Removing harvest residues from hardwood stands affects tree growth,wood density and stem wood nutrient concentration in European beech (Fagus sylvatica) and oak (Quercus spp.)
Snjoy Roy, Jen-Michel Lebn, Bernhrd Zeller, Gregory vn der Heijden,Arnud Reichrd, Mrie-Christine Gehin, Philippe Sntenoise,c, Lurent Sint-Andre
a INRAE, BEF, F-54000 Nancy, France
b IGN, Laboratoire de l’Inventaire Forestier, Nancy, F-54000, France
c Universit′e de Lorraine, AgroParisTech, INRAE, SILVA, F-54000 Nancy, France
Keywords:Harvest residues Fagus sylvatica Quercus petraea Dendroecology Dendrochemistry Radial growth Wood density Tree growth Ring width Translocation
ABSTRACT Background:Higher exportation of harvest residues from forests due to increased demand for woody biomass,has reportedly diminished soil mineral resources and may lead to degraded tree nutrition as well as growth.However,as nutrients become less available in the soil, the remobilization of nutrients in biomass tissues (plant internal cycling) helps sustain tree nutrition. Our study aims to quantify the impact of Removing Harvest Residues and Litter (RHRL) during five years on tree growth, wood density, and stem wood nutrient concentrations in young beech and oak forest stands.Result: Our study found that, RHRL significantly decreased tree growth ring width by 14%, and wood density by 3%, in beech trees, in near bark rings. RHRL also significantly reduced nutrient concentration in near bark and near pith areas of both studied species. Mg, Na and S were found lower by 44%, 76%, and 56%, respectively, in near bark area of beech trees. In near bark area of oak trees, K, Ca, Mg, Na, S, and Fe were lower by 20%, 25%,41%, 48%, 41%, and 16%, respectively. K and Mg concentrations decreased more strongly in near pith area compared to near bark area suggesting internal translocation of these two elements.Conclusion:In beech trees,wood density proved to be an important factor while quantifying the effect of removing harvest residuals on tree growth and biomass. Soil nutrient loss intensified the remobilization of nutrients contained in older tree rings (close to the pith) towards newly formed rings (close to bark). In our study, in beech trees,K was found to be the most recycled major nutrient.These results demonstrate the potential of such analysis for providing valuable insight into the effect of RHRL in premature stands on the physiological adaptive strategies of trees and an indication of soil fertility status.
1. Background
Removing harvest or logging residues from the forest has been promoted as a supplementary bioenergy source in Europe and around the world.This practice derived from the response to alleviate anthropogenic pressure on fossil fuel consumption, to mitigate climate change effects,and to increase the contribution of renewable bio-resources to the energy mix. The EU policy for renewable energy, established in the EU Energy and Climate Change Package(CCP)and the Fuel Quality Directive(FQD),has promoted renewable energy,with a mandatory binding target of 20%in the overall energy mix of the EU by the end of 2020 and at least 32%by the end of 2030(Phillips et al.,2018).However,there has been ongoing controversies concerning this strategy, because a substantial amount of recent and previous researches have indicated that removing harvest residues from the forest can affect forest ecosystem functioning and productivity (e.g., Conifers: Helmisaari et al., 2011; Kaarakka et al.,2014;hardwood species:Vanguelova et al.,2010;Wall,2012;Tamminen et al.,2012;Hume et al.,2018;Maillard et al.,2019)by reducing nutrient availability in the soil(see metanalysis from Thiffault et al.,2011;Achat et al., 2015a; Egnell, 2017; Clarke et al., 2021). Most studies haveobserved the effects of removing harvest residues on tree diameter and/or height and/or volume growth. However, to our knowledge, no study has yet considered wood density as a contributing factor explaining the effect of removing harvest residues on tree biomass despite wood density is an equally important variable to translate tree volume into biomass. Due to highly associated costs of measurements, biomass estimates in practice rely on combining tree volume data with an average density value per species (IPCC, 2006), but different ecological factors such as water and nutrient availability, site elevation may strongly contribute to explaining variation of the inter and intra-specific wood density values (Castro et al., 2020; Kerfriden et al., 2021). Studies have indicated that the availability of soil nutrients affects the width of tree growth rings (e.g., Sheppard et al., 2001; Ponton et al., 2019). A sharp decline of soil nutrients was found to reduce diameter growth in hardwood species (Kint et al., 2012). Yet, little is known about the effect of nutrient availability on tree wood density.However,such findings can be important,not only to understand the relationship between tree growth and wood density and its contribution to wood carbon estimation (Kerfriden et al.,2021)in both conifer and hardwood species but also because wood density is an important parameter to produce desired quality timber,with higher mechanical properties(Saranp¨a¨a,2003).
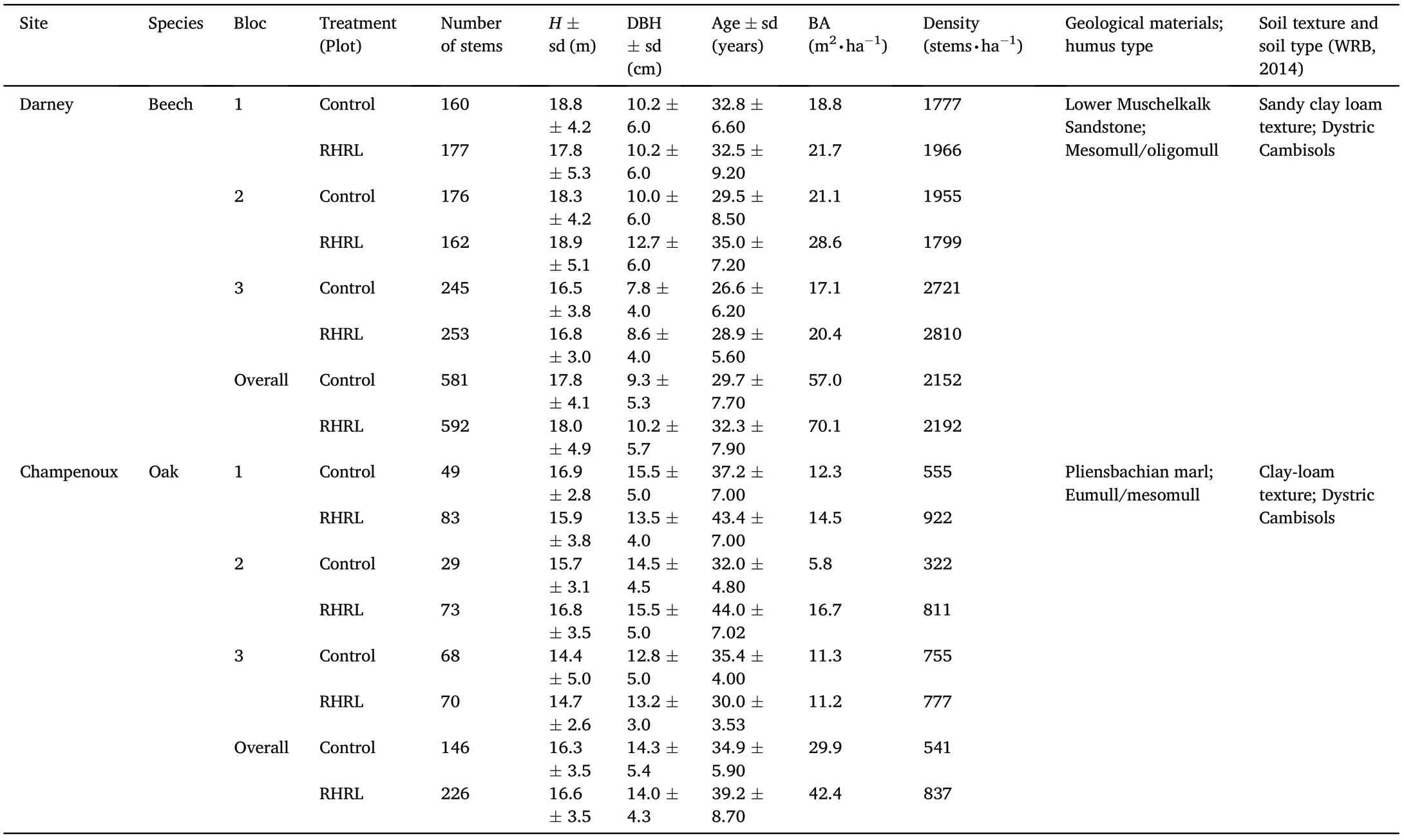
Table 1Forest inventory and soil information of the two sites. H denotes height (m)and BA is basal area(m2∙ha-1). Age is expressed as the number of years.
The pools of nutrients accumulated in aboveground biomass are crucial in maintaining tree physiology,vitality,and growth.Trees absorb most of their required nutrients from the soil and distribute them within different tree organs through the xylem sapflow. Soil being the mai n reserve of nutrients for trees,a scarcity in soil mineral pool,is expected to engender reduction of nutrient contents in the plant woody tissues.However, previous studies have shown that (i) part of nutrient cations(Mg, Ca, and K) in biomass tissues are stored in an adsorbed/exchangeable and mobilizable form(van der Heijden et al.,2015,2017),(ii)trees may remobilize nutrient reservoirs from dead/dying tissues, a process known as plant internal cycling(L′evy et al.,1996; Meerts,2002). Some studies have also shown that trees may compensate for low nutrient availability in the soil and thus sustain their growth by intensifying the plant internal cycling (e.g., Weatherall et al., 2006; Weatherall et al.,2006a,2006b,Achat et al.,2018b).
The objective of this study was to identify and quantify how the reduction of nutrient availability in the soil,resulting from the removal of harvest residues and litter,affects tree growth,wood density and nutrient concentration in tree stems as well as the remobilization of nutrients stored in the pith of the stem wood.For this purpose,we studied two sites belonging to the MOS(Manipulation de la Mati‵ere Organiques des Sols)network(Akroume et al.,2016),which was established in 2013 to study the impact of removing harvest residues on ecosystem functioning,including the effects on soil biodiversity(Elie et al.,2018;Maillard et al.,2019).We analyzed the oldest(close to pith)and the newly formed(close to bark) tree rings in increment cores extracted from European beech(Fagus sylvatica)and oak(Q.petraea, Q.robur) trees at both sites.
The hypotheses we have formulated, on how trees respond to soil fertility loss due to intensive harvest residue and litter removal are (a)tree rate of height and diameter increment decreases, (b) the density of newly formed tree rings decreases (we expect a decline in ring-width,which is expected to further affect wood density, in both species) and(c)internal reservoirs of nutrients stored in the tree stem are remobilized to compensate the decreased availability of nutrients in the soil.
2. Materials and methods
2.1. Study sites

Fig. 1. The effects of treatment, i.e., RHRL-removing harvest residues and litter (percentage change in the RHRL plots calculated with Control plots as denominator), after 3 years of the establishment of experimental plots, on the soil physical and chemical properties at the site Darney in two different soil layers (0–5 and 5–10 cm). The value in the [] represents the P-value of the Pearson correlation. P-values,only for significant parameters are included (*P ≤0.05;**P ≤0.01).Abbreviations:N,total nitrogen;C,total carbon; SOM, soil organic matter; P, available phosphorus; CEC, cation exchange capacity.
This study was carried out in north-eastern France(Champenoux and Darney)at two experimental sites,which belong to the experimental plot network named MOS (Manipulation de la Mati‵ere Organiques des Sols:https://www6.inrae.fr/in-sylva-france/Services/IN-Situ/Reseau-MOSManipulation-de-la-matiere-organique-du-sol). The forest stands at the site in Champenoux is dominated by oak(Quercus petraea L.and Quercus robur L.)mixed with hornbeam(Carpinus betulus).In contrast,the Darney site is mainly composed of European beech (Fagus sylvatica L.). The stands at both sites were naturally regenerated. The average age of the stands was 37(sd =7)years for the oak trees and 31(sd =8)years for the beech trees (Table 1). Prior to the establishment of the experimental plots, standard forest management practices were carried out at both sites following local management plans: selective thinnings occurred every 7 years and the harvested stems were extracted from the stands leaving harvest residues on site. The different treatment plots were installed just after a thinning in November 2013 in Champenoux and January 2014 in Darney. Since then, no thinning operations have been carried out.The full description of the experimental design can be found in Akroume et al.(2016).
2.2. Experimental design and treatments
In this study, we focused on two contrasted treatments: (1) conventional forest management(control treatment), in which the logging residues(branches under 7-cm diameter)and litter were kept on the forest floor after harvesting, and (2) RHRL (Removing Harvest Residues and Litter),every year the fallen tree residues and litter were removed using a leaf blower or manually leaving a bare forest soil surface. Although,complete removal of litter is not a common practice,but an experimental one, its purpose is to achieve a nutrient and carbon loss equivalent to repeated intensive harvesting and biomass exportation, only over a shorter time period. This treatment is thought to heavily impact many ecosystem features and properties,i.e.,forest productivity,nutrient loss,etc. As the loss in leaf nutrients removal can supplant or exceed the amount accumulated in residual barks,twigs,and branches altogether for N,Mg,or P as shown in Mendham et al.(2014).Each experimental site covered 2 ha and treatment plots(area 40 m×40 m each)were assigned randomly in a bloc design. Blocs and plots were defined and inter-plot homogeneity was checked using near- and mid-infrared spectroscopy in soil and litter samples (i.e., there were no statistically significant differences between plots and blocs before the treatments were applied).At each site, treatment plots were replicated three times, leading to 6 studied plots(3 blocs×2 treatment plots)per site and 12 studied plots in total.Sampling and measurements were carried out within a 30 m×30 m subplot in each of the treatment plots to avoid potential border effects.
2.3. Soil information
The soil at both Darney and Champenoux was described as Dystric Cambisols according to the World Reference Base for Soil (FAO, 2014).For all stands,the humus form was a mesomull(Jabiol et al.,2009),with clay loam and sandy clay loam texture in the respective sites (Table 1).Generically,the soil fertility of the Darney site was comparatively poorer than the Champenoux site (Akroume et al., 2016). To evaluate the treatment effect,i.e.,RHRL on soil properties,a study was conducted by Maillard et al.(2019)at the Darney site in beech trees after 3 years of the establishment of experimental plots.The study found that the total soil N,soil organic C,soil organic matter(SOM),and pH were not impacted by removing harvest residuals,but removing harvest residuals significantly affected the other soil parameters measured in the 0–5 cm soil layer(Fig. 1), leading to an average decrease of available P concentration(-30%),CEC(-12%)and C/N(-8%)in the RHRL plots compared with the control plots. The study also found a significant drastic effect of removing harvest materials upon the microbial metabolic profiles and enzymatic activities in both 0–5 and 5–10 cm soil layers.
2.4. Tree sampling
Tree diameter at breast height (DBH at 1.3 m) was measured for all the trees inside the 30 m×30 m subplots with a diameter tape(12 plots,3118 trees in total). Trees were selected randomly for height measurement based on 5 cm2-interval basal area classes. The height of approximately 35 trees per plot(452 trees in total)were then measured using a vertex(brand:Hagl¨of–Sweden,product code:15-105-1008).
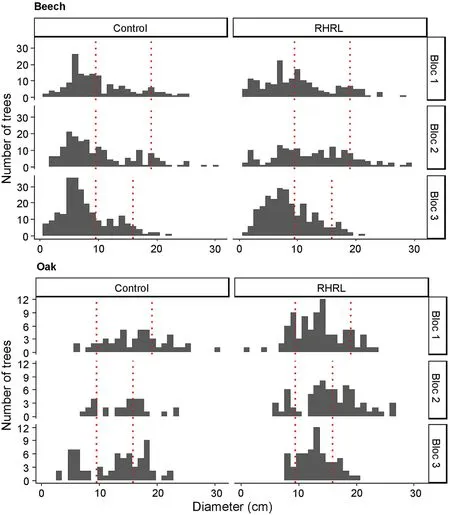
Fig.2. Distribution of tree densities to residue neutral blocs: trees with diameter outside the red lines were selected for sampling in each blocs. For the oak trees,the limits indicated by the red lines were 9.5 cm ≥selection ≥19.1 cm;9.5 cm ≥selection ≥15.9 cm;and 9.5 cm ≥selection ≥15.9 cm for bloc 1, bloc 2 and bloc 3, respectively. For the beech trees, the limits were 9.5 cm ≥selection ≥19.1 cm;9.5 cm ≥selection≥19.1 cm; and 9.5 cm ≥selection ≥15.9 cm. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Increment cores of 4.3-mm diameter were collected at breast height from trees using a gasoline-driven increment borer. To avoid heterogeneity between treatments,the sampled trees were selected based on their diameter distribution (Fig. 2). At the Champenoux site, 10 oak tree increment cores (5 from dominant trees and 5 from suppressed trees)were collected from each plot,leading to 30 cores per treatment,whereas 15 beech increment cores were collected from each plot at the Darney site(10 dominant and 5 suppressed,leading to 45 cores per treatment).Cores from both dominant and suppressed trees were taken, to assess the treatment effect according to the tree social classes. Sample chemical analysis required a minimum core length of 5 cm. For trees with a diameter less than 10 cm,the increment borer crossed the pith point.The average length of the increment cores was 10.6 cm (sd =4 cm) for the beech trees, and 9.5 cm (sd =3 cm) collected for the oak trees. The samples were stored in honeycomb polycarbonate panels immediately after extraction for further ring width, wood density, and nutrient analysis.
2.5. Ring width, wood density, and nutrients measurement
The honeycomb polycarbonate panel plates were scanned (device:printer Toshiba e-studio 2518A;resolution:600×600 dpi)immediately after extraction to obtain an image (dimension: 5672 × 2953 pixels) of the green core length. The samples were oven-dried for 24 h at 103°C and scanned again.The length from the pith to the bark of the oven-dried core images was measured using ImageJ software. We repeated the measurement on the green core images in case the pith location was difficult to identify in the images of the oven-dried samples, and shrinkage was taken into account.The shrinkage was calculated from the images, in percentage, as the difference between the green core length and the oven-dried length of a full-length core,over the green length.In addition,the number of rings from the pith to the bark in the oven-dried image was counted via microscopy to determine the tree age in the number of years.
To assess treatment effects on tree ring width, nutrient content, and wood density between the near pith and near bark area,we cut a 2.5-cm long sample from both ends of each core(Saint-Andr′e et al.,2002).This length was selected based on preliminary tests and to ensure that a minimum of 160 mg of wood powder was obtained after crushing the samples.In trees<10 cm diameter,the near pith samples were extracted keeping the pith location at the middle point. There was a total of 300 tree core subsamples (150 trees). The number of rings was counted for each tree core subsample to determine average ring widths near the pith and near the bark. The wood density (kg∙m–3) of the subsamples was measured for the whole core and for the 300 subsamples based on the oven-dried cores using the Xylotech platform,INRAE,Champenoux,with a medical X-ray scanner (model: GE BrightSpeed Excel). The polycarbonate plates(each containing 15 cores)were assembled by means of an adhesive tape to form packages of 17 plates.Six packages were passed together through the medical CT scanner following the specification made in Freyburger et al.(2009).For each package of 17 plates,the X-ray scanner software produces about 400 CT images(512×512 pixels each),with a slice thickness of 0.625 mm.A specific software was developed to detect automatically individual alveolar cells in a plate, containing the wood cores and to compute the wood density of each sample (Jacquin et al.,2017).Detailed methodology and specifications of the scan options and image properties are described within the XyloDensMap project(Leban et al.,2017;Jacquin et al.,2017,2019).

Fig.3. The effect of treatment(i.e.,control-classical silviculture and RHRL-removing harvest residues and litter)on tree diameter at breast height(cm)and height(m)in the beech and oak trees. The effect is shown with mean indicators for the average value of diameter and height.
The subsamples were then digested using an Anton Paar Microwave Reaction System MultiWave 3000 with nitric acid,and the analysis of the major elements- Al, Ca, Fe, K, Mg, Mn, Na, and S was carried out by inductively coupled plasma atomic emission spectrometry (Agilent Technologies,700 series ICP EOS)after digestion.P could not be assessed in our study, as the concentration in wood recorded was below the detection limits.
2.6. Data analysis
Minimum (Min), maximum (Max), mean, median, and standard deviation(SD),values were calculated for both explanatory and dependant variables.Tree basal area(m2∙ha-1)and stem density(number of stems per ha)were calculated for each bloc,and site for both species.Treatment effects on tree height and diameter were assessed with boxplots and height-diameter curves for each species.To determine the treatment effect on the properties of near bark and near pith samples at each site,generalized linear regression models (GLMs) were performed using R software (R core team, 2017) for the ring widths, wood densities, and nutrient concentrations. Because the study focuses on harvest residues export, treatment effects were always considered a fixed variable in all models.The goodness-of-fit of the models was assessed with the R2and RMSE indicators. The final selection of the model with the best fit was made using the Akaike information criterion (AIC), based on maximum likelihood;the lower the value is,the better the model fit.The variation explained by each significant parameter in the selected models was calculated from their coefficient values.
3. Results
3.1. Effect on tree growth
For the height and diameter,we found no RHRL effect for either the beech trees at the Darney site or the oak trees at the Champenoux site(Fig.3).For the beech trees at the Darney site,the growth ring width in the RHRL plot was significantly lower (p =0.1), on average 0.18 mm smaller (14% relative decrease) in the newly formed rings compared to the control plots(Fig.4).We also observed a significant decrease(17.37 kg∙m–3or 3% relative decrease, p =0.05) in wood density between RHRL and control plots(Fig.4).
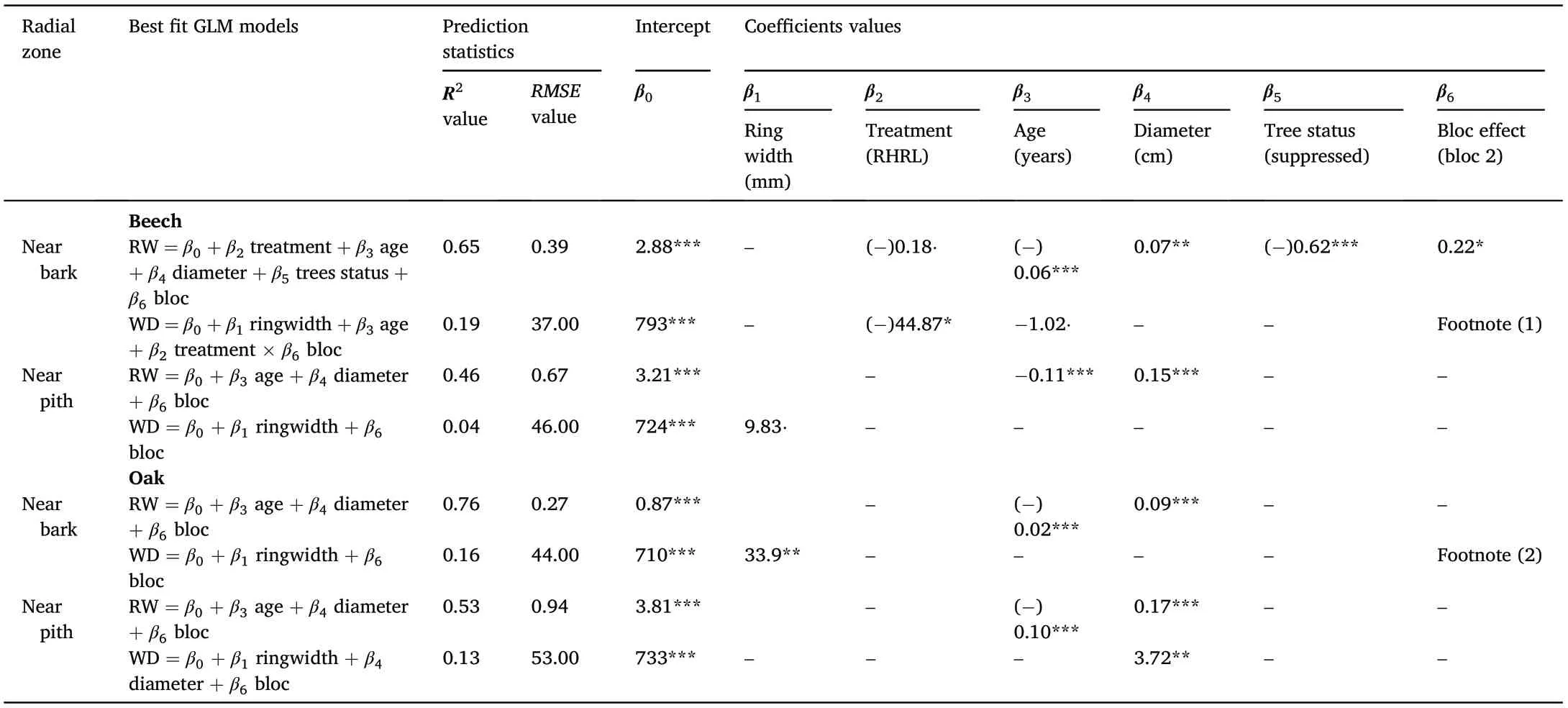
For the oak trees at the Champenoux site, growth ring width in the RHRL plot was also slightly smaller in the newly formed rings compared to the control plot but this difference was not statistically significant(Fig. 4). There was no treatment effect on wood density in oak trees.However,for near bark samples,the growth ring width was statistically significant (p =0.01) in the generalized linear model predicting wood density(Table 2).Among the total variation of 25%was explained by the model,over 15%came from ring width alone(see Table 2).
3.2. Effect on stem wood nutrients
In the beech trees of the Darney site, the concentrations of Mg, Na,and S in the near bark area were significantly lower in the RHRL plot,by 44%, 76%, and 56%, respectively; the concentrations of K and Ca were also lower, but non significantly. Al, Mn, and Fe concentrations were significantly higher,by 65%,26%,and 137%,respectively.
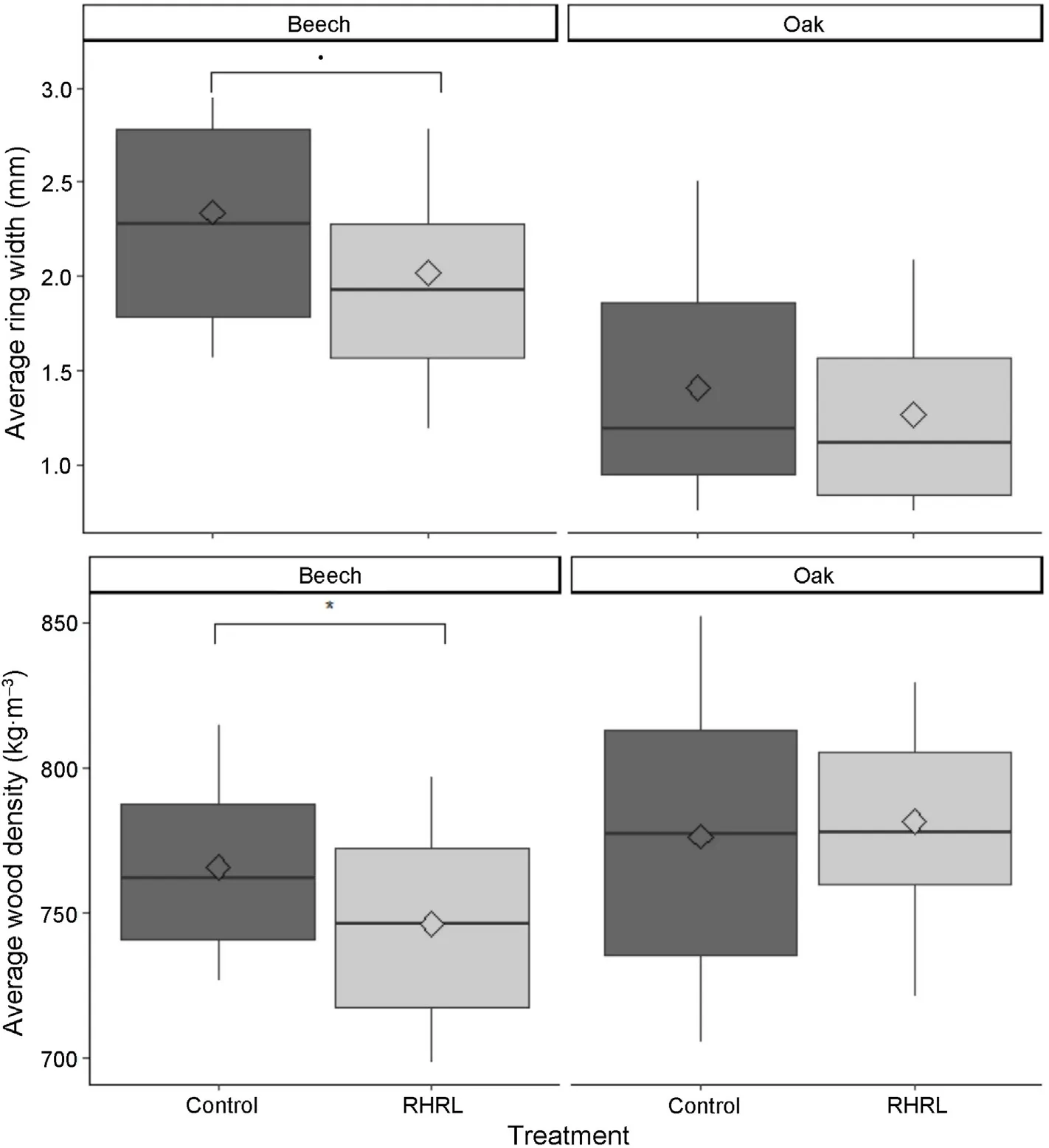
Fig.4. The effect of treatment(i.e.,control-classical silviculture and RHRL-removing harvest residues and litter)on beech and oak tree ring width and wood density at the newly formed rings. The effect is shown over the population with mean indicators. The P-value of the Pearson correlation, only for significant parameters are included above the corresponding boxplots (. P ≤0.1; *P ≤0.05; **P ≤0.01).
In case of oak trees of the Champenoux site,the K,Ca,Mg,Na,and S concentrations in the near bark area were significantly lower in the RHRL plot, by 20%, 25%, 41%, 48%, and 41%, respectively. Fe, Mn or Al concentrations were also lower in the RHRL plot but the differences were not statistically significant.
Near pith area in the RHRL plots,the concentrations of K,Mg,Na,Al,and S were significantly lower than the Control plots in the beech trees(Ca was lower,but non-significant)and the concentrations of K,Ca,Na,and Fe were significantly lower in the oak trees (Mg, S and Al concentrations were lower,but non-significant)(Table 3.1 and 3.2).
The relative decrease of nutrient concentration (Control – RHRL)/Control), calculated from the mean values, revealed, in the beech trees the relative decrease (in percentage) of near pith concentrations was 370%,8%,and 47%,for K,Mg and Ca respectively,and was greater than that of near bark tissues(Fig.5a),indicating relocation of nutrients from pith tissues in the RHRL plots.This observation was not repeated for oak(Fig. 5b). We also calculated the mean difference in the nutrient concentration between the near bark and near pith tissues(‘near bark’minus‘near pith’) in respect to treatment and species, and for beech trees, we found this difference to be significantly greater in K(<0.001)and in Mg(but non-significant), in the RHRL plots compared to the control(Table 3.2).
Only for the oak trees in the Chamepnoux site,including the diameter improve the goodness-of-fit of the model predicting the treatment effect on nutrient concentrations in the near bark area.We found a significant positive response from diameter to explain nutrient concentrations regarding all the elements analyzed except for Ca and Al. The response from diameter was highest for K and S accounting for approximately 82%and 63% of the total variation explained by full respective models(Table 3.2).
4. Discussion
4.1. Tree growth and wood density
In our study, RHRL treatment did not affect the tree dimensions(height or diameter)after 5 years of treatment.Nevertheless,we showed that the RHRL treatment decreased tree growth(newly formed rings)in the beech plots. A number of studies have also reported that increasing biomass exportations from forest stands resulted in a decrease in the growth rate of trees(see Achat et al.,2015a;Achat et al.,2015b;Chave et al.,2009).However,these studies reported growth rates of very young trees after a clear-cut,while our study focused on 30-year-old trees.Even if we found a significant difference in the width of the last formed rings,the amplitude of the difference is low by comparison with the diameter incremented before the treatment. This explains why we found no significant difference in DBH between treatments. We expect a significant difference after another five years of treatment. The reduced growth in beech trees is most likely due to the decreased availability of nutrients in the soil.For the Darney site,Maillard et al.(2019)showed that the RHRL
Footnote(1):In the model,the coefficient values for‘treatment×bloc interaction’were 44.87*and 44.29*in the RHRL‘bloc 2’and‘bloc 3’respectively.This indicates that the effects in bloc 2 and bloc 3 from RHRL treatment increase by approximately 44.87 and 44.29 units compared to the effects from control treatment of the respective blocs.Footnote(2):the coefficient value for‘ringwidth×bloc interaction’was-58*.This indicates in the respective bloc,the effect of ringwidth on wood density is lower by 58 unit in response to the RHRL treatment compared to control.
Table 3.1 The best GLM models fitted for each of the nutrients observed in our study,in the near bark and near pith area of beech and oak trees.The full models are enlisted here with the indication of intercept(β0)and other variable coefficients(β1,β2,β3… etc.). The value of the intercept and coefficients for each model are provided in Table 3.2. Prediction statistics (R2and RMSE value) for each best fit model are included in Supplementary materials Table A1.treatment significantly decreased the contents in soil nutrients and the cation exchange capacity.Other studies have reported that depletion or enrichment of soil nutrients conveys a direct relationship with tree ring width and wood density,in oak(e.g.,Berg‵es et al.,2008;Kint et al.,2012;Ponton et al., 2019) and beech(e.g.,Elhani et al.,2005) trees.
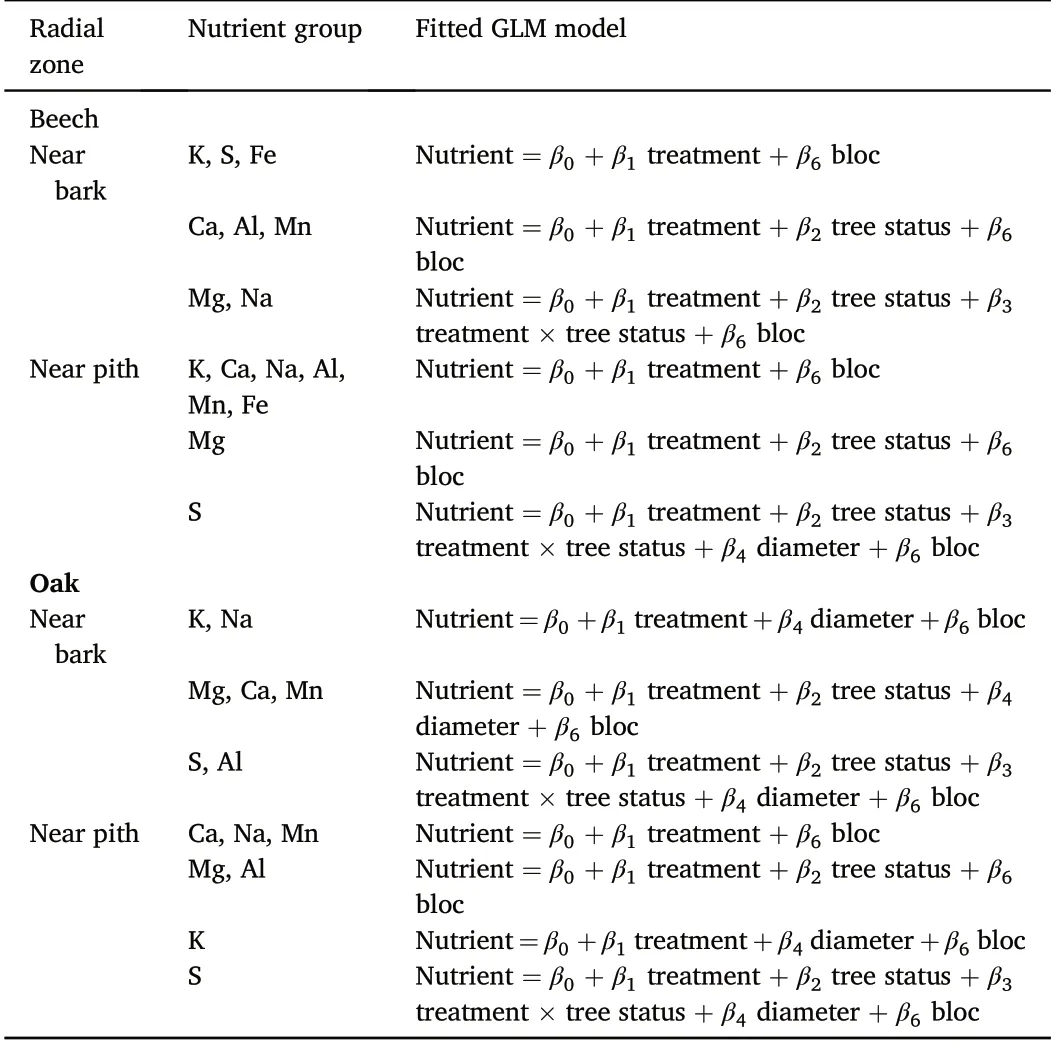
Radial zone Nutrient group Fitted GLM model Beech Near bark K, S, Fe Nutrient =β0 + β1 treatment +β6 bloc Ca, Al, Mn Nutrient =β0 + β1 treatment +β2 tree status +β6 bloc Mg, Na Nutrient =β0 + β1 treatment +β2 tree status +β3 treatment × tree status +β6 bloc Near pith K, Ca, Na,Al,Mn, Fe Nutrient =β0 + β1 treatment +β6 bloc Mg Nutrient =β0 + β1 treatment +β2 tree status +β6 bloc S Nutrient =β0 + β1 treatment +β2 tree status +β3 treatment × tree status +β4 diameter + β6 bloc Oak Near bark K, Na Nutrient =β0+β1 treatment+β4 diameter+β6 bloc Mg, Ca, Mn Nutrient =β0 + β1 treatment +β2 tree status +β4 diameter + β6 bloc S, Al Nutrient =β0 + β1 treatment +β2 tree status +β3 treatment × tree status +β4 diameter + β6 bloc Near pith Ca, Na,Mn Nutrient =β0 + β1 treatment +β6 bloc Mg, Al Nutrient =β0 + β1 treatment +β2 tree status +β6 bloc K Nutrient =β0+β1 treatment+β4 diameter+β6 bloc S Nutrient =β0 + β1 treatment +β2 tree status +β3 treatment × tree status +β4 diameter + β6 bloc
In our study, the RHRL treatment decreased the rate of ring width increment and wood density in oak trees but non-significantly. In general, tree growth of diffuse-porous species such as beech is found to be more responsive to stress than ring-porous species such as oak (Meyer et al., 2020). This may explain the differences observed in this study between the beech stands at the Darney site and the oak stands trees at the Champenoux site. This difference could also be explained by the differences in soil nutrient availability between the two sites. The availability of soil nutrients at the Darney site was lower than that of the Champenoux site.This may have led to more acute effects at the Darney site.For both oak and beech trees,the RHRL group is overall older,and the stem density is substantially higher in oak plots 1 and 2(Table 1).The higher stem density might have resulted in reduced rate of radial growth,although non-significant, in those respective plots (Supplementary materials, Fig.S1).However,no discernible interaction between treatment and age was detected among plots in our models.
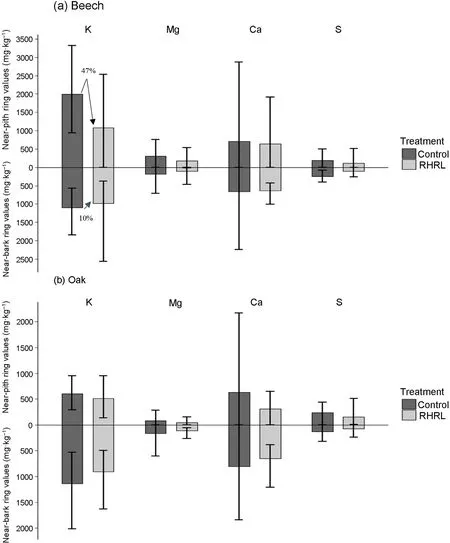
Fig. 5. Nutrient concentration (mg∙kg–1)of K, Mg,Ca,S occurring at the near pith and near bark tissues in(a)beech and(b)oak trees in respect to treatments, i.e.,control-classical silviculture and RHRL –removing harvest residues and litter. The number in (%) indicates relative decrease of the corresponding nutrient concentration (mean values) calculated as (Control-RHRL/Control) in the near pith and near bark zone.
Wood density variability is found to be less affected by radial growth components in the diffuse-porous species,compared to ring-porous species (Bouriaud et al., 2004; Diaconu et al., 2016). Along with other components such as cambial age and climatic variables,ecological factors(e.g., soil fertility nutrients, etc.) are also found to explain variability in radial growth and wood density.However,in the related studies,factors such as climate, water, and nutrients were not explicitly distinguished(Berg‵es et al., 2008), and site condition variability was largely not accounted for; for instance, in most cases, extreme site conditions were not sampled or were undersampled(e.g.,Polge and Keller,1973;Becker,1979; Guilley et al., 2004). In our study, for the beech trees, the ring width in the treatment plots (i.e., RHRL) explains less than 1% of the wood density reduction in the newly formed rings; to the remaining variation(of the 2.56%observed)comes from the treatment itself(after the ring width effect is removed). There might be a modification of the cell properties in addition to the reduction in the ring width. However,additional anatomical studies are required to verify this hypothesis.This could not be conducted in our study because the subsamples were milled to analyze their nutrient content.It is also likely that a 5-years period of intensive harvest might not be long enough to observe the effect and distinguish the variability in wood density among species; however,preliminary effects were seen in beech at the Darney site,suggesting that this species may be used as a sentinel.
4.2. Nutrient status
Two particular phenomena about the dynamics of nutrients as a consequence of removing harvest materials could be identified from our study:(i)there was a sharp decrease in nutrients for both near bark and near pith regions in the treatment plots and (ii) the results suggest that certain nutrients were translocated from the near pith region to the nearbark region in the beech trees to compensate for the lower availability of nutrients in the soil.
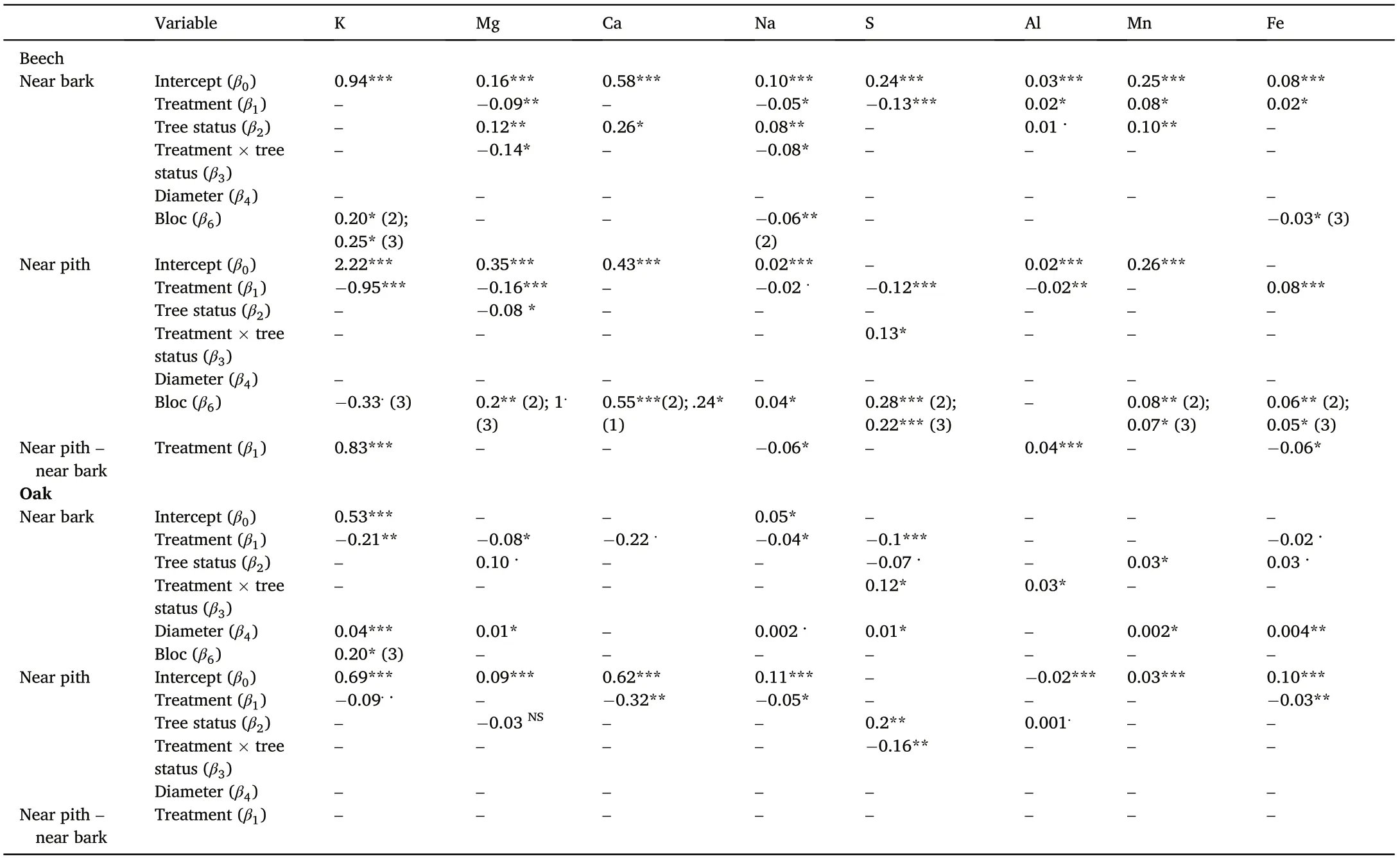
Table 3.2 Intercept and coefficient values from the best fit GLM models to determine the effects of treatment (i.e., control-classical silviculture and RHRL-removing harvest residues and litter)and other variables on the nutrient concentrations(g∙kg-1)in the stem near bark and stem near pith regions in the beech and oak trees.RW is the ring width(mm),‘diameter’is diameter of the tree at breast height(cm),‘tree status’is a binary variable–dominant or suppressed.For the‘treatment’and‘tree status’variables,the values are presented in response to RHRL and suppressed trees respectively.For block effect,the values inside the first bracket indicates corresponding number of the bloc,where the effect was found(e.g.,bloc 2 is presented as“(2)”).Significance levels of the p values:0,‘***’=0.001,‘**’=0.01,‘*’=0.05,and‘.’=0.1.Prediction statistics(R2 and RMSE value) for each best fit model are included in Supplementary materials Table A1.
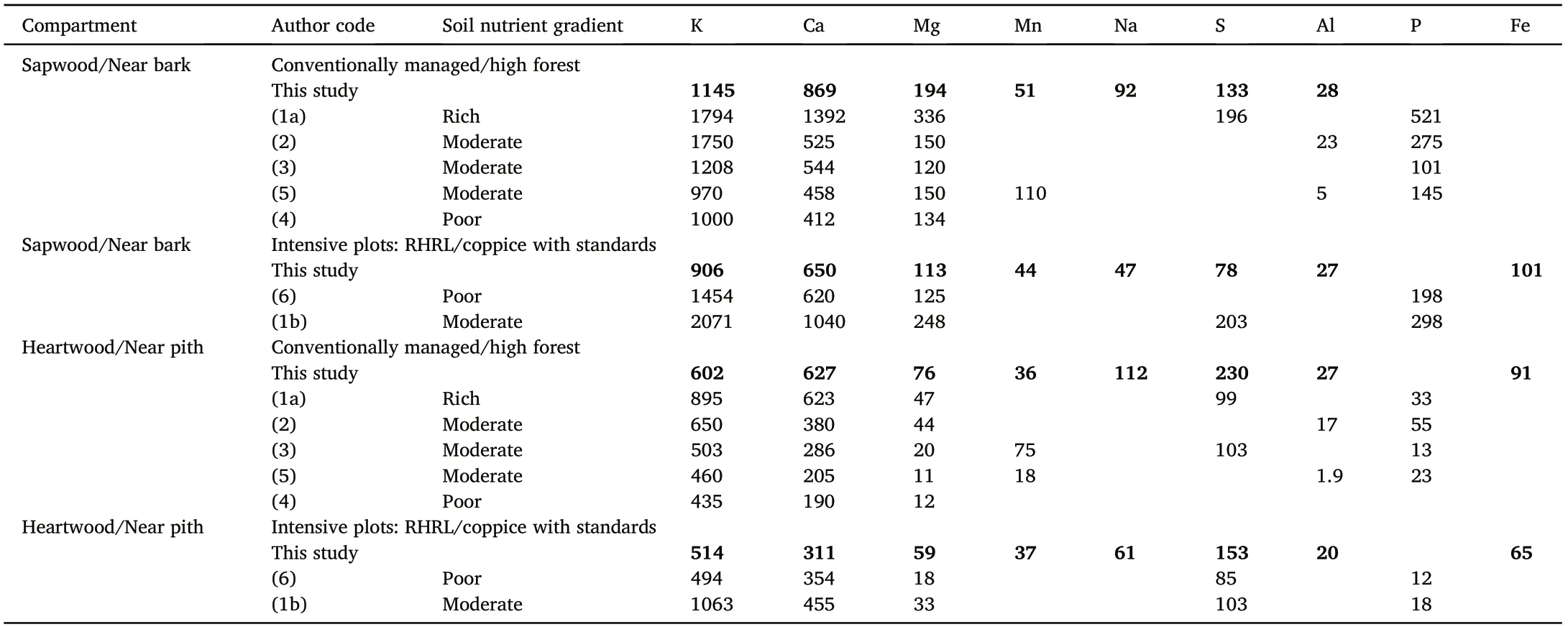
Table 4 Comparison of the nutrient concentrations(mg∙kg–1)in the stem sapwood/near bark and stem heartwood/near pith zones of the oak trees(Quercus spp.)sampled in our study with those from the reference studies. The data were assembled along with information on the soil nutrient gradients found in the reference studies. Detailed information on the authors, associated stands and site quality for each reference study used is provided in table format in Supplementary materials Table A2.
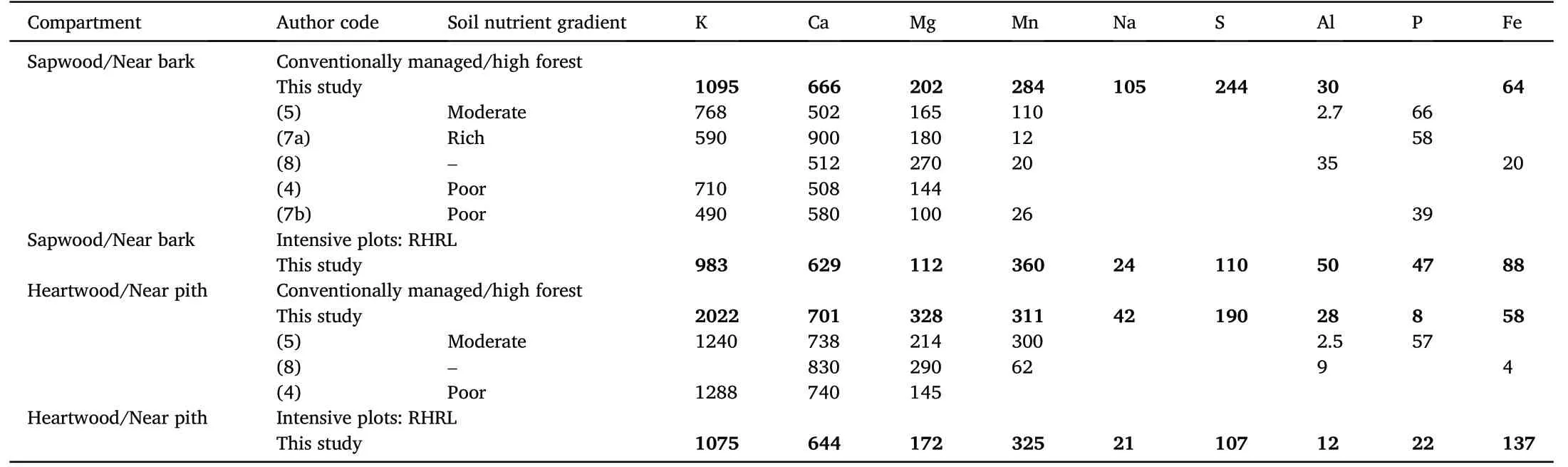
Table 5 Comparison of the nutrient concentrations(mg∙kg–1)in the stem sapwood/near bark and heartwood/near pith zones in the beech trees(Fagus sylvatica)sampled in our study with those from reference studies. The data were assembled along with information on the soil nutrient gradients found in the reference studies. Detailed information on the authors, associated stands and site quality for each reference study used is provided in table format in Supplementary materials Table A2.
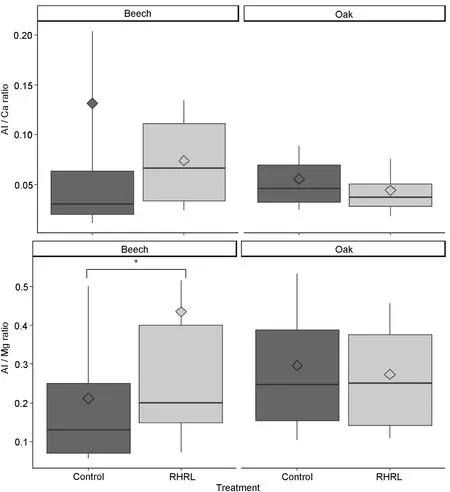
Fig.6. Indicator of soil acidity:Al/Ca and Al/Mg ratio in response to treatment(i.e.,control-classical silviculture and RHRL–removing harvest residues and litter)for each species seen at the newly formed rings(near bark area),with mean indicators.The P-value of the Pearson correlation,only for significant parameters are included above the corresponding boxplots (∙P ≤0.1; *P ≤0.05; **P ≤0.01).
The loss of nutrients in the soil as a consequence of removing harvest materials from forests had been reported in many parts of the world.Tamminen et al. (2012), Brandtberg and Olsson (2012), Kaarakka et al.(2014),and Vangansbeke et al.(2015)found depletion of one or multiple soil base cations(K,Ca,and Mg)due to whole-tree harvesting(WTH)in the Scandinavian trial studies. The same was also reported in North America (e.g., Ponder et al., 2012, from the North American long-term soil productivity (LTSP) network; Johnson et al., 2015; Johnson et al.,2016)and also from the tropical studies(see workshop proceedings from Nambiar, 2008; Kumaraswamy et al., 2014). The concentration of nutrients in the stem heartwood can act as an indicator of the nutrient status in the soil (L′evy et al., 1996). We may assume that the loss of nutrients from trees in response to our treatment, was a consequence of losing nutrients from the soil,due to removing harvest residues.Maillard et al.(2019) (see subsection 2.2) conducted a parallel study on the same experimental site and found significant loss of soil nutrients and cation exchange capacity (CEC) in treatment plots at the Darney site. There were few/no cases in the existing literature that studied the harvest removal effects on wood chemistry and nutrient translocation, but for both species,the wood nutrient values reported in our study were closer to those, where trees were growing in moderate to poor soil fertility(Tables 4 and 5).
In the RHRL plots,the relative decrease of near pith concentrations of K was above threefold higher than that of near bark tissues in beech trees.For Mg and Ca, the relative decrease was also comparatively higher by 8% and 47% respectively. Besides, the difference in nutrient concentration(‘near bark’minus‘near pith’)was also significantly greater for K(p< 0.001) and for Mg (non-significant) in RHRL plots compared to the control plots. This trend indicates that these elements may have been internally translocated from the near pith tissues to the near bark tissues,probably to compensate for the nutrient loss, as described by Meerts(2002). The difference was not significant for Mg, with a 54% proportional increase computed from the mean values. However, the internal compensation might not be as efficient for Mg as it is for K,and Mg was still significantly lower in the near bark samples from the RHRL plot than it is in the samples from the control plots. However, our study supports the hypothesis stated by Penninckx et al.(2001)that the efficiency of the resorption of Mg is higher in sites with the lowest availability, as a possible mechanism to compensate for the deficit from soil resources.Calcium, which is less mobile than Mg or K in trees (Mc Laughlin and Wimmer, 1999; Lautner and Fromm, 2010), had a decreasing trend regarding treatment and also for the abovementioned difference value.Ca is found to be the least translocated element in trees, if not translocated at all(e.g.,Colin-Belgrand et al.,1996;Fife et al.,2008)and often reported to be immobilized in great portion in the tree bark and stem wood tissue (Ferguson, 1980; Katainen and Valtonen, 1985). However,part of the Ca in aboveground biomass may be stored in an exchangeable/adsorbed and mobilizable form(van der Heijden et al.,2015,2017).Overall, in our study, the behaviour of Mg and Ca were similar in the RHRL plots in respect to remobilization from the near pith tissues. The Sulphur is found to be extensively lost from both the near bark area(55%)and near pith area(45%)in the beech trees and from the near bark area (41%) in the oak trees. The forest floor is recognized as the major pool of S available to trees (Cronan et al., 1978; Yanai, 1998), and the availability of S in a soluble form is highly correlated with fungi and bacterial interactions in forest soils (Strickland and Fitzgerald, 1984).Thus, the removal of soil litter, as well as the significant reduction in topsoil enzymatic activities due to the removal of harvest residues(Maillard et al.,2019),can explain the extensive loss of S in the treatment plots.
In the oak trees, the concentrations of K, Ca, Mg, Na and S were significantly depleted in both the near bark and near pith tissues(except for Mg and S in the heartwood) in the treatment plots. While the concentrations of K,Ca,and Mg were found to be significantly higher in the near bark area than that in the near pith area, which can indicate potential nutrient translocation (L′evy et al., 1996; Andr′e and Ponette,2003),but these trends were not significant of the treatment.In contrast to most existing literature(Peri et al.,2006;Andr′e et al.,2010;Peri and Lasagno, 2010), we found a positive effect of tree diameter on the nutrient concentration in the oak near bark zone, but this could be because our stands are much younger than those assessed in published studies.Colin-Belgrand et al.(1996)found the same effect on stemwood in relatively young chestnut trees.
After 3 years of carrying out treatments in the same experimental plots as those used in our study,Maillard et al.(2019),did not find any change in the soil pH of the treatment plots,but the CEC of the soil was significantly decreased. At present, after 5 years, we have found a significant increase in the ratio of Al/Mg(p =0.001)in the treatment plots(Fig. 6), which potentially indicates increased acidity in the soil (L′evy et al., 1999). The aberrant increase in the concentration of Fe in the treatment plots may also be related to high soil acidity (Meisch et al.,1986);however,this may be more likely due to instrument error related to the use of an increment borer to collect the stem wood samples(Augusto and Bert,2005).
5. Conclusion
We found that removing harvest residues did not affect tree dimension(height and diameter)in the premature oak and beech stands in just 5 years of treatment, but the dendroecological analysis of the tree increment cores confirmed a significant reduction in rate of radial growth and wood density in the newly formed rings of the beech trees.Nutrient concentrations were also reduced especially in the near bark tissues(and mostly for the base cations important to trees) in both species. In addition, the dendrochemical analysis has provided insight relating tree physiological adaptation (nutrient translocation) against poor site fertility and an indication of the acidity in the soil.
Authors’ contributions
Jean-Michel Leban, Laurent Saint-Andre and Bernhard Zeller conceived and designed the experiment, edited and reviewed the manuscript draft.Sanjoy Roy contributed the study design,collected and prepared the data,analyzed the data,conducted laboratory experiments,prepared figures and tables, drafted the manuscript. Gregory van der Heijden edited and validated the manuscript with critical comments and reviewed the manuscript draft. Arnaud Reichert contributed the data collection and field visits. Marie-Christine Gehin contributed the laboratory analysis. Philippe Santenoise contributed the data analysis. All authors checked and approved the final manuscript.
Funding
This work was supported within the XyloDensMap project, INRAE funded by the French Ministry of Agriculture under the convention n°A6.01/2017.
Availability of data and materials
The data set generated for the study area is available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Acknowledgments
The authors are grateful to Dr. Serajis Salekin and Jeremie Bel for their insightful comments and kind support at different stages of the manuscript formation. This research was carried out in the laboratory Biogeochimie des Ecosyst‵emes forestier (BEF), INRAE (Institut national de la recherche agronomique),Champenoux,FR.The research benefited from the unit's established experimental sites and the in situ and laboratory facilities for data and sample collection as well as for spectrometry and mineral analysis. We also benefited from the Silvatech platform (a shared platform between Silva and BEF, INRAE) for X-ray scanning of wood cores and wood density measurements by image analysis.We also acknowledge the French National Research Agency through the Cluster of Excellence ARBRE (ANR-11-LABX-0002-01) and the mobile lab (MPOETE) of ANAEE-France (ANR-11-INBS-0001) for support on the experiment.
Abbreviations
IPCC Intergovernmental Panel on Climate Change
RHRL Removing Harvest Residues and Litter
CEC Cation Exchange Capacity
sd standard deviation
SOM Soil Organic Matter
WTH Whole Tree Harvesting
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://do i.org/10.1016/j.fecs.2022.100014.
- Forest Ecosystems的其它文章
- Modeling of cold-temperate tree Pinus koraiensis (Pinaceae) distribution in the Asia-Pacific region: Climate change impact
- Carbon stocks in a highly fragmented landscape with seasonally dry tropical forest in the Neotropics
- Intraspecific variation in shoot flammability in Dracophyllum rosmarinifolium is not predicted by habitat environmental conditions
- Current climate overrides past climate change in explaining multi-site beta diversity of Lauraceae species in China
- Ecological niche modeling of the main forest-forming species in the Caucasus
- Impact of nitrogen input from biosolids application on carbon sequestration in a Pinus radiata forest

