Impact of nitrogen input from biosolids application on carbon sequestration in a Pinus radiata forest
Jianming Xue, Mark O. Kimerley, Russell B. MKinley
a Scion, PO Box 29237, Christchurch, 8440, New Zealand
b Retired (previously Scion), Rotorua, 3010, New Zealand
c Scion, Private Bag 3020, Rotorua, 3046, New Zealand
Keywords:Biosolids application Stand density and growth Wood density C sequestration
ABSTRACT Background: Forest management practices (e.g. choice of stand density, fertilisation) are just as important in carbon (C) forestry as in other types of forestry and will affect the level of C sequestration and profitability.Because C stored in wood is approximately proportional to the product of its volume and density,it is necessary to account for both volume growth and wood density when assessing the effects of fertilisation on C sequestration in pine forests.Methods: The effects of nitrogen (N) input from biosolids application on forest C sequestration were quantified from an intensively monitored biosolids field trial in a Pinus radiata plantation on a sandy soil in New Zealand.The field trial tested the application of three biosolids rates: Control (no application), Standard (300 kg N⋅ha-1 applied every three years), and High (600 kg N⋅ha-1 applied every three years), across three levels of stand density: 300, 450, and 600 stems⋅ha-1. Carbon sequestration was estimated using the C-Change model from annual plot measurements of stand density, stem height and diameter, and annual breast height wood densities obtained from increment cores.Results:By age 24 years,N-fertilised trees had sequestered 40 t C⋅ha-1 more than unfertilised trees,an increase of 18%.Fertilisation increased stem volume by 23%but reduced stem wood density by 2.5%.Most of the increased C sequestration occurred between age 6 and age 17 years and the Standard rate gave the same increase in C sequestration as the High rate. On average, there was no significant difference in growth rate between fertilised and unfertilised trees after the 17th growth year,but the increased growth ceased earlier at higher stand densities,and later at lower stand densities.Conclusions: This study indicates that 2–3 applications of the Standard rate would have been sufficient to achieve the increased C sequestration,with an applied N to C conversion ratio of 43–65 kg C⋅kg-1 N.Our results highlight that N fertilisation will become more widespread under greenhouse gas emissions trading schemes which encourages forest management practices that improve C sequestration in young forests in New Zealand in particular and other countries in general.
1. Introduction
Although the burning of fossil fuels has been the largest anthropogenic cause of increased atmospheric CO2, deforestation has played an important secondary role. Forests store large amounts of carbon (C),which is released into the atmosphere during deforestation or as a result of forest degradation.It is estimated that global emissions from land use change have accounted for 33% of total anthropogenic emissions over the period 1850–1990,with the great majority of this loss due to a 20%reduction in global forest area over this period(Houghton,1999,2003).It follows that protecting existing forests and planting new forests can play an important role in reducing emissions of atmospheric CO2, thus mitigating global warming.
New Zealand has contributed to this historic trend of forest destruction with the area of land under natural forest reducing from approximately 85% prior to human habitation (McGlone, 1989) to its current area of 7.8 million ha(Holdaway et al.,2017)representing 29%of land area.This has been partly offset over the past century by the planting of commercial plantations of fast-growing exotic trees, which currently cover 1.7 million ha(Ministry for Primary Industries,2016)or 7%of land area.Grasslands that have replaced the natural forest typically store less than 10 t C⋅ha-1in their biomass (IPCC and Penman, 2003) compared with the natural forest biomass, which stores an average of more than 200 t C⋅ha-1(Holdaway et al., 2017). Forest soils also generally store more organic C than grassland soils (Cotrufo et al., 2019). The exotic plantation forests with average age of less than 15 years store about 100 t C⋅ha-1on average but older exotic forest can store twice or more times the average figure (Beets et al., 2011, 2012). Exotic plantation forests,with 90% by area being Pinus radiata D. Don, therefore can quickly accumulate far more C than is lost from the biomass of the grassland they replace or the loss in soil organic C that can occur soon after forest establishment which is believed to average about 10–20 t C⋅ha-1over the first rotation(Oliver et al.,2004).
The New Zealand government's principal policy response to climate change is the New Zealand Emissions Trading Scheme(ETS)(Jiang et al.,2009). This promotes the reduction in greenhouse gas emissions while maintaining economic productivity.By putting a price on greenhouse gas emissions, it provides incentives both to reduce emissions and to plant forests to absorb CO2.Under the scheme,C credits can be sold based on estimates of C sequestration as a forest develops. At present under the ETS, all harvested wood taken off site is conservatively assumed to be immediately released back into the atmosphere, while harvest residues that remain on-site are considered to decay over a period of several years.The scheme can be integrated into a commercial forest operation in forests established after 1989, with forest owners receiving a regular cashflow by selling credits while their forest grows but having to repurchase a portion of them at harvest. The ETS may also encourage other types of forest management including continuous-cover forestry where stands are not clear-felled but which may incorporate periodic production thinnings to remove trees as stands become over stocked,and forests managed solely for the purpose of sequestering C. Forestry entered the scheme in 2008,but prices of units have mostly been too low to greatly influence forest management practices. However, recent restrictions on the supply of units may give the scheme greater relevance to forest owners(Ministry for the Environment, 2016).
Forest management practices are just as important in forests established for C sequestration as in other types of forestry(Pukkala,2018;Yu et al., 2021). The choice of species, site preparation and establishment methods, fertilisation, control of pests and diseases, choice of stand density and timing and intensity of thinning and harvesting operations,will all affect the level of sequestration,and profitability under the ETS.Manipulation of stand density and choice of genetic material are two key mechanisms through which forest managers can influence tree growth and wood properties (Smith et al., 1997; Moore et al., 2015) and C sequestration (Erkan and Aydin, 2016; Kamara et al., 2020). In New Zealand,P.radiata is well suited to C forestry because of its rapid growth rate and the extensive knowledge base concerning its management.The deployment of genetically improved varieties of P. radiata can greatly improve C sequestration (Kimberley et al., 2015) at a modest expense,and therefore benefit C forestry as much as conventional forestry. One major difference between the economic returns under a scheme such as the ETS and conventional forestry is that the benefits of improved growth are realised soon after they occur rather than at harvest. Therefore, an operation carried out early in a rotation that provides a short-term increase in growth provides an immediate economic return under the ETS in contrast to conventional forestry where its profitability is reduced by the need to discount revenue from a future harvest back to the time of the operation.
Nitrogen(N)fertilisation of P.radiata is an example of the operation,which is usually carried out on young trees and often only provides a short-lived growth response. Added N can increase P. radiata growth rates significantly on nutrient-poor sites (Mead and Gadgil, 1978) but also on higher-productivity sites (Woollons and Will, 1975). However,growth is typically increased only for 3–5 years following fertilisation before returning to its original base rate,and fertilisation is usually most effective in young trees or immediately following thinning(Woollons and Will, 1975). To evaluate the potential benefits of N fertilisation in a P.radiata C forest,it is important to have a good understanding of growth responses to fertilisation across the range of stand ages and densities.Although it is well documented that N fertilisation increases stem volume growth, a negative relationship between wood density and N supply in P. radiata has also been established.For example,an application of 200 kg N⋅ha-1reduced density of wood formed in the year of application by 50–60 kg⋅m–3(Cown and McConchie, 1981) while a reduction in wood density of as high as 100 kg⋅m–3was recorded in a stand growing on a nutrient-poor site subject to long-term application of N fertiliser (Beets et al.,2001).Many studies have investigated responses of forest growth(Harrison et al., 2001; Lteif et al., 2007; Scharenbroch et al., 2013) to short-term (1–5 years) application of biosolids. However, only a few studies have investigated the impacts of long-term (>10 years) application of biosolids on tree growth in plantation forests(Henry et al.,1994;Prescott and Blevins,2005).Similar to the effect of chemical N fertiliser,basic wood densities at a mid-rotation assessment of the trial which is the subject of the present study were also significantly lower in the trees fertilised with biosolids(Wang et al.,2006).Because C stored in wood is approximately proportional to the product of its volume and density,it is necessary to account for both volume growth and wood density when assessing the effects of fertilisation on C sequestration.
In this study we analysed results from an intensively monitored field trial established in a P. radiata stand on a nutrient-poor sandy soil and tested the effects of long-term additions of N-rich biosolids. The objectives of this study were (1) to quantify any increase in C sequestration achieved by N input from application of biosolids, accounting for the effects on both volume growth and wood density,and(2)to determine if the response to N input from application of biosolids varied across a range of stand densities and to test for changes in the response over time as a stand developed. We hypothesized that (1) biosolids application would have positive effect on tree growth but some negative effect on wood density(2)high stand density(i.e.stocking rate),would reduce the diameter growth of individual trees but increase total stem volume and C sequestration per hectare, and (3) the impacts of both biosolids application and stand density would change over the time.
2. Materials and methods
2.1. Site description, trial design and establishment
The trial was designed to test the sustainability of repeated applications of treated biosolids from the Nelson regional wastewater treatment plant to a 1,000-ha P.radiata forest plantation on Rabbit Island(41°16′S,173°08′E) near Nelson City, New Zealand. The Island is very flat(maximum altitude 10 m) and is made up of predominantly Tahunanui Sands with naturally low fertility.Organic material is almost absent from soil profiles due to previous burning practices for re-establishment.Average annual mean temperature is 12.7°C and annual rainfall is 960 mm (1981–2010). The trial was established in 1997 in a stand planted in 1991, and tree growth has been monitored regularly along with a number of environmental variables,such as soil and groundwater quality. The soil at the trial site consists of coarse coastal dune sands,classified as a sandy raw soil(Hewitt,1998),which has very low total C and N(Table 1) but provides free rooting access to the shallow groundwater 2.0–4.2 m below the surface (Wang et al., 2004). The plantation was planted at a stand density of 1,000 stems⋅ha-1, and all trees in the trial were pruned in up to four lifts to 6 m height between ages 5 and 10years.

Table 1 Selected soil properties of the study site at Rabbit Island.
The trial was a split-plot, randomised block design with four replicates.Three biosolids treatments,applied in main-plots,were(1)Control(no biosolids),(2)Standard treatment(300 kg N⋅ha-1every three years)as recommended by the Guidelines for the Safe Application of Biosolids to Land in New Zealand (NZWWA, 2003), and (3) High treatment (600 kg N⋅ha-1every three years). Each main-plot contained three tree stocking rates(subplots)at 300,450 and 600 stems⋅ha-1.There were 36 subplots in total with each subplot measuring 25 m × 25 m, plus 5 m buffer zones. Treated biosolids (i.e. stabilised domestic sewage sludge containing around 3% total solids) from the Nelson regional municipal wastewater treatment plant were applied in 1997, 2000, 2003, 2006,2009 and 2012 at the same rates to the same plots using a purpose built spreader that sprays the biosolids about 25 m on each side, with the cumulative application rates of 18 and 36 Mg dry biosolids per ha for the Standard rate and High rate treatments, respectively. Stand density treatments were applied by thinning to waste in 1996 with final stand densities in each subplot varying slightly from the nominal values.Selected properties of biosolids applied (mean values) are listed as follows: pH 8.5, solids 2.55%, total N 9.75%, NH4-N 3.81%, total P 1.4%,and heavy metals of As 10.4,Cd 3.2,Cr 77,Cu 287,Pb 36,Hg 1.3,Ni 21 and Zn 559 mg⋅kg–1.
2.2. Measurement of tree growth, stem wood density and needle nitrogen concentration
Tree height and diameter at breast height(1.4 m)of all trees in each subplot were measured annually from age 6 and 24 years. For each subplot,stand density,mean top height(MTH,mean height of the largest 100 trees per hectare), and basal area (BA) were calculated. The stem volume in each subplot was estimated from the BA and MTH using a P. radiata stand-level volume function (Kimberley and Beets, 2007). In May 2015,breast height pith-to-bark 5 mm increment cores were taken from four randomly chosen trees in each of all 36 subplots. This gave a total of 144 cores,or 48 cores per biosolids application treatment.Breast height wood densities were obtained for each growth ring(mid-winter to mid-winter) of 144 core samples by direct scanning densitometry.Briefly,the densitometry process requires the cores to be resin-extracted by refluxing in methanol in a Soxhlet apparatus and conditioned to 10%moisture content.Resin-extracted cores are then precision-machined to a tangential thickness of 1.5 mm prior to scanning with a radioactive source (Fe55) and a scintillation counter (Cown and Clement, 1983).Density data were collected in radial increments of 0.3 mm and processed to yield equivalent basic density values by growth ring in conjunction with other ring parameters such as ring width and latewood percent.Means of wood density for each ring were obtained for each subplot.To assess changes in tree nutrition,the current-year needles were sampled in most years from the youngest second-order branches in top third of the crown of selected trees and analysed by main plot for N concentration.
2.3. Estimates of C stock by pool and total C sequestration
Annual estimates of C sequestration were obtained for each subplot using the C-Change model (Beets et al., 1999). Inputs required by this model were a yield table of annual stand densities and stem volumes obtained from plot measurements as described above, and densities of stem wood annual growth increments (growth sheaths), which were predicted from breast height densities using the sheath density model described by Beets et al. (2007). C-Change uses growth partitioning functions to allocate C to live biomass components and an ‘accounting’approach to estimate C flows into dead organic matter pools,and decay functions to estimate losses of C to the atmosphere from decay of organic dead pools (Beets et al., 2011). C-Change estimates C in the above-ground biomass(AGB),below ground biomass(BGB),dead woody litter(DWL)and fine litter(FL)pools and the total C of these pools,but does not estimate C in the soil organic pool or in understory vegetation.
2.4. Statistical analysis
Two-way mixed model (split-plot design) analysis of variance(ANOVA) and least significant difference (LSD) tests were used to determine the statistical significance of the biosolids application rate(as main plot) and tree stocking rate (as subplot) effects. The biosolids application rate and tree stocking rate were treated as fixed effects and block as a random effect.Data were analysed using the MIXED Procedure(SAS/STAT Version 9.3), with the following model:
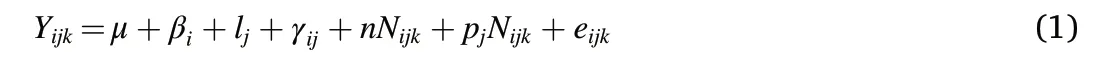

The dependent variables(Yijk)analysed were:total C,C in each major pool,stem volume,stem wood density,and foliar N concentration.These variables were analysed for each annual measurement. The C CAI (current annual increment, calculated for each growth year (mid-winter to mid-winter)from values at the beginning and end of the period),was also analysed along with the basic density of the stem wood growth sheath laid down in each year. To account for underlying differences in wood density between trees at the start of the trial,wood density in the growth year prior to trial establishment was used as a covariate in all analyses of wood density.
When a forest is within an accelerating growth phase as is typical of young trees, a simple comparison of CAI between fertilised and unfertilised trees will tend to exaggerate growth differences and the length of response time simply because the underlying growth rate over a given period is positively associated with the tree size at the beginning of that period. We therefore used the age-shift method to better identify the point when growth rate differences between treatments decline or cease(South et al.,2006).To apply this method,the age at which each subplot reached a given level of C sequestration was used as the dependent variable in Model (1). This age was calculated for each subplot from its annual C estimates by linear interpolation.Ages for C stocks ranging from 10 to 160 t C⋅ha-1in steps of 10 were used in this analysis.
3. Results
3.1. Effect of biosolids application on C stock, wood density, stem volume and foliar N nutrition
At age 24 years, the P. radiata trees treated with the Standard biosolids application rate had sequestered significantly more C than untreated trees but there was no additional increase in sequestration for trees receiving the High application rate (Table 2). The total C sequestered in fertilised trees averaged 260 ± 10 t C⋅ha-1(mean of Standard and High application rates with 95%confidence interval)compared with220±13 t C⋅ha-1in unfertilised trees,a difference of 40±16 t C⋅ha-1or 18% ± 7%. Stem volume of fertilised trees was 23% ± 7% higher than unfertilised trees but stem wood density of fertilised trees was 11 ± 8 kg⋅m–3or 2.5%±1.9%lower than unfertilised trees.Thus,in terms of C sequestration, the positive effects of N addition from biosolids application on stem volume growth greatly outweighed the negative effects of reduced wood density.As is typical in young P.radiata stands,the great majority of C is sequestered in the above and below ground biomass pools with only a small component in the dead woody litter and fine litter pools(Table 2).Annual means of total C,stem volume and stem wood density for each biosolids application rate are shown in Fig. 1. Overall, the dynamic change patterns of total C stocks in biomass for biosolids treatments(Fig.1a)were similar to those of stem volume(Fig.1c)at the same treatments. The reduction of stem wood density by nitrogen addition from biosolids application started at tree age of 7 years, gradually increased from age 8 years old to age 13 years old and then peaked(i.e.largest reduction) at age 14 years old without further worsening afterwards (Fig.1b).

Table 2 Effect of biosolids application rate on carbon stored in the above ground biomass(AGB), below ground biomass (BGB), dead woody litter (DWL) and fine litter(FL),and stem volume and stem wood density of P.radiata trees at age 24 years.Values in a column followed by the same letter do not differ significantly (LSD test,α =0.05).
A more detailed view of the biosolids treatment effects on tree growth,wood density and the C sequestration in biomass is provided by examining annual growth increments (Fig. 2). There was an immediate increase in the rate of C sequestration in fertilised trees after the initial biosolids application at age 6 years,with only slightly decreasing in the third year after fertilisation(Fig.2a).The difference in sequestration rate increased again following the second application and widened in subsequent years until the 11th growth year. After this, the difference in sequestration rate between fertilised and unfertilised trees gradually declined.Apart from a dip in the third year after the initial application,the percentage increase in sequestration remained fairly constant, averaging 51% until the 11th growth year, and then declining. In absolute terms it was lower initially because of the low baseline sequestration rate of young trees.The difference in sequestration rate between fertilised and unfertilised trees was statistically significant (p < 0.05) in all growth years between the 7th and 17th, but statistically non-significant in all subsequent years. The response to the High application rate was not significantly greater than the response to the Standard application rate(p> 0.05) in any year other than the 8th growth year two years after the initial application.
The density of stem wood laid down in each year showed the steady increase with tree age typical of P.radiata,but with considerable year-toyear fluctuation presumably due to climatic effects (Fig. 2b). Although there was a tendency for fertilisation to decrease wood density,the effect was intermittent. It was statistically significant (p < 0.05) in the year following the initial biosolids application and disappeared in the following few years before reappearing in some subsequent years. A statistically significant(p<0.05)effect was only apparent for wood laid down in growth years 7,13,14,17 and 20.

Fig. 1. Inter-annual dynamics of (a) total carbon sequestration in biomass, (b)stem wood density, and (c) stem wood volume for three biosolids application treatments over 24 years. The following symbols and lines are used for each biosolids application rate: Control – dots and solid line; Standard – stars and dashed line; High – triangles and dotted line. Error bars show standard errors.
Foliar N concentration of the Control treatment averaged 1.22%over the course of the trial with little consistent change over time (Fig. 2c).Application of biosolids significantly increased foliar N concentration,with the Standard treatment averaging 1.39% and the High treatment 1.50%over the course of the trial.The differences among the treatments remained consistent over time apart from a temporary reduction in the second and third year following the initial biosolids application, which was rectified by the second application.
3.2. The interactive effect of stand density and biosolids application on C stock annual increment
The wide range of stand densities in the trial plots, initially ranging between 250 and 800 stems⋅ha-1, made it possible to analyse the interactions between biosolids N loading rates and stand density over the course of the trial. There was a highly significant (p < 0.0001) positive relationship between stand density and C sequestration in every growth year. The relationship between C sequestration, stand density and biosolids treatment are illustrated in Fig. 3, which shows effects at four different stages of stand development.When the trees were young,there was a strong positive relationship between C sequestration and a multiplicative response to N across the stand density range,but sequestration in these young trees was low in absolute terms (Fig. 3a). By the 13th growth year,the larger trees were sequestering C at a higher rate,and the response to N was high and additive across the stand density range(Fig.3b).By the 17th growth year,sequestration was even higher but the response to N had decreased and was only apparent at low stand densities(Fig. 3c). Finally, in the 23rd growth year, sequestration remained very high but there was no longer any response to N input(Fig.3d).
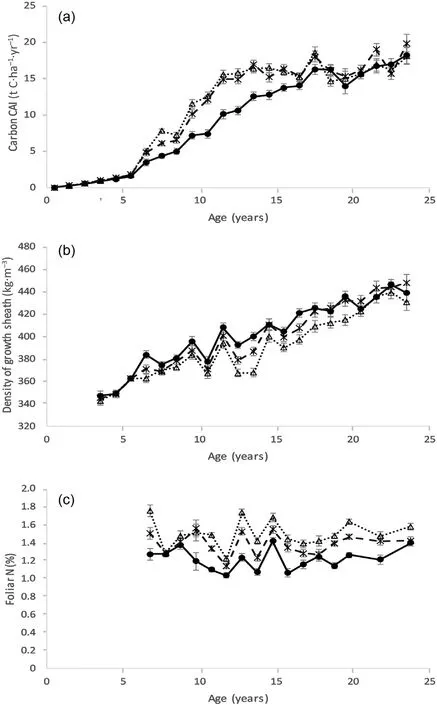
Fig.2. Inter-annual dynamics of(a)carbon CAI(current annual increment),(b)density of growth sheaths (i.e. stem wood annual growth increments), and (c)foliar N concentration for three biosolids application treatments over 24 years.The following symbols and lines are used for each biosolids application rate:Control – dots and solid line; Standard – stars and dashed line; High – triangles and dotted line. Error bars show standard errors.
3.3. Age-shift response to biosolids application
For simplicity, results are shown for the Control versus the mean of the Standard and High rates of biosolids application (Fig. 4). Age-shift analysis shows that fertilised trees sequestered 80 t C⋅ha-1two years earlier than unfertilised trees (Fig. 4a). At a stand density of 600 stems⋅ha-1, fertilised and unfertilised trees sequestered C at the same rate once they achieved 80 t C⋅ha-1(Fig.4b).However,at a stand density of 300 stems⋅ha-1, fertilised trees continued to store C at a faster rate(Fig. 4b), with 3.5 years earlier than unfertilised trees for sequestering 160 t C⋅ha-1(Fig. 4a). The difference between low and high stand densities in the increased rate of C sequestration by fertilised trees was statistically highly significant at C stock levels between 100 and 140 t C⋅ha-1(Fig.4b).
4. Discussion
The fertilised trees receiving the Standard biosolids application rate(i.e. 300 kg N⋅ha-1every 3 years) achieved the same increase in C sequestration as those receiving double this rate(i.e.600 kg N⋅ha-1every 3 years).Under the Standard application rate,total 1,800 kg N⋅ha-1were added to the plots from six biosolids applications. In response, the fertilised P. radiata trees sequestered an additional 40 t C⋅ha-1when compared to the Control treatment,giving a ratio of increased C per unit of applied N of 22 kg C⋅kg-1N.However,this increased C sequestration could have been attained using much lower levels of N. Most of the increase occurred prior to age 15 years when only 900 kg N⋅ha-1in total had been applied. The increased C sequestration could therefore be achieved with two or at most three applications of the Standard biosolids rate,implying an applied N to C conversion ratio of 43–65 kg C⋅kg-1N.
There has been debate about the level of C sequestration in forests associated with N fertilisation, particularly in regard to increased C sequestration in Northern Hemisphere temperate and boreal forests from deposition of anthropogenic sources of atmospheric N (Magnani et al.,2007; de Vries et al., 2008; Sutton et al., 2008; Pukkala, 2017). An analysis of increased forest growth versus levels of N deposition across a range of European forests suggested that a substantial proportion of the C sink activity apparent in these forests could be due to this increased supply of N and implied a N to C conversion ratio in the order of 200(Magnani et al., 2007). However, a revised analysis of the data taking account of climatic effects suggested a lower ratio of 30–70 kg C⋅kg-1N(de Vries et al.,2008),which is similar to the ratio we calculated from our trial. Analysis of fertiliser trials in Northern Hemisphere forests show ratios similar to ours (Chen et al.,2011;Albaugh et al., 2012). A model analysis of 23 European forest sites predicted a ratio of 50–75 kg C⋅kg-1N (Sutton et al., 2008) while a ratio for increased NEP (net ecosystem productivity) of 55–79 kg C⋅kg-1N was obtained in three Vancouver Island Pseudotsuga menziesii (Mirb.) Franco stands in the four years following fertilisation with urea(Dou et al.,2015).
The estimates of C sequestration obtained from the C-Change model used in this study account for the C in the stems,roots and crowns of the growing P.radiata trees,which transfers from the live trees into litter and dead wood pools and losses from these pools from decay. Because the rapidly growing P. radiata is by far the dominant element in terms of changes in C storage in this young forest ecosystem,the estimates of the total C sequestration given in Table 2 are likely not to differ greatly from the true NEP(net ecosystem productivity).The estimates take no account of losses in C from previous vegetation nor to changes in the soil organic C pool which are also likely to be minor(Beets et al.,1999,2011).They also take no account of C accumulation in understorey vegetation,which however forms a very minor component of the forest.
Our estimates of increased sequestration from N fertilisation are likely to understate the true increase in NEP for two other reasons.Firstly,we estimated stem volumes from DBH and height measurements but the predicted gains in volume from fertilisation may be underestimated if the extra wood accumulates disproportionately in the upper stem as reported by Woollons and Will (1975), although not found by Hunter et al. (1987). Secondly, as N fertiliser tends to impede organic matter decomposition in temperate forest soils(Janssens et al.,2010),it is likely that the forest soil respiration under fertilised trees may have been lower than in unfertilised trees(Zhang et al.,2021)and thus soil C sequestration may have been higher(or less negative)in fertilised plots.
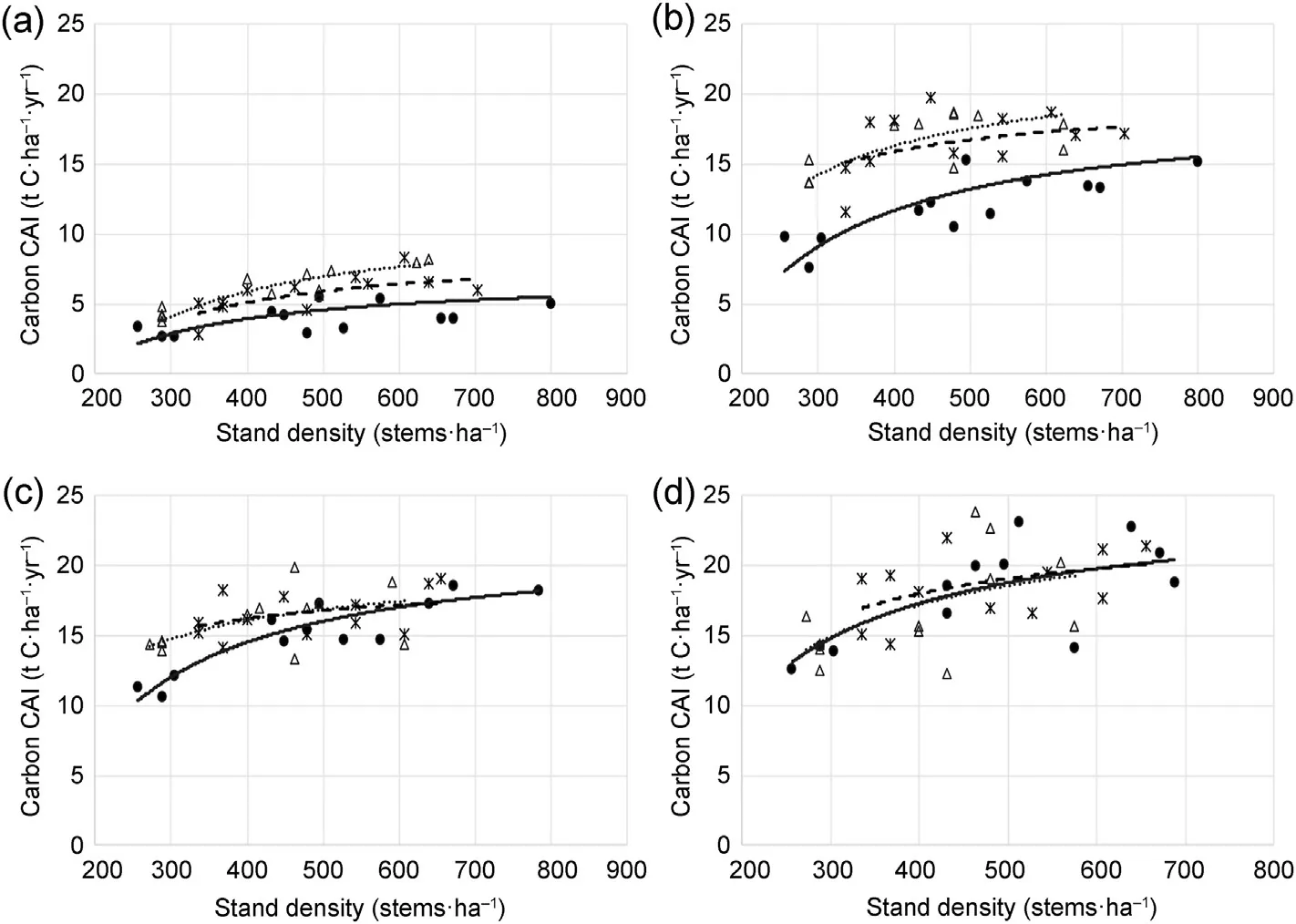
Fig.3. Effect of stand density and biosolids application treatments on carbon CAI(current annual increment)in different years(a)the 7th growth year,(b)the 13th growth year,(c)the 17th growth year,and(d)the 23rd growth year.Points show individual plots and lines show predictions from Model 1.The following symbols and lines are used for each biosolids application rate: Control – dots and solid line; Standard – stars and dashed line; High – triangles and dotted line.
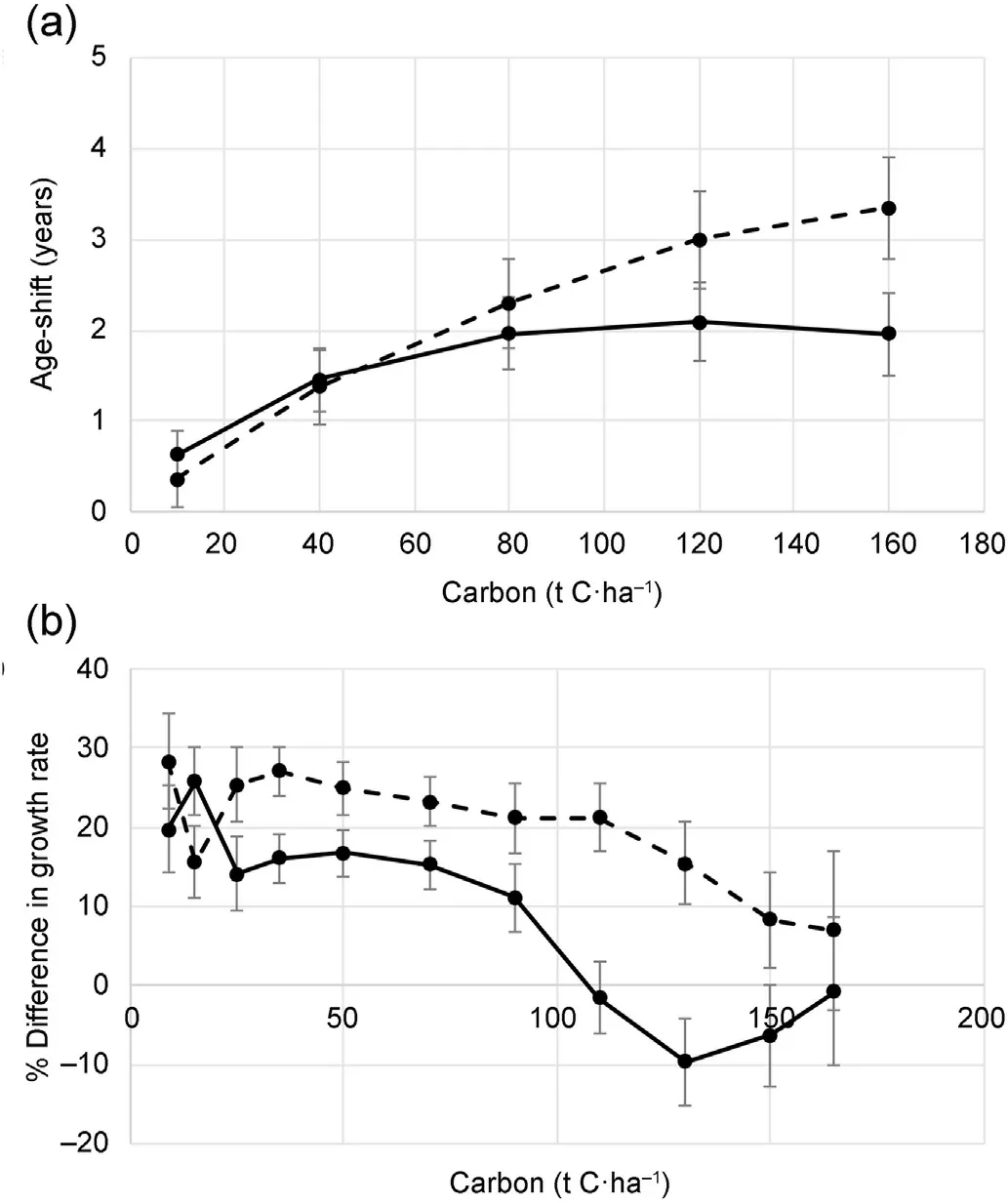
Fig. 4. Age-shift analysis comparing fertilised and unfertilised trees at stand densities of 600 stems⋅ha-1 (solid line) and 300 stems⋅ha-1 (dashed line).Shown are(a)the difference in the age at which fertilised and unfertilised trees achieve a given level of carbon sequestration,and(b)the percentage difference in growth rate between fertilised and unfertilised trees at a given level of carbon sequestration. Error bars show standard errors.
The increases in volume of 133 and 163 m3⋅ha–1for the Standard and High biosolids application rates respectively in our study are at the upper end of the reported range of growth responses in P. radiata N fertiliser trials in New Zealand.Hunter and Hoy(1983)found stem volume gains over the 5 years following fertilisation with 300 kg N⋅ha-1in two trials in moderately and severely nutrient-deficient coastal sand soils of 50 and 70 m3⋅ha–1respectively. Mead and Gadgil (1978) reported responses in 11 South Island trials 3–5 years following fertilisation with 200 kg N⋅ha-1+100 kg P⋅ha-1ranging from 0 to 93 m3⋅ha–1and averaging 45 m3⋅ha–1.Woollons and Will(1975) reported growth responses of 36–63 m3⋅ha–1over 7 years in moderately high producing pumice soil forests in the Central North Island from fertilisation with two application of urea in consecutive years totalling 500 kg N⋅ha-1.Growth responses have mostly been to stem diameter rather than height with Woollons and Will(1975)reporting no increase in height growth on productive sites although Hunter and Hoy (1983) reported height responses of 1.8 and 3.7 m on moderately and severely nutrient-deficient sites respectively. In our study,increases in volume were mainly from improved diameter growth with a height gain of only 0.9 m.
These results demonstrate that N fertiliser can increase P. radiata growth rates not only in low productivity sites but also in those of moderately high productivity. Our trial is on a moderately low productivity site with a 300 Index (a productivity index used for P. radiata representing the volume mean annual increment at a 300 stems⋅ha-1stand at age 30 years(Kimberley et al.,2005))of 20 m3⋅ha–1⋅yr–1(Wang et al., 2013), which is about 20% below the average for New Zealand.The larger than usual fertiliser response in volume growth observed in our trial is partly due to the high level of applied N in multiple doses,but mostly due to the form of fertiliser used. The uptake efficiency of N in commonly used N fertilisers such as urea or ammonium nitrate is typically in the range 20%–30% with significant losses from volatilization and especially immobilization in the surface horizons, with bioavailability of N often declining to background levels within six months of application (Myrold and Nason, 1992). However, the biosolids used in our trial provide a more gradual release of N.Around 50%–90%of N in biosolids is in organic compounds. Biosolids decompose over several years,gradually releasing nitrogen,sulfur,and micronutrients(Lu et al.,2012).Results from earlier trials also suggest that the timing of fertiliser application can greatly influence the response and non-optimum timing may account for poor responses in some trials.Woollons and Will(1975)found a response to fertiliser only in very recently thinned stands and reported minimal response in a stand thinned 3–5 years before fertilisation. This suggests that N fertiliser is most effective during canopy development or recovery following thinning(Mead and Gadgil,1978).
Improved growth in P.radiata from N fertilisation is primarily due to increased foliage mass rather than improved foliar efficiency.An analysis of a P.radiata fertiliser trial in a recently thinned stand by Carlyle(1998)showed that growth was highly correlated with LAI (leaf area index)which was in turn highly correlated with N uptake in the year of fertilisation.An index of LAI×foliar N concentration was no better correlated with growth than LAI alone.Because LAI remained elevated in fertilised trees even after N uptake had returned to baseline levels, growth rates remained higher for several years following fertilisation. At some maximum LAI,the forest canopy can be considered“closed”.In P.radiata stands, above-ground production increases approximately linearly with LAI to a value as high as 10 and shows a declining rate of increase up to a LAI value of 20 or more (Beets and Pollock, 1987). Foliage mass in P.radiata increases with age until an equilibrium level is reached(Beets and Whitehead, 1996). Although we have no direct measurements of foliage mass in our trial, our results suggest that fertilisation increases foliage mass in trees with underdeveloped canopies but not after canopy closure. In other words, fertilisation accelerates the rate of increase in needle mass in a developing canopy but does not affect the final level of foliage mass in the closed canopy. Therefore, growth rates in our trial after age 17 years were no higher in fertilised than in unfertilised trees.Age-shift analysis indicates that the C sequestration rate in fertilised trees in our trial accelerated by about 20%immediately following fertilisation and remained at this elevated level before declining to the same level as unfertilised trees presumably at canopy closure (Fig. 4). This occurred earlier in more highly stocked stands in terms of both age and C storage.Canopy closure in more densely stocked stands occurs at a younger age when trees are shorter and therefore have less stored C. This was also reflected in the relationship between carbon CAI (current annual increment),stand density and biosolids treatment at different stages of stand development(Fig.3).Because of the natural sigmoidal shape of the tree growth curve, some apparent narrowing in the rate of growth and therefore carbon CAI between biosolids-treated and-untreated trees was expected, especially at more densely stocked stands. This was simply because the treated trees were ahead of the Control trees in development.Due to the limited growth space, the development gap between biosolids-treated and-untreated trees was narrower and closed earlier at more densely stocked stands(Fig.3).
The management implications of this study are that fertilising P.radiata forest with N after canopy closure will have limited effects on tree growth especially at high stand density. This supports the New Zealand forest industry operational strategy of N fertilisation in conjunction with pruning and thinning of pine stands with marginal foliar N nutrition after canopy closure.However,broadcast application of N fertilisers at a young age when trees are too small to provide for rapid expansion of foliage mass will also have only a limited effect,although a targeted application of N at planting may be beneficial. From our analysis,it seems that broadcast fertilisation should be carried out when the stored C in a stand is about 10 t C⋅ha-1. At this point, the canopy is developed enough to allow rapid expansion of foliage in response to added N. This will occur at about age 7 years in well stocked stands, a little earlier on better sites or later on poorer sites,and a little later in low stocked stands. It will often coincide with a final thinning to waste in a conventional silvicultural regime, but a good response should also be attained in unthinned stands with this level of stored C. To achieve the full advantage of increased growth, there is a window of opportunity when N fertiliser will promote accelerated growth before canopy closure.This window appears to lie between C stocks of about 10 and 60 t C⋅ha-1.It may be beneficial to apply more than one application of fertiliser during this period as suggested by Woollons and Will(1975),especially at lower stand densities. Fertilisation following a late production thinning should also be beneficial as canopies can take several years to close following thinning. The period when a stand benefits from fertiliser application will be longer and the increase in C sequestration greater in lower stocked stands.Conversely,at higher stand densities the timing of fertilisation is more critical because of the shorter length of time available for the stand to respond to fertiliser application before canopy closure.We do not here consider the question of optimum stand densities in forests managed for C sequestration other than to note that higher levels of C sequestration in our trial occurred at higher stand densities,not only at young ages but even at age 24 years.This accords well with the analysis by Madgwick et al. (1977) who studied foliage mass in P.radiata pine between ages 6 and 22 and found that it diminished with reducing stand densities below 400 stems⋅ha-1. In conventional forest management, there is an upper limit to stand density due to the requirement to produce trees of good diameter within a short rotation length,but much higher stand densities may be optimal for maximising C sequestration.
Under the ETS scheme in New Zealand,N fertilisation could be quite profitable in P. radiata forests established after 1989 which are eligible for inclusion in the scheme.The current cost of aerial application of urea fertiliser is approximately$NZ 600 per tonne or$NZ 1.30 kg-1N,and at the current price of per ETS unit of approximately$20 per tonne of CO2,the cost of fertilisation will be covered by the value of sequestered C if the N to C conversion ratio is greater than 14 kg C⋅kg-1N, well below the range 43–65 kg C⋅kg-1N we found in our biosolids trial. This is the calculation for a ‘pure’ C forest established with no intention of harvesting.However,the profitability of fertilisation will be even better for a conventionally managed forest under the ETS.In this case,the decision of whether and when to harvest is governed by the value of harvested wood relative to the cost of repurchasing the ETS units associated with harvesting. If the value of wood is worth less than the cost of repurchasing units,an economically rational forest owner would not harvest,and the forest would remain a ‘pure’ C forest at least until it is profitable to harvest.However, if it is profitable to harvest,the net increase in wood value achieved from fertilisation comes at no cost if the cost of fertilisation has already been covered by the sale of additional C units from the improved growth realised within a few years of fertilisation.
Apart from economic considerations, the C payoff from fertilisation can also be extremely positive. An analysis by Albaugh et al. (2012)showed that the increased C sequestration achieved from fertilising pine plantations in southeast USA is typically 19 times greater than the C emissions from the production and aerial application of the fertilisers.However, over-use of N fertilisers in forests can also have adverse environmental effects.Nitrogen loses from forest systems are generally lower than from other land uses (Di and Cameron, 2002), although there is potential for increased leaching losses of nitrate and other forms of N following N fertilisation of forests(Davis,2014).Leaching of N into rivers and lakes can cause eutrophication resulting in excessive growth of algae and aquatic weeds,affecting fish populations and the recreational value of water. Contamination of drinking water supplies by nitrate can also cause health risks(Cameron et al., 2013).
5. Conclusions
Biosolids-applied trees had sequestered 40 t C⋅ha-1more than untreated trees at age 24 years,an increase of 18%.Age-shift analysis shows that fertilised trees sequestered 80 t C⋅ha-1two years earlier than unfertilised trees.Most of the increased C sequestration occurred between age 6 and age 17 years and the Standard rate gave the same increase in C sequestration as the High rate.An applied nitrogen(N)to C sequestration ratio of 43–65 kg C⋅kg-1N was achieved by fertilising a young P.radiata stand with N-rich biosolids during its canopy development phase on a lowmedium productivity sandy soil. Returns using more conventional N fertilisers are likely to be somewhat lower. Because their canopies took longer to develop, responses of tree growth and C sequestration to biosolids-derived N at low stand densities were greater and effective over a longer period of time than at higher stand densities.These findings will have important implications for developing forest management practices that improve C sequestration in young forests in New Zealand in particular and other countries in general under emissions trading schemes.
Funding
The Ministry of Business,Innovation and Employment,New Zealand provided funding (contract no. C03X0902) for this research. PF Olsen Limited, Tasman District Council and the Nelson Regional Sewerage Business Unit)provided in-kind support.
Availability of data and materials
The data that support the findings of this study are available from the New Zealand Ministry of Business,Innovation and Employment(MBIE),but restrictions apply to the availability of these data, which were used under license for the current study,and so are not publicly available.Data are however available from the authors upon reasonable request and with permission of MBIE.
Authors’ contributions
JX, MK and RM developed the idea and conjointly prepared the manuscript;MK designed analysis approach;JX collated the data;MK,JX and RM analysed the data.All authors contributed critically to the drafts and gave final approval for publication.
Ethics approval and consent to participate
Not applicable.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgements
The authors thank PF Olsen Limited, Tasman District Council, the Nelson Regional Sewerage Business Unit and the New Zealand Ministry of Business, Innovation and Employment for funding (contract no.C03X0902) or in-kind support; Doug Graham, Alan Leckie and David Henley for foliage and soil sampling and growth measurement; Peter Wilks at PF Olsen for trial maintenance; and all those who have contributed to the project over the years.
- Forest Ecosystems的其它文章
- Removing harvest residues from hardwood stands affects tree growth,wood density and stem wood nutrient concentration in European beech (Fagus sylvatica) and oak (Quercus spp.)
- Modeling of cold-temperate tree Pinus koraiensis (Pinaceae) distribution in the Asia-Pacific region: Climate change impact
- Carbon stocks in a highly fragmented landscape with seasonally dry tropical forest in the Neotropics
- Intraspecific variation in shoot flammability in Dracophyllum rosmarinifolium is not predicted by habitat environmental conditions
- Current climate overrides past climate change in explaining multi-site beta diversity of Lauraceae species in China
- Ecological niche modeling of the main forest-forming species in the Caucasus

