the ligand,which in turn will be the case and the coordination number will decrease.
The substitution effect of the metal ions Al3+and Tb3+was verified by ICP-OES(inductively coupled plasma-optical emission spectroscopy)tests.Table S1 summarizes the ion concentrations of Al3+and Tb3+in the xerogels before and after 3,7,and 15 d of replacement.The concentration of Al3+decreased as the replacement period increased,while the concentration of Tb3+gradually increased,reaching a peak at 7 d of replacement.The results indicate that the ions in the gels have been successfully exchanged.The substitution of Al3+may be due to the stronger interaction between the Tb3+and the main group of the polycarboxylic acid.
2.2 Fluorescence characteristics,stability,and cytotoxicity of MOG(Tb)
After ion exchange,we can see that the product has obvious Tb3+characteristic emission(Fig.2).The obvious characteristic peak of MOG(Tb)at 418 nm is due to the5D3→7F5transition of Tb3+.The characteristic peak at 538 nm can be attributed to the5D4→7F5transition.Compared with the weak fluorescence emission of H3TCA at 425 nm,due to the charge transfer between the ligand and the metal,the coordination between Tb3+and H3TCA makes the xerogel have stronger fluorescence at 418 and 538 nm(Fig.S2).The fluo-rescence quantum yields were calculated,andΦMOG(Al)=0.261,ΦMOG(Tb)=0.273.It can be seen that Tb3+as the central ion has a better luminescence effect than Al3+,and the energy transfer efficiency with the ligand is also higher.
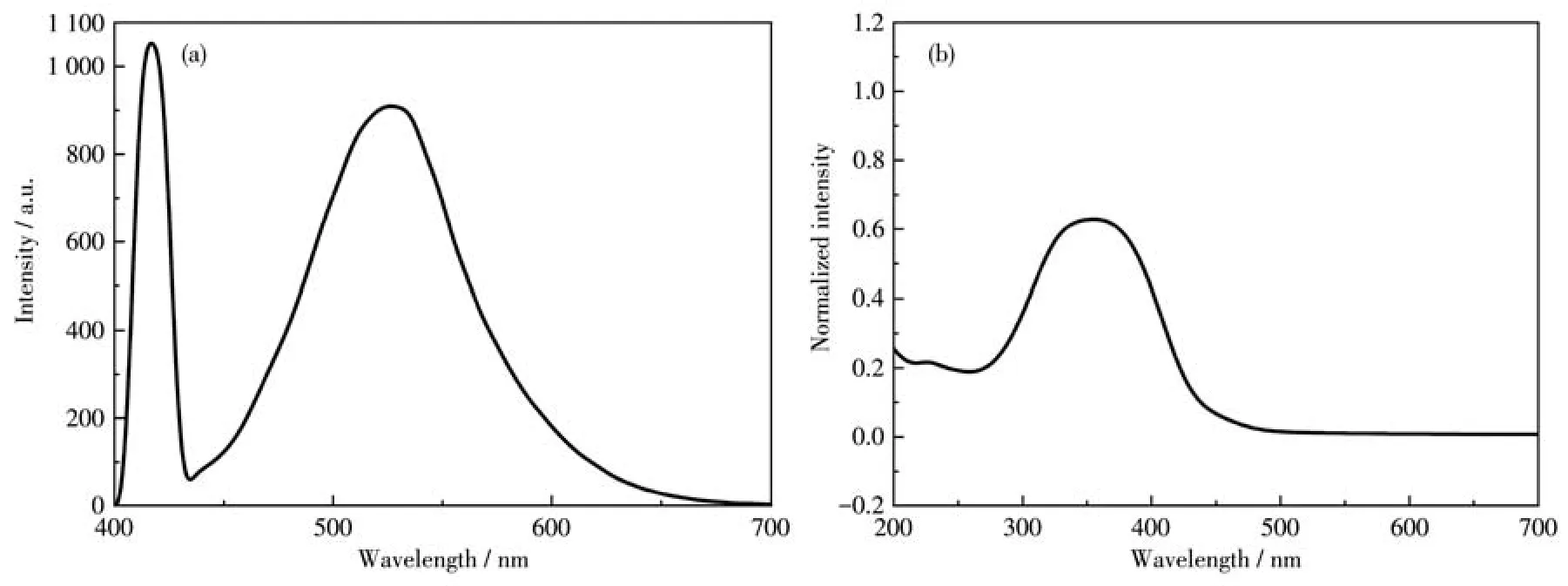
Fig.2 (a)Emission and(b)excitation spectra of MOG(Tb)
In addition,the framework and structure of MOG(Tb)remained intact after soaking in pH=2,3,5,7,9,11,and 12 for 48 h(Fig.3),and it can be seen from Fig.S3 that the N2adsorption-desorption isotherms did not change significantly after the xerogel was immersed in the aqueous solutions with pH=2 and 12.To further examine the stability of the structure,MOG(Tb)was sonicated in aqueous solutions with different pH values,and then Al3+and Tb3+in the aqueous solutions were detected by titration and chromogenic methods,respectively.NaOH solution was added dropwise to the aqueous solutions with pH=2,3,5,7,9,11,and 12 after ultrasonic treatment.It can be seen that there was a small amount of white precipitate in the aqueous solution with pH=2 and 12,but there was no precipitation in other solutions(Fig.S4).It can be concluded that a small amount of Al3+was released into the aqueous solution with pH=2 and 12.Then,ammonium oxalate was used to detect the free Tb3+content in aqueous solutions with different pH values after ultrasonication.0.05 mL of the aqueous solution was pipetted with a micropipette and placed on filter paper.After drying with hot air,saturated ammonium oxalate solution was sprayed.If there are Tb ions,it will show yellow-green fluorescence under ultraviolet light.We can see that at pH=2,a few Tb3+ions were released in an aqueous solution(Fig.S5).

Fig.3 PXRD patterns of MOG(Tb)in aqueous solutions with pH=2,3,5,7,9,11,and 12
As shown in Fig.S6,after 24 h of incubation,the survival rate of PK cells was still greater than 76%,and the survival rate of BHK cells was greater than 83%.These results indicate that MOG(Tb)has relatively low cytotoxicity.
2.3 Detection of antibiotics and NACs
Due to the superior water stability and excellent fluorescence emission of the xerogel,we were able to use MOG(Tb)to detect some antibiotics.We used nine antibiotics for the assay,namely sulfamethazine(SMZ),sulfadiazine(SDZ),FZD,MDZ,dimetridazole(DTZ),ornidazole(ODZ),chloramphenicol(CAP),florfenicol(FFC),and penicillin G potassium salt(PCLP).As can be seen in Fig.4,MDZ and FZD had the highest quenching efficiencies of 91.2% and 94.7%,respectively.Interestingly,PCLP enhanced the fluorescence of MOG(Tb).The quenching efficiencies of DTZ and ODZ were average.The quenching efficiencies of the other types of antibiotics were weaker.To quenching intensity of these antibiotics were ranked in the order of FZD>MDZ>ODZ>DTZ>CAP>FFC>SDZ>SMZ>PCLP.PXRD testing of the antibiotic-tested MOG(Tb)xerogel powder in turn showed that the structure and skeleton of the xerogel remained unchanged significantly(Fig.S4).Compared to Fig.2a,the leftward shift of the peak near 420 nm in Fig.4a may be due to the up-conversion process.When MOG(Tb)is mixed with the antibiotic solution,the luminescent center can absorb more photon energy and then produce emitted photons of higher energy than each excited photon.The absorption process for each photon is a binary primitive reaction due to the presence of an excited intermediate state.The“new peak”at 740 nm was the emission spectrum,which is due to the transition of electrons to different excited-state energy levels,absorbing different wavelengths of energy and producing different absorption bands,resulting in fluorescence of a certain wavelength.
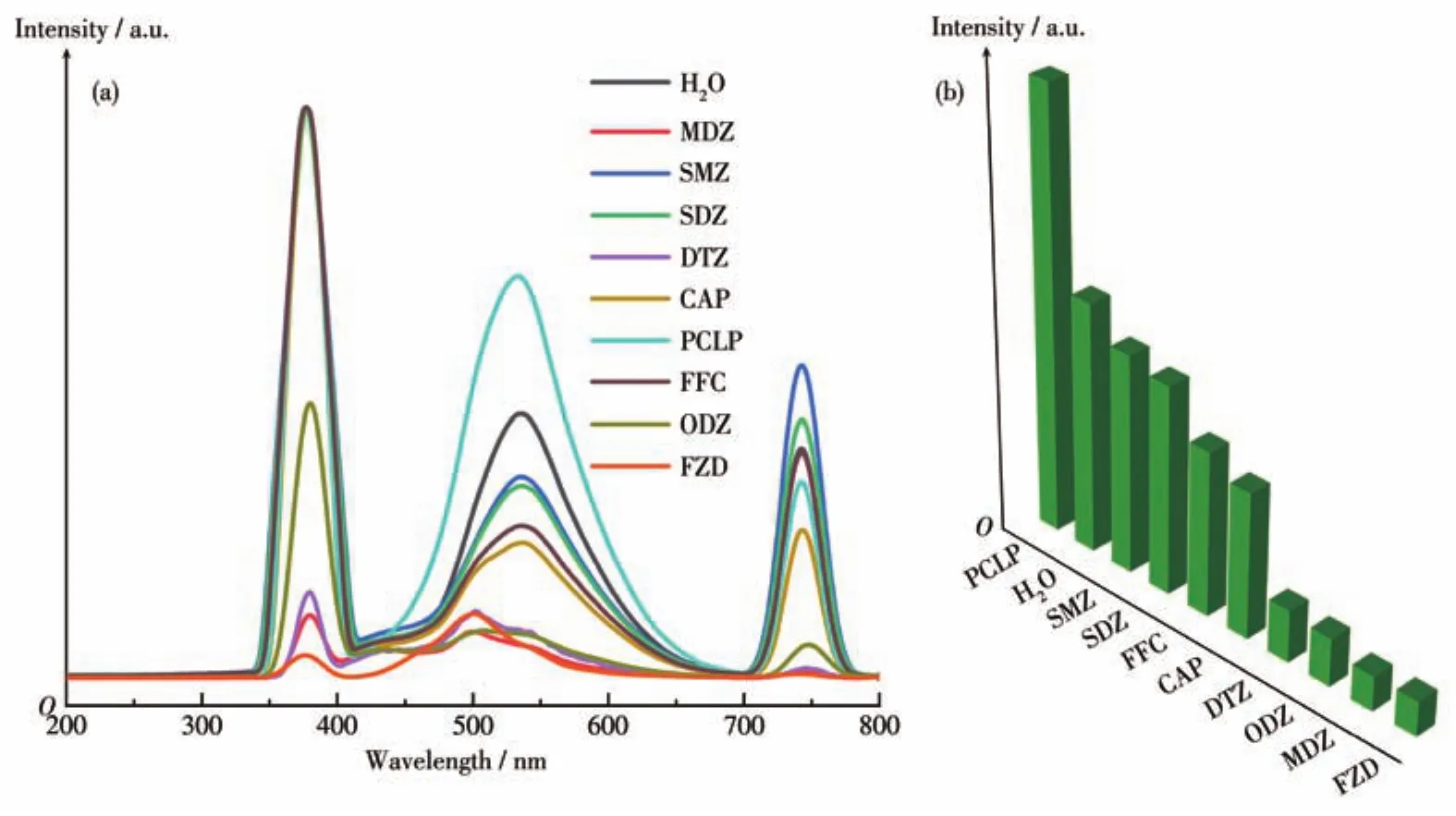
Fig.4 (a)Emission spectra and(b)fluorescence intensity at 538 nm of MOG(Tb)in various antibiotic aqueous solutions
To more clearly explain the fluorescence quenching effect of MOG(Tb)on MDZ and FZD,we tested the change of xerogel′s fluorescence intensity as the antibiotic concentration gradually increased where each concentration gradient was increased by adding a 2 μL antibiotic solution(Fig.5a and 5c).The quenching effect can be rationally calculated using the Stern-Volmer(SV)equation:I0/I=1+KSVcQ,whereI0andIare the fluorescence intensity of MOG(Tb)without adding and adding a certain concentration of the antibiotic solution,respectively,cQis the concentration of the antibiotic aqueous solution,andKSVis the quenching constant.KSVcan be obtained by plotting 1/(I0-I)against 1/cQand performing a linear fit.
For MOG(Tb),the quenching constants of FZD and MDZ were 7.76×104and 8.03×104L·mol-1,respectively(Fig.5b and 5d).The values of the limit of detection(LOD=3σ/KSV)for FZD and MDZ were 0.668 and 1.27 μmol·L-1(Table S2).The quenching constant of FZD and MDZ to MOG(Tb)was also higher than some reported MOF materials(Table 1),which is sufficient to prove its excellent quenching ability.

Fig.5 Emission spectra of MOG(Tb)with the gradually increasing(a)FZD and(c)MDZ concentrations;Linear fitting of SV equation for the fluorescence of MOG(Tb)quenched by(b)FZD and(d)MDZ in aqueous solution

Table 1 Summary of queching constants(KSV)of related materials for FZD,MDZ,2,4-DNT,and 4-NP
The selective detection ability of the xerogel for antibiotics is also very important in practical applications.To demonstrate the selective detection of FZD and MDZ by MOG(Tb),we performed control experiments.First,the fluorescence intensity of MOG(Tb)dispersed in water was tested,and then other antibiotics(30 μL)and the corresponding FZD and MDZ aqueous solutions(30 μL)were added to observe the change in fluorescence intensity.Fluorescence quenching occurred immediately after the addition of selected antibiotics FZD and MDZ to MOG(Tb)aqueous solutions containing other antibiotics,as shown in Fig.6,with negligible effects of other antibiotics.The above experiments demonstrate the high selectivity and sensitivity of MOG(Tb)for FZD and MDZ.Furthermore,we also found that the drop wise addition of PCLP solution to MOG(Tb)solution resulted in enhanced fluorescence(Fig.4a).Although the specific fluorescence enhancement mechanism is unclear,we believe that the improved fluorescence properties can be attributed to the interaction of the carboxyl group of H3TCA in MOG(Tb)with PCLP,resulting in the aggregationinduced emission(AIE)effect of the triphenylamine central structure.
Many researchers use unsaturated fatty acids for the detection of NACs,however,this method must be performed in organic solvents.It is more troublesome in practical application.Therefore,MOG(Tb)xerogels with excellent water stability and high-intensity fluorescence are particularly suitable for the detection of NACs.We selected 2,4-DNT,2,4,6-trinitrophenol(TNP),nitrobenzene(NB),4-NP,phenol(PHL),benzoic acid(BC),chlorobenzene(CB),toluene(MB),2,3-dimethyl-2,3-dinitrobutane(DMNB)to study their effects on the fluorescence of MOG(Tb)in water.As shown in Fig.7,the quenching effect of 4-NP on the fluorescence of MOG(Tb)was the strongest,followed by 2,4-DNT.The quenching efficiencies of 2,4-DNT and 4-NP were 92.5% and 95.7%,respectively,while those of the other analytes were less than ideal.Their quenching efficiencies can be ordered as 4-NP>2,4-DNT>NB>DMNB>MB>BC>TNP>CB>PHL.Moreover,after being immersed in an aqueous solution of antibiotics or small organic molecules for 24 h,the framework and structure of MOG(Tb)were still closely arranged with almost no change(Fig.S7 and S8).
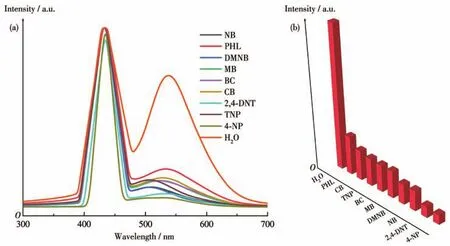
Fig.7 (a)Fluorescence spectra and(b)fluorescence intensity at 538 nm of MOG(Tb)in aqueous solutions of various small organic molecules
Fluorescence quenching titration(Fig.8a and 8c)and SV linear fitting(Fig.8b and 8d)were performed for 2,4-DNT and 4-NP,respectively.TheKSVfor 2.4-DNT and 4-NP were 2.65×104and 8.58×104L·mol-1,respectively,and the LOD values for 2,4-DNT and 4-NP were 1.49 and 0.253 μmol·L-1,respectively(Table S2).TheKSVvalues of MOG(Tb)for 2.4-DNT and 4-NP were higher or comparable to those of previously reported MOF materials,which demonstrates its good detecting ability(Table 1).In addition,we also analyzed and discussed the effect of other small organic molecules(TNP,DMNB,BC,MB,NB,CB,PHL)on the detection of 2,4-DNT and 4-NP by MOG(Tb).The fluorescence from MOG(Tb)was almost completely quenched when 2,4-DNT and 4-NP(30 μL)were added to an aqueous MOG(Tb)solution containing other analytes(Fig.9).Negligible effects of other small organic molecules on the analysis can be demonstrated,demonstrating the excellent selectivity and sensitivity of the xerogel for 2,4-DNT and 4-NP.

Fig.8 Emission spectra of the fluorescence quenching titration of MOG(Tb)by(a)2,4-DNT and(c)4-NP;SV linear fitting for the fluorescence of MOG(Tb)quenched by(b)2,4-DNT and(d)4-NP in aqueous solution

Fig.9 Fluorescence intensity of MOG(Tb)at 538 nm in the presence of(a)2,4-DNT/(b)4-NP and other small organic molecules
After detection,the xerogel could be washed with ethanol,filtered,and dried,and it still had a reproduc-ible fluorescence quenching effect for detecting the analytes(Fig.10).After 7 cycles of quenching experiments,the framework and structure of MOG(Tb)were still intact(Fig.S9).It could also be seen from the SEM image of MOG(Tb)that the structures were closely arranged(Fig.S10),proving that MOG(Tb)has good recyclability and stability.

Fig.10 Reproducible fluorescence quenching effect for MOG(Tb)detecting the analytes
2.4 Mechanism of fluorescence quenching
We further explored the mechanism of fluorescence quenching MOG(Tb).UV-Vis spectra of the nine antibiotic analytes mentioned above and the nine NACs and non-NACs were examined(Fig.S11 and S12).It can be seen that the absorption bands of FZD and MDZ overlapped most of the excitation band of MOG(Tb)compared to the other antibiotic analytes,which may lead to lower absorption of the ligands of MOG(Tb).This implies that the stronger quenching of MOG(Tb)by FZD and MDZ is due to an energy transfer process between the ligands and metal ions[50].For NACs and non-NACs,2,4-DNT and 4-NP had the highest recombination and the mechanism can be inferred that the fluorescence quenching of MOG(Tb)by FZD,MDZ,2,4-DNT,and 4-NP can be attributed to their competition with the ligands of MOG(Tb)[48].FZD,MDZ,2,4-DNT,and 4-NP absorb the excitation energy and reduce the ligand absorbed light,so the energy transfer from the ligand to the Tb3+ion is reduced and the characteristic fluorescence of Tb3+is subsequently quenched.
3 Conclusions
In summary,we prepared a gel named MOG(Al)based on the triphenylamine structure as the main ligand and then formed a xerogel named MOG(Tb)with strong fluorescence emission through metal ion exchange.And it has sensitive detectability for FZD,MDZ,2,4-DNT,and 4-NP,and the detection limits are 0.668,1.27,1.49,0.253 μmol·L-1respectively.The quenching mechanism can be attributed to the interaction with the ligand competitive absorption.The preparation of MOG(Tb)can provide more possibilities for the development of MOG in the future.
Conflicts of interest:The authors declare no competing financial interest.
Supporting information is available at http://www.wjhxxb.cn

