Ecological niche modeling of the main forest-forming species in the Caucasus
R. Pshegusov, F. Tembotova, V. Chadaeva, Y. Sablirova, M. Mollaeva, A. Akhomgotov
Tembotov Institute of Ecology of Mountain Territories of Russian Academy of Science, 37a I. Armand Street, Nalchik, 360051, Russia
Keywords:Distribution modeling Pure forests BAM diagram Maxent Environmental predictor
ABSTRACT Background: Ecological niche modeling of the main forest-forming species within the same geographic range contributes significantly to understanding the coexistence of species and the regularities of formation of their current spatial distribution. The main abiotic and biotic environmental variables, as well as species dispersal capability, affecting the spatial distribution of the main forest-forming species in the Caucasus, have not been sufficiently studied.Methods: We conducted studies within the physiographic boundaries of the Caucasus, including the Russian Federation, Georgia, Armenia, and Azerbaijan. Our studies focused on ecological niche modeling of pure fir,spruce, pine, beech, hornbeam, and birch forests through species distribution modeling and the concept of BAM(Biotic-Abiotic-Movement) diagram. We selected 648 geographic records of pure forests occurrence. ENVIREM and SoilGrids databases, statistical tools in R, Maxent were used to assess the influence of abiotic, biotic, and movement factors on the spatial distribution of the forest-forming species.Results: Geographic expression of fundamental ecological niches of the main forest-forming species depended mainly on topographic conditions and water regime. Competitor influence reduced the potential ranges of the studied species by 1.2–1.7 times to the geographic expression of their realized niches and led to differences in the distribution of species with similar requirements for abiotic conditions.Movement factor significantly limited the areas suitable for pure forests (by 1.2–1.8 times compared with geographic expression of realized ecological niches), except for birch forests.Conclusion: Distribution maps, constructed by abiotic, biotic, and movement factors, were the models of the occupied distributional area of the forest-forming species in the Caucasus.Biotic and movement factors should be considered in modeling studies of forest ecosystems if models are to have biological meaning and reality.
1. Background
Ecological niche modeling (ENM) or Species distribution modeling(SDM), based on Machine Learning Algorithms and statistical data processing,is currently an important part of research on the species potential distribution. The efficiency of ENM as a method for assessing the geographic distribution of plant species was confirmed by numerous studies of different tree and grass species.For many species,the predicted geographic ranges corresponded to the actual distribution of plants and their ecological and biological characteristics (Ebeling et al., 2008;Carvalho et al., 2017; Li et al., 2017; Zurell and Engler, 2019; Bowen and Stevens, 2020). In addition,one of the reasons for the popularity of the ENM method in species distribution studies is the availability and accessibility of global databases on biological diversity and environmental variables (Ortega-Huerta and Peterson, 2008; Peterson et al.,2011).
An emerging field of ENM is the study of ecological niches of sympatric species within the same geographic range.Such studies contribute significantly to understanding the coexistence of species and the formation of their current spatial distribution (Pirayesh Shirazinejad et al.,2017; Hemami et al., 2020). The studies are especially relevant for dominant species, which largely determine the structure, species composition, and dynamics of plant communities. In the Caucasus, the main forest-forming species are Abies nordmanniana(Steven)Spach,Picea orientalis (L.) Peterm., Pinus sylvestris L., Fagus orientalis Lipsky, Carpinus betulus L., Betula litwinowii Doluch., and Betula pendula Roth. These species are of great economic importance and high conservation value for mountain regions of the Caucasus.At the same time,Abies nordmanniana and Picea orientalis are endangered due to bacterial diseases and outbreaks of forest pests.Despite previous studies on GIS mapping of spruce,fir, pine, and beech forests in different regions of the Caucasus(Komarova et al.,2016;Sablirova et al.,2016;Shevchenko and Geraskina,2019;Aliev et al.,2020),there is no unified concept of their spatial distribution and coexistence. We lack a clear understanding of climatic, landscape,soil, and biotic variables that contribute to the potential distribution of the species in the Caucasus Mountains. It remains unknown which environmental conditions and territories are most suitable for the restoration of threatened fir and spruce forests.A deeper understanding of spatial distribution patterns of the studied species is important for the development of strategies for the protection and restoration of mountain forests in the Caucasus.
Our study focused on the ecological niche modeling approach through SDM and the BAM(Biotic-Abiotic-Movement)concept(Sober′on and Peterson,2005;Peterson,2006;Peterson et al.,2011;Peterson and Sober′on, 2012). SDM is a scientifically proven numerical method that uses species records(observations of the species presences and absences,species richness) as a dependent variable and geographic layers of environmental information (climatic, landscape, soil data) as independent variables to predict the potential distributions of species and their habitats in space and time (Elith et al., 2006; Elith and Franklin, 2013;Duarte et al., 2019; etc.). BAM diagrams have proven to be effective in studies for which models of distributions and niches were used: justification of the geographic area for model development(Barve et al.,2011;Myers et al., 2015; Banerjee et al., 2019); assessment of niche conservatism and niche shifts during biological invasions (Jim′enez-Valverde et al., 2011; Guisan et al., 2014; Battini et al., 2019; Flores-Tolentino et al., 2019); investigation of the importance of biotic factors in the distribution of invasive species (Sim~oes and Peterson, 2018), etc.Whereas species distribution models based on the statistical relationship between species distribution and environment variables often consider only abiotic factors (Carvalho et al., 2017; Shevchenko and Geraskina,2019; Bowen and Stevens, 2020; etc.), the BAM concept takes into account three sets of factors that determine the geographic distributions of species. In addition to abiotic factors (A), BAM diagrams consider interactions between species (B factors – competition, predation, mutualism, etc.), which are known to significantly affect the spatial distributions of species (Sober′on and Peterson, 2005; Peterson and Sober′on, 2012; Sim~oes and Peterson, 2018; etc.). We also assumed that competitors, along with abiotic conditions, have an important effect on the spatial distribution of the forest-forming species in the Caucasus.The possibility of inclusion the geography of other species in single-species models in accordance with the Biotic-Abiotic-Mobility framework(Sober′on and Peterson, 2005) corresponds to the tool capabilities of SDM. The third critical determinant of the species dispersal capacities,which should prove particularly important for regional-scale studies of the areas with complex geography (Sober′on and Peterson, 2005; Barve et al.,2011;Peterson and Sober′on,2012),is the set of areas accessible to the species(movement factor,M).Given the size of the studied territory and the complex landscape configuration of the Caucasus Mountains,which generates barriers to species distribution, the niche modeling outputs in this research should be reduced to the areas within M.Currently, there is no single conceptual approach to the geographic specification of M areas. According to a review by Barve et al. (2011),researchers indicated M regions 1)within geopolitical units without any biologically meaningful basis;2)within biotic regions(areas with sets of species different from those of other areas); 3) within areas of the historical species distribution modeled on the characteristics of their present-day ecological niches.M areas were also limited to regions with the previously identified climate classes suitable for the studied species(Banerjee et al., 2019), or regions with fossil records of the species(Myers et al.,2015).Our approach to the formalization of the movement factor suggested that the territories with the highest predicted probability of pure forest occurrences are the most accessible for the studied species.
The BAM concept has the advantage of distinguishing the effects of the three components (A, B, and M) (Peterson, 2006). Along with a hierarchical relationship between the fundamental niche, the realized niche, and the “occupied distributional area” (Sober′on and Peterson,2005;Peterson and Sober′on,2012),this is consistent with the purposes and methodological approaches of our study. We used a comparative analysis of species distribution models constructed on different sets of environmental data. These were abiotic conditions for A Models(geographic expressions of the fundamental niche); competitors and abiotic conditions for BA Models(geographic expressions of the realized niche);abiotic conditions,competitors,and accessibility of areas for BAM Models (occupied distributional area). The main modeling tool was Maxent(Maxent software for species habitat modeling),from which we produced the species distribution models and analyzed the factors determining this distribution.
This study was aimed to figure out how abiotic,biotic and movement factors affect the spatial distribution of the main forest-forming species in the Caucasus, which helps in the adoption of measures for sustainable conservation and restoration of forests.Inclusion of the BAM concept in the traditional SDM contributes to a better understanding of spatial distribution patterns and coexistence of the studied species.
2. Methods
2.1. Study area
The physiographic boundaries of the Caucasus (38°–47°N and 36°–50°E)consist of the Greater Caucasus,the Lesser Caucasus,and the Transcaucasian Depression(Kura-Araks Lowland in the east and Colchis Lowland in the west), including the territory of the Russian Federation,Georgia,Armenia, and Azerbaijan(Fig.1).
The study area covered about 390 thousand km2in the range from-28 m (Caspian Lowland) to 5,642 m (Mount Elbrus) above sea level.The prevailing climate of the Greater Caucasus is generally humid subtropical in the South-Western Caucasus (Black Sea coast of Russia) and humid warm summer continental in the North-Western Caucasus. The climate of the Central and Eastern Greater Caucasus(Russian territory in the north and Georgian territory in the south of the Main Caucasian Ridge)is continental and cool,humid(or even alpine)in the highlands,warm, humid in the middle mountains, and hot, dry on the plains. The prevailing climate in the foothills and low mountains of the Lesser Caucasus (mountain system in Georgia, Armenia, and Azerbaijan) is humid subtropical in the west and dry subtropical in the east. In the middle mountains, a continental climate prevails (drier to the east and southeast). The climate of the Kura-Araks Lowland and the Colchis Lowland,separating the Greater Caucasus and the Lesser Caucasus, is dry continental and humid subtropical,respectively.
Fir and spruce forests dieback throughout the native range due to deforestation, bacterial diseases, pest outbreaks, and climate change(Tufekcioglu et al., 2008; Akatov et al., 2013; Bebiya,2015; Akinci and Ers¸en, 2016; Gornov et al., 2018; Güney et al., 2019; Pukinskaya et al.,2019). In the Caucasus, the ranges of Abies nordmanniana and Picea orientalis mainly cover the mountains of the western part (Litvinskaya and Salina,2012). The ecological plasticity of Pinus sylvestris determines the vast area of the species in the Caucasus (Ermakov et al., 2019), but the main productive pure pine forests cover the slopes of the Central Greater Caucasus. The native range of Fagus orientalis occupies the Greater and Lesser Caucasus, as well as Crimea, Northern Iran, Turkey, Greece, and Syria(Aliev et al., 2020; Dagtekin et al.,2020). Carpinus betulus is common species in the Caucasus, Mainland Europe, and Asia Minor. The native range of Betula litwinowii mainly covers the Greater Caucasus,where birch forests are usually confined to the steep slopes at the upper border of the forest belt (Akhalkatsi et al., 2006; Kessel et al., 2020).Betula pendula is common species from the northern regions to the southern mountain areas of Europe(Beck et al.,2016).

Fig. 1. Location of the study area and climate classification scheme of the Caucasus. We constructed the climate classification scheme using monthly mean temperature and precipitation data from WorldClim v2.0. based on the SagaGis algorithm of Conrad et al. (2015). K¨oppen-Geiger climate classification and map color scheme was used from Peel et al.(2007):BSk is a cold semi-arid climate (cold steppe climate);Cfa is a humid subtropical climate; Cfb is an oceanic climate; Csa is a Mediterranean hot summer climate;Csb is a Mediterranean warm or cool summer climate;Dfa is a hot summer continental climate;Dfb is a warm summer continental or hemiboreal climate; Dfc is a cool summer continental climate;Dsa is a hot dry summer continental climate; Dsb is a warm dry summer continental or hemiboreal climate;Dsc is a cool dry summer continental climate;ET is an alpine climate(tundra climate).(For interpretation of the references to color in this figure legend,the reader is referred to the Web version of this article.)
2.2. Species occurrence data and environmental variables
We studied highly productive pure forest stands of the main forestforming species. In their final stages of development, these stable plant communities are reliable indicators of the most suitable habitats for the studied species. Accordingly, using pure forests as species occurrence data in distribution modeling increases the probability of detecting species in the predicted area.
Species occurrence data were sourced from the Global Biodiversity Information Facility (GBIF) and the expedition research in the Central and Western Greater Caucasus in 2012–2021.We filtered the occurrence data from the GBIF database to exclude geographic records outside the natural range of the species (urban and rural areas, trees in landscape design), obviously incorrect records (plain steppes, alpine grasslands,glaciers,etc.),as well as those that were not the presence points of pure forests(records were verified based on forest management maps and field observations) (Table 1). Geographic records were further checked for duplicates and were spatially rarefied (using the "clean duplicate" function from library ntbox in R(Osorio-Olvera et al.,2020)).We selected no more than one point per grid cell (cell size of 1 km2) to avoid model over-fitting and to ensure the validity of the statistical analysis.In total,648 species occurrence records were retained in the study area.
We used a set of 16 climatic and two topographic variables from the ENVIronmental Rasters for Ecological Modeling database (ENVIREM,2021). Many of these environmental variables, such as evapotranspiration parameters, are directly related to the ecological or physiological processes that determine the distribution of plant species (Title and Bemmels, 2018). We also used 11 edaphic variables from the SoilGrids database (SoilGrids250m version 2.0) (Poggio et al., 2021). Edaphic variables such as bulk density,cation exchange capacity,coarse fragmentvolumetric,the proportion of clay,sand and silt particles,total nitrogen,soil pH,soil organic carbon content,organic carbon density,and organic carbon stocks were extracted for Interval II (depth of 5–15 cm) and adapted to an ASCII standard format with a spatial resolution of 30 s(~1 km2).
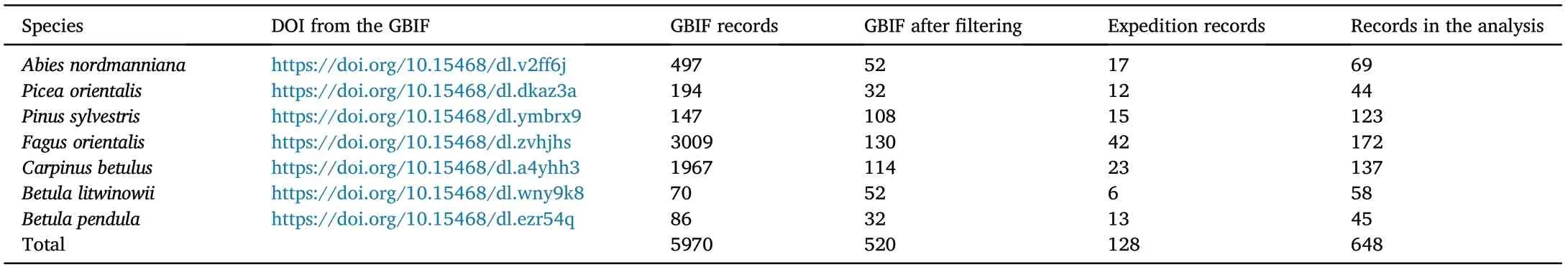
Table 1 Species occurrence data used in the study.
To prevent overfitting of the models and select predictors most important for modeling,VIF(Variance Inflation Factor)test in R was run to assess variable correlation, including latent correlations. VIF test constrained predictors to only 12 non-correlated variables for model outputs (threshold VIF ≤3). They were four climatic variables, one topographic variable,and seven edaphic variables(Table 2).
2.3. Model development and evaluation
Model development was conducted in R package dismo (Hijmans et al.,2017)using Maxent(ver.3.4.3)(Steven et al.,2017)for each of the studied species. Maxent is one of the most efficient modeling methods,especially in prediction based on presence-only data (Elith et al., 2006;Phillips and Dudík,2008;Dube et al.,2015;Yi et al.,2018;Iverson et al.,2019; Komori et al., 2019). Maxent generates the probability of species occurrences from the distribution of predictor values.The territories with the highest probability of species occurrences are considered the most suitable. Extrapolation of the probabilities of species occurrence to the study area with the logistic format of output data results in a probability distribution map in the range from 0 to 1.Maxent defines the importance of environmental variables in species distribution and constructs the response curves that illustrate the relationship between a particular variable and the predicted probability of suitable conditions for a species.The program was used with the Auto(LQHP)feature type,which allows the set of features to depend on the number of presence points, using general empirically derived rules:all feature types when there are at least 80 training samples; linear, quadratic and hinge features for 15–79 samples; linear and quadratic features for 10–14 samples; only linear features when there are less than 10 samples. We used a five-fold cross-validation method in which 75% of occurrence records of each species (Table 1) were the training samples, and 25% were kept as test samples(Phillips and Dudík,2008).
The determination of thresholds should not be arbitrary and should consider the relative importance of omission and commission errors(Hernandez et al., 2008; Nenzen et al., 2011; Liu et al., 2013; Norris,2014). Various thresholds were used to convert the continuous probabilities calculated by Maxent into discrete presence/absence predictions(Liu et al.,2005,2013),and there is not a perfect way to determine the best threshold for suitable habitats (Glover-Capfer, 2015). Accuracyassessment methods rely on presence/absence data,making it difficult to select a threshold without reliable absence data (Li and Guo, 2013). In our study, the models identified pure forests, and it was important to reduce the risk of mistaken identification (including mixed stands) by selecting a sufficiently high threshold for determining habitats with a high degree of suitability (Pearson et al., 2004). Accordingly, we based the threshold for potentially suitable habitats on a fixed probability of suitability >0.5 (50% probability of presence in suitable areas) (Elith et al., 2010; Kramer-Schadt et al., 2013). Optimal habitats were determined using a fixed threshold>0.8(Buhl-Mortensen et al.,2019),which corresponds to models with a low layer of false-positive results.
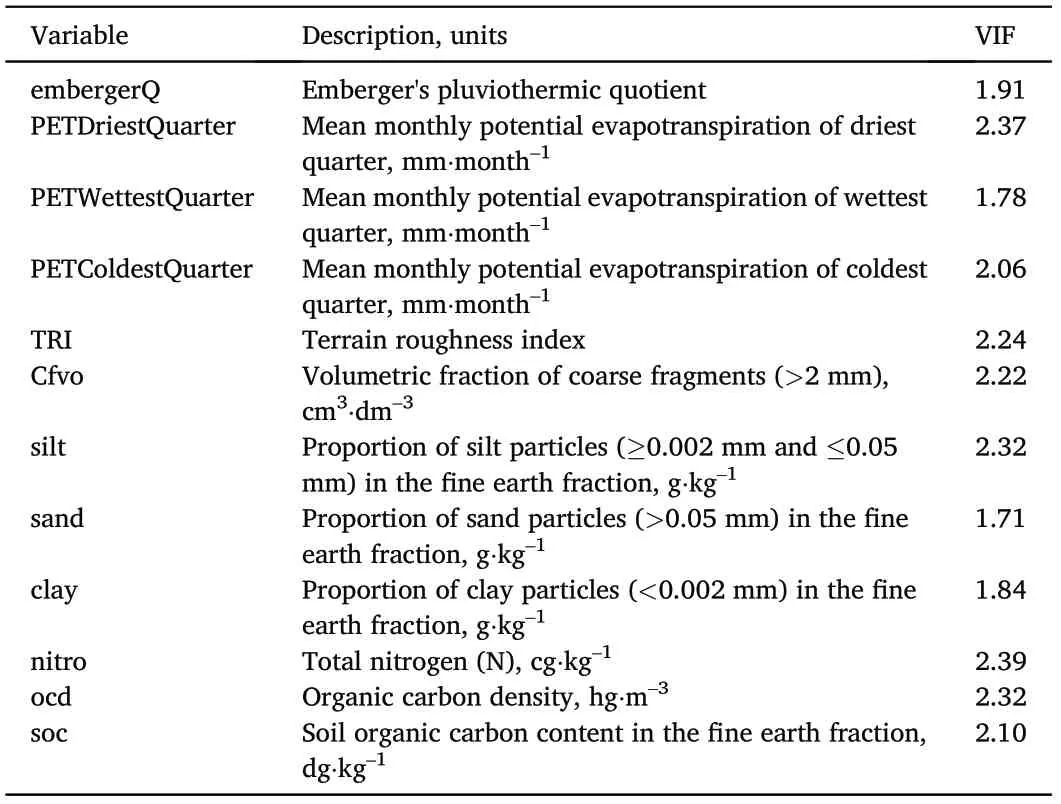
Table 2 Environmental variables selected by VIF test.
Visualization of the probabilities of species occurrence to the study area was carried out according to the ranked values of the standard Maxent palette in gradation of colors from blue (occurrence “0”) to red(occurrence “1”) by converting the output Maxent file to a netCDF file with subsequent visualization in the PanoplyWin program(PanoplyWin,2021).
At the first stage of modeling(Step 3),the input data used to run the species distribution models were the occurrence records and the environmental variables selected by VIF test (Fig. 2). According to BAM diagram (Sober′on and Peterson, 2005; Peterson, 2006; Peterson et al.,2011; Peterson and Sober′on, 2012; etc.), A Models represented areas with suitable abiotic conditions that could be considered as the“geographic expression of the fundamental ecological niches of the species”.The analyzed abiotic factors imposed physiological restrictions on the ability of species to persist (survival and growth) in the identified territory.
Species distribution modeling, along with abiotic factors, should include an analysis of biotic factors (Sober′on and Peterson, 2005;Peterson and Sober′on, 2012; Sim~oes and Peterson, 2018), which are positive and negative interactions with other species.Species distribution models constructed with abiotic and biotic variables (BA Models) corresponded to the “geographic expression of the realized niche of the species” (an area with suitable abiotic conditions and interspecific interactions that do not prevent positive fitness (Sober′on and Peterson,2005; Peterson and Sober′on, 2012; etc.)). One of the most important biotic factors for plant species is competitors, which can significantly limit the actual distribution of species by limiting population processes.To consider the influence of competing species, in accordance with the correlative approach to ecological niche modeling, it is possible to include the geography of other species in single-species models(Sober′on and Peterson,2005).To do this,we re-modeled the spatial distribution of each species using the abiotic environmental variables and the previously obtained probability distribution maps of other species as biotic environmental layers.
In our study, movement factor (“species dispersal capability”,“accessibility of areas”) represented the part of the Caucasus that was most accessible to the studied species(“the regions that are accessible to dispersal by the species from some original area over a relevant period of time” (Sober′on and Peterson, 2005; Peterson and Sober′on, 2012)).Optimal areas were territories with a probability of species occurrence of 0.8–1 in BA Models(strict threshold of optimal habitat for pure forests).We displayed the accessibility of areas through the distance from these territories, where the probability of species occurrence was higher than 0.5(0.5 threshold of habitat suitability).We used the obtained raster of distances as an additional layer for modeling.BAM Models corresponded to the geographic expression of the intersection of M, B, and A areas,which was termed “geographic distribution of the species”, “actual distribution of the species” or“distributional area”(Sober′on and Peterson,2005), and later “occupied distributional area” (Peterson et al., 2011;Peterson and Sober′on,2012),“actual occupied area”(Jim′enez-Valverde et al.,2011)or“realized distribution”(Battini et al.,2019).In this study,we used the term “occupied distributional area” for the geographic expression of BAM Models.

Fig. 2. Theoretical framework of the study. Step 1 – selecting environmental layers for modeling; Step 2 – removal of correlated variables using VIF test; Step 3 –modeling by abiotic environmental variables(A Models);Step 4–extraction of species distribution models as biotic environmental layers;Step 5–modeling by abiotic and biotic environmental variables (BA Models);Step 6–extraction of species distribution models with a probability of species occurrence of 0.8–1 from BA Models and creating a raster of distances from the optimal habitats; Step 7 – modeling based on abiotic, biotic and movement components of species ecological niches(BAM Models).
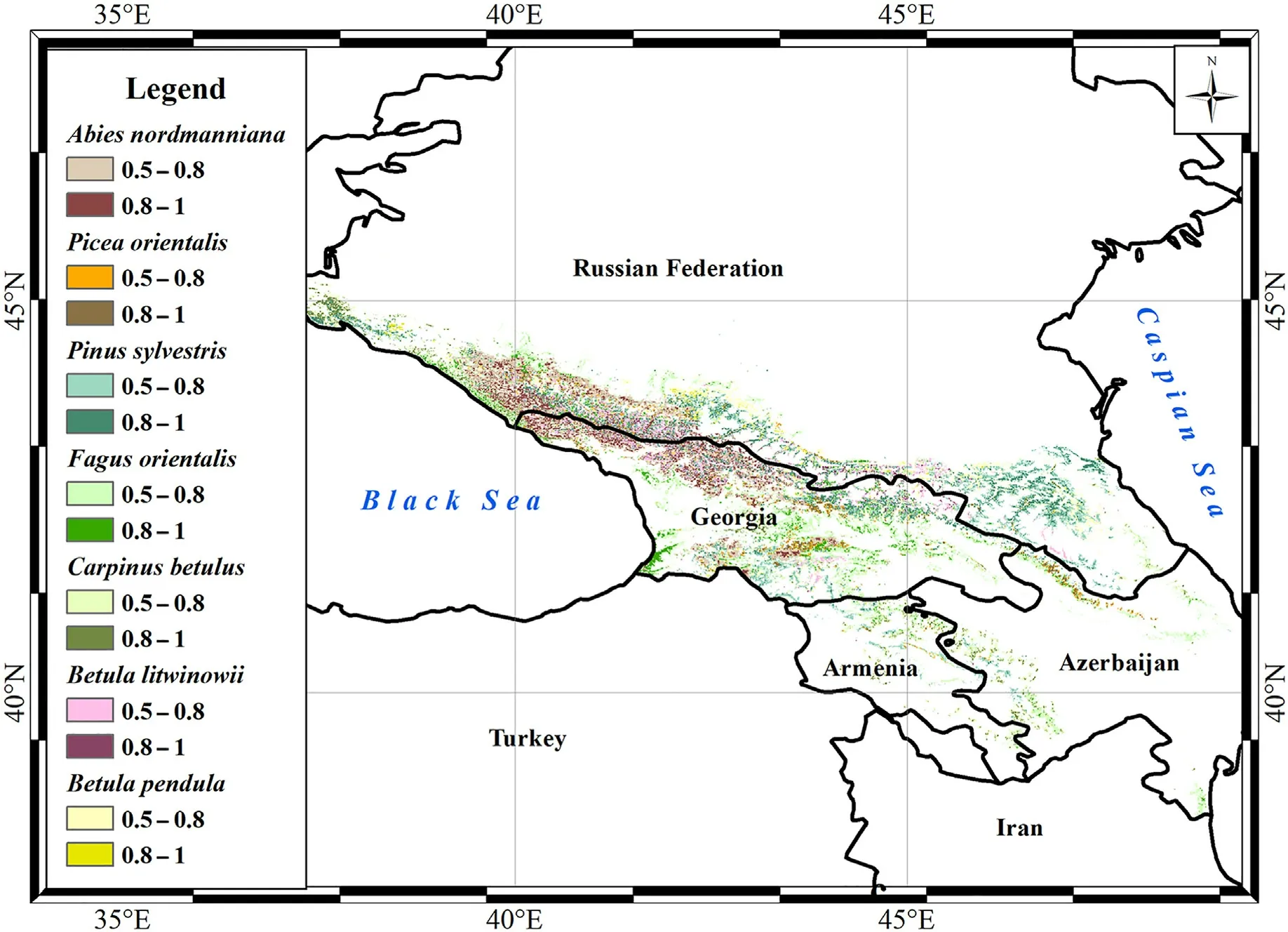
Fig. 3. Distribution map of habitats acceptable (0.5–0.8 threshold of habitat suitability) and optimal (0.8–1 threshold of habitat suitability) for the main forestforming species in the Caucasus by BAM Models.
The model evaluation consisted of the area under the receiver operating characteristic (Area under the curve, AUC) as a measure of predictive success.AUC values provide information about the sensitivity and specificity of the model for classifying data compared to random ones(Fielding and Bell, 1997). We used AUC values from the test (AUCTest)and training(AUCTrain)data.Minimum difference between training and test data indicated that the models were not over-parameterized to be overly specific to the training data (Warren and Seifert, 2011). AUC values for each model were averaged across five replicates that differed in 25% of the test data, which were occurrence records randomly separated from the original presence points.
3. Results
3.1. Species distribution modeling by abiotic environmental variables
Models constructed with 12 selected abiotic variables showed a reliable prediction.AUCTrain and AUCTest ranged from 0.92 to 0.98 and from 0.87 to 0.95, respectively (Table 3). The differences between AUCTrain and AUCTest were quite low(0.03–0.06).
For all seven species,one of the variables with the greatest percentage contribution was Terrain roughness index(TRI)(Table 4).TRI quantifies local vertical topographic heterogeneity by calculating the average elevation difference between a particular site and its eight neighbor sites(Riley et al.,1999;R′ozycka et al.,2016).Using a 0.5 threshold of habitat suitability, suitable TRI values for Abies nordmanniana, Picea orientalis,and Betula pendula ranged from nearly level (80–90) to moderately rugged(350–425)areas(Table 4,SI Figs.1,2,7)according to Riley et al.(1999).Suitable conditions for Fagus orientalis and Carpinus betulus were the lower TRI values ranging between level (35–50) and intermediately rugged(200–235)areas(SI Figs.4 and 5).The upper TRI values for Pinus sylvestris and Betula litwinowii corresponded to highly rugged areas(Table 4,SI Figs.3 and 6).
For five species, one of the main environmental predictors was Emberger’s pluviothermic quotient(embergerQ)(Table 4).EmbergerQ is based on the combined dynamic of evapotranspiration and the extreme annual temperature amplitude and synthesizes temperature and humidity of climate with higher values for more humid conditions(Emberger,1955; Daget et al., 1988). In our study, pure spruce, beech and birch(Betula pendula) forests occurred mostly in sub-humid and humid areas(0.5 threshold of habitat suitability) (Table 4, SI Figs. 2, 4, 7). Betula litwinowii and Abies nordmanniana preferred only humid habitats with embergerQ of at least 100 and 120,respectively(Table 4,SI Figs.1 and 6).
In terms of percentage contribution and permutation importance,mean monthly potential evapotranspiration of the driest quarter (PETDriestQuarter)and the wettest quarter(PETWettestQuarter)contributed significantly to the distribution models of pine and hornbeam forests,respectively. Potential evapotranspiration indicates the maximum amount of moisture that evaporates per unit time by a unit surface of vegetation cover in the absence of moisture deficiency (Allen et al.,1998). It largely depends on precipitation, solar radiation, air temperature, and wind speed. The suitable values of PETDriestQuarter and PETWettestQuarter for Pinus sylvestris and Carpinus betulus,respectively,were in the range of rather low values(Table 4,SI Figs.3 and 5).
Edaphic factors were less important in the geographic distribution of the studied species, except the proportion of sand particles in the fine earth fraction for Abies nordmanniana.Suitable soils for fir forests should contain 35%–50% sand, which corresponds to loamy soils (Kaufmann and Cutler,2008).
According to A Models, Carpinus betulus, Fagus orientalis, and Pinus sylvestris were able to occupy more than 40 thousand km2each(Table 5).The potential ranges of these species largely overlapped throughout the Caucasus and covered the mountain regions of the Greater and Lesser Caucasus. At the same time, 7.5 thousand km2of optimal areas for hornbeam forests were mainly concentrated from the foothills to the middle mountains of the Western Greater Caucasus and, partly, on the Black Sea coast of Georgia with a humid subtropical climate. In the Central and Eastern Greater Caucasus and the Lesser Caucasus, the habitats optimal for Carpinus betulus were limited to relatively small areas in the foothills, low and middle mountains with a warm summer continental or hemiboreal climate.Probability distribution map of hornbeam forests by A Model was presented in Supplementary Information (SI Fig.8a).
Optimal habitats of Fagus orientalis (about 12 thousand km2) were scattered in the foothills, low and middle mountains of the South-Western Caucasus and the Black Sea coast of Georgia (humid subtropical climate),in the middle mountains of the North-Western Caucasus and the Georgian part of the Central Greater Caucasus (warm summer continental and humid subtropical climate) (SI Fig. 9a). With a high probability, pure beech forests were predicted in the west of the Lesser Caucasus and the Eastern Caucasus on the border of Russia and Azerbaijan(humid subtropical and warm summer continental climate).
Areas optimal for Pinus sylvestris (about 9 thousand km2) were widespread in the middle mountains and highlands of the Greater Caucasus with an oceanic climate (the Western Caucasus) or, most commonly, a warm summer continental or hemiboreal climate (the Central and Eastern Caucasus) (SI Fig. 10a). In the Eastern Greater Caucasus, pure pine forests were predicted in the low mountains of the Caspian Sea coast (hot summer continental climate). In the Lesser Caucasus, areas optimal for Pinus sylvestris were found in the mountain regions with a warm summer continental climate.
According to A Models, Abies nordmanniana and Picea orientalis occupied the smallest potentially acceptable and optimal areas among the forest-forming species in the Caucasus(Table 5).Optimal areas of fir and spruce forests covered the middle mountains and highlands of the Western Caucasus and the southern slopes of the Central Greater Caucasus (humid subtropical and warm summer continental climate) (SI Figs. 11a and 12a). On the northern slopes of the Central Greater Caucasus, habitats optimal for Abies nordmanniana and Picea orientalis were limited to small areas in the upper reaches of mountain gorges.A Models predicted the smallest acceptable and optimal areas for fir and spruce forests in the Eastern Greater Caucasus and Lesser Caucasus with a more continental climate.
The area of acceptable habitats for Betula pendula exceeded that for Betula litwinowii,while the areas of optimal habitats for both species were almost the same. Areas optimal for Betula pendula were concentrated in the middle mountains and highlands of the Greater Caucasus and thewestern part of the Lesser Caucasus with a warm summer continental or hemiboreal climate (SI Fig. 13a). The main distribution area of Betula litwinowii covered the mountain regions of the Greater Caucasus, where the probability of species occurrence was higher in the middle mountains and highlands of the North-Western Caucasus and the Georgian part of the Central Greater Caucasus with a humid subtropical and warm summer continental climate(SI Fig.14a).
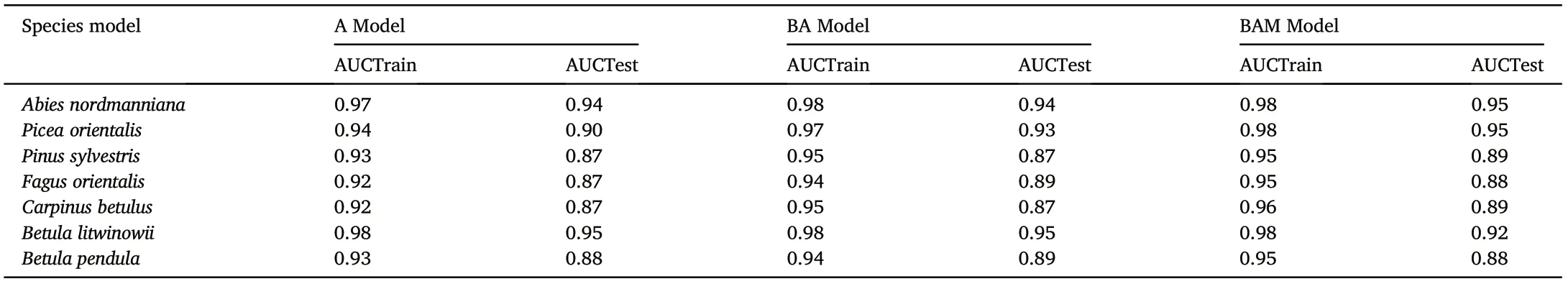
Table 3 Evaluation of Maxent models using AUC values averaged over five replicate runs.

Table 4 Contribution of environmental variables to the Maxent models of distribution of the main forest-forming species in the Caucasus.

Table 5 Areas of acceptable and optimal habitats for the main forest-forming species in the Caucasus by the Maxent models.
3.2. Ecological niche modeling by abiotic and biotic environmental variables
Models constructed with abiotic and biotic variables also showed a reliable prediction.AUCTrain and AUCTest ranged from 0.94 to 0.98 and from 0.87 to 0.95, respectively, and the differences between AUCTrain and AUCTest values were fairly low(0.04–0.08)(Table 3).
According to ВA Models, the most important environmental predictors in the potential distribution of the main forest-forming species in the Caucasus were biotic factors (competitors), which significantly masked the influence of abiotic variables(Table 4).The competition from Picea orientalis had the largest percentage contribution in ВA Model of Abies nordmanniana distribution. The habitats were considered suitable for Abies nordmanniana (0.5 threshold of habitat suitability)if the probability of Picea orientalis occurrence on the sites was 0.3 and higher(Table 4, SI Fig. 1). This proved the similarity of ecological niches of these species. At the same time, the main competing species for Picea orientalis in the Caucasus was Fagus orientalis. Pure spruce forests formation was possible in the sites with a probability of beech forests occurrence of 0.6–1 (SI Fig. 2). Pinus sylvestris was a relatively weak competitor for other forest-forming species except for Carpinus betulus,Betula pendula,and B.litwinowii(SI Figs.5–7).In turn,the studied species had a relatively weak influence on the distribution of pine forests (percentage contribution of about 11%–17%). The main competing species for Fagus orientalis was Carpinus betulus,to a lesser extent Picea orientalis(SI Fig.4).Betula litwinowii and B.pendula competed with each other for distribution in the Caucasus.
The acceptable values of climatic variables in BA Models were almost the same as in A Models, but TRI values for some species increased(Table 4, SI Figs. 2, 3, 6, 7). The contribution of abiotic factors to the construction of species distribution models significantly decreased. The exceptions were embergerQ for Abies nordmanniana and TRI for Pinus sylvestris, the percentage contribution of which decreased only to 10%and 20%,respectively.
According to ВA Models,the areas of acceptable and optimal habitats of the most main forest-forming species in the Caucasus decreased by 1.1–1.7 times(Table 5).For Carpinus betulus,the optimal area decreased to the greatest extent in the Eastern Greater Caucasus and Georgia,including the central part of the Greater Caucasus and the west of the Lesser Caucasus (SI Fig. 8b). The areas of optimal habitats of Fagus orientalis decreased fairly evenly throughout the entire potential range of pure beech forests(SI Fig.9b).BA Model predicted reduction in the area optimal for pine forests in the North-Western Caucasus (SI Fig. 10b).Optimal habitats of Abies nordmanniana and Picea orientalis decreased throughout the entire potential range, but especially in the Lesser Caucasus (SI Figs. 11b and 12b). BA Models still predicted small optimal areas for both species in the upper reaches of mountain gorges on the northern slopes of the Central Caucasus. BA Model demonstrated the largest reduction in the optimal habitats of Betula pendula (twice as compared to A Model),which were concentrated mainly in the highlands(SI Fig. 13b). Areas optimal for Betula litwinowii most significantly decreased on the northern slopes of the Central and North-Western Greater Caucasus,as well as in the northeast of Georgia(SI Fig.14b).
3.3. Ecological niche modeling by abiotic, biotic, and movement environmental factors
In our study,the movement factor was characterized by the distances(km)from optimal habitats(sites with a probability of species occurrence of 0.8–1.0),where the probability of species occurrence was higher than 0.5.The distances were determined in a straight line,taking into account the terrain.BAM Models constructed with abiotic,biotic,and movement factors showed a reliable prediction(Table 3).The movement factor was the most important ecological predictor in the potential distribution of hornbeam,fir,and pine forests(Table 4).However,the total contribution of the biotic factor in BAM Models of Pinus sylvestris (37.3%) and Abies nordmanniana was not much less than that of the movement factor. TRI and embergerQ still retained an influence on the distribution of pine and fir forests, respectively. According to BAM Models, competition from other forest-forming species remained the most important factor in the distribution of beech,spruce,and birch forests in the Caucasus(Table 4).
Betula litwinowii and B.pendula were the most“mobile”forest-forming species in the Caucasus.The distance of territories suitable for pure birch forests was up to 20 km from optimal habitats(Table 4,SI Figs.6 and 7).BAM Models predicted a slight decrease in the areas acceptable and optimal for Betula litwinowii and B. pendula compared to BA Models(Table 5,SI Figs.13c and 14c).
Ten-kilometer distances of suitable habitats from the sites with optimal environmental conditions significantly limited the initially large potential ranges of Fagus orientalis and Pinus sylvestris (by 1.8 and 1.5 times compared to BA Models) (Table 5, SI Figs. 9c and 10c). The relatively small predicted range of Abies nordmanniana decreased only by 1.2 times.BAM Model predicted new small areas optimal for this species in the west of the Lesser Caucasus(SI Fig.11c).Nevertheless,the total area of habitats optimal for Abies nordmanniana,as well as for Pinus sylvestris,significantly decreased.Areas optimal for Fagus orientalis with a relatively low influence of movement factor decreased to the least extent(only by 1.03 times compared to BA Model).
The smallest predicted distribution from optimal habitats was for pure hornbeam(0–1 km)and spruce(0–6 km)forests(Table 4,SI Figs.2 and 5). According to ВAM Models, Carpinus betulus and Picea orientalis tended to reduce the area of potential distribution in the Caucasus by 1.7 and 1.4 times compared to BA Models. The areas of optimal habitats of Picea orientalis did not decrease significantly, while for Carpinus betulus,the reduction was 1.2 times(Table 5,SI Figs.8c and 10c).
4. Discussion
4.1. A Model
The aim of this study was to assess the influence of abiotic,biotic,and movement factors on the spatial distribution of the main forest-forming species in the Caucasus by modeling the geographic expression of their ecological niches. We revealed a significant effect of topographic conditions and water regime on the potential distribution of the studied species. Our results showed that the acceptable habitats for pure fir forests were relatively gentle slopes (between nearly level and moderately rugged) with loamy soils in humid conditions (Table 4, SI Fig. 1).Pure spruce forests also potentially occurred on relatively gentle slopes in sub-humid and humid conditions (SI Fig. 2). Optimal habitats of both species were mainly located in the middle mountains and highlands of the Western Caucasus and the Georgian part of the Central Greater Caucasus with a humid subtropical and warm summer continental climate(SI Figs.11a and 12a).These results are in line with Shevchenko and Geraskina (2019), who observed that in the North-Western Greater Caucasus, the modern potential areas of Abies nordmanniana and Picea orientalis almost completely coincided. The authors concluded that the main limiting factors in the distribution of these drought-sensitive species in the region were the precipitation in the driest month,as well as the altitude (Shevchenko and Geraskina, 2019). Previous research also revealed high sensitivity to climate humidity of Abies nordmanniana in the Caucasus(Litvinskaya and Salina,2012)and Picea orientalis in Turkey(Ucarcı and Bilir,2018). According to Akatov et al.(2013),the suitable average annual precipitation for Abies nordmanniana ranged from 700 to 2,500 mm.Our result is also consistent with a previous study of fir forests in northwestern Turkey (Coban, 2020), which showed that pure fir forests mainly occurred between 1,000 and 1,600 m above sea level on mountain slopes with a steepness of about 1100°––2200°°.Litvinskaya and Salina(2012) observed that in the Western Greater Caucasus, optimal conditions for Abies nordmanniana and Picea orientalis forests were formed at altitudes of 1,200–1,600 m and up to 1,500–1,700 m,respectively.Usta and Yılmaz (2020) found that in the Trabzon mountains (northeastern Turkey), slope steepness and altitude positively correlated with the distribution of Picea orientalis.The authors suggested that negative anthropogenic interventions could limit spruce forests to steep slopes unsuitable for agriculture and settlement(Usta and Yılmaz,2020).Our studies of the importance of edaphic factors in fir forest distribution were also supported by Litvinskaya and Salina (2012), who highlighted that Abies nordmanniana is sensitive to deteriorating soil conditions and prefers loamy soils.
In our studies, Pinus sylvestris mainly depended on the topographic factor TRI;the percentage contribution of climatic factors to the species distribution model was relatively low (Table 4, SI Fig. 3). Acceptable habitats of pure pine forests were located in a wide range of mountain slope steepness and altitude from nearly level to highly rugged areas with fairly low mean monthly potential evapotranspiration of the driest quarter. Areas optimal for Pinus sylvestris mainly included the middle mountains and highlands of the Greater Caucasus with warm summer continental,hemiboreal,oceanic,or hot summer continental climates(SI Fig. 10a). The wide ecological range of the species by temperature and humidity gradients, climate continentality, underlying rocks, and soil is in line with previous studies of pine forests in the Dagestan Republic(Eastern Greater Caucasus,Russia)by Ermakov et al.(2019).The authors showed that pine forests were distributed in the middle mountains and highlands at an altitude of 1,600–2,500 m(Ermakov et al.,2019),which is consistent with our results. Researchers also highlighted the drought resistance of Pinus sylvestris(Usta and Yılmaz,2020)and its tolerance to excessive moisture (Rakhmatullina et al., 2017). Rakhmatullina et al.(2017)and Arslan and ¨Orücü(2019)used Maxent models to analyze the contribution of environmental factors to the distribution of pine forests in the Southern Ural (Republic of Bashkortostan, Russia) and Turkey,respectively. They revealed a significant influence of the maximum temperature of the warmest month, which may be due to climatic differences between these regions and the Caucasus.
Our results showed that the potential ranges of Fagus orientalis and Carpinus betulus largely overlapped throughout the study area,while the area of optimal habitats for beech forests was almost twice that for hornbeam forests (Table 5). Optimal areas for both species covered the foothills,low and middle mountains(from level to intermediately rugged areas) of the Western Greater Caucasus and the Black Sea coast of Georgia(Table 4,SI Figs.4,5 and 8a,9a).Moreover,the Georgian part of the Central Greater Caucasus,the Eastern Caucasus,and the west of the Lesser Caucasus also included optimal sites for beech forests. The low frost resistance of these species (Shevchenko and Geraskina, 2019)probably explains the relatively low upper limit of the distribution of beech and hornbeam forests in the Caucasus Mountains.Usta and Yılmaz(2020) also reported that slope steepness and altitude were negatively correlated with the distribution of Carpinus orientalis on the Karada˘g Mass, Turkey. Fagus orientalis preferred mainly sub-humid and humid bioclimatic conditions,while Carpinus betulus occurred in conditions with rather low suitable values of mean monthly potential evapotranspiration of the wettest quarter. This result coincided with Jensen et al. (2008),who showed that in central and northern Europe, a drier and warmer climate (annual precipitation of less than 600 mm and mean July temperature above 18°C) favored the distribution of Carpinus betulus,whereas beech forests prevailed in more humid regions. Based on modeling the range of Fagus orientalis with environmental data of the present,past,and future climates,Dagtekin et al.(2020)also showed that drier climate and higher temperatures will limit the future distribution of this species. Previous studies in the North-Western Greater Caucasus(Shevchenko and Geraskina, 2019) and Anatolia, Turkey (Koç et al.,2021)confirmed that the water regime significantly affected the current distribution of beech and hornbeam forests. Shevchenko and Geraskina(2019) reported that beech forests were mainly distributed in areas where the annual precipitation was not less than 600 mm (Shevchenko and Geraskina, 2019). According to Packham et al. (2012), beech trees have a shallow root system which makes them sensitive to moisture deficiency during the drought period.
Birch forests of Betula pendula occurred mainly in the sub-humid and humid bioclimatic zones of the Greater and Lesser Caucasus with a warm summer continental or hemiboreal climate, while Betula litwinowii preferred humid habitats in the North-Western and Central Greater Caucasus with a humid subtropical and warm summer continental climate(Table 4,SI Figs.6,7 and 13a,14a).Both species were common in the middle mountains and highlands; however, the probability of Betula litwinowii occurrence was higher in more rugged areas.This result supported previous reports that on the northern and southern slopes of the Greater Caucasus,Betula litwinowii usually formed the upper border of the forest belt (1,500–2,800 m above sea level) on steep slopes (Akhalkatsi et al., 2006; Kessel et al., 2020). In addition, Akatov (2009) and Hansen et al.(2017)concluded that in the Western and Central Greater Caucasus, the upper limits of Betula litwinowii tended to increase in an uphill direction.Beck et al.(2016)associated Betula pendula distribution in southern Europe (mainly in mountain regions) with its sensitivity to summer drought, which did not contradict our conclusion about the importance of the water regime in the distribution of the species.
4.2. BA Model
In our study, the contribution of biotic ecological predictors significantly exceeded the contribution of abiotic variables to the construction of the models of the species distribution in the Caucasus(Table 4).Areas of geographic expression of realized ecological niches of species were 1.2–1.7 times smaller than the areas of geographic expression of their fundamental ecological niches (Table 5). This result is consistent with previous opinions and conclusions (Keane and Crawley, 2002; Sober′on and Peterson, 2005; Peterson et al., 2011; Peterson and Sober′on, 2012;Atwater et al., 2018; Sim~oes and Peterson, 2018; etc.) that positive and negative interactions between species should be considered in ENM or SDM studies if models are to have biological meaning and reality.
The present study revealed that in the Caucasus, Picea orientalis was the main competitor to Abies nordmanniana in the same areas,while the main species limiting the distribution of Picea orientalis was Fagus orientalis (to a lesser extent Abies nordmanniana and Pinus sylvestris)(Table 4). In turn, the main competitor of Fagus orientalis was Carpinus betulus,while the ecological niche of Carpinus betulus was most similar to that of the Pinus sylvestris. The ecological niches of both birch species were similar; Pinus sylvestris was also a competitor species to Betula pendula and B.litwinowii.
Our results (SI Figs. 11b and 12b), as well as species distribution modeling in the North-Western Caucasus (Shevchenko and Geraskina,2019),showed that the potential ranges of Abies nordmanniana and Picea orientalis almost completely coincided. This finding supported previous reports (Nishimura, 2006; Litvinskaya and Salina, 2012) on the convergence of suitable environmental conditions for spruce and fir.Therefore,Shevchenko and Geraskina (2019) suggested that Abies nordmanniana and Picea orientalis could form mixed communities within the entire range of dark coniferous forests of the North-Western Caucasus. At the same time, in the Caucasus, the areas of pure spruce forests, as well as fir-spruce co-dominated forests, were relatively small (Litvinskaya and Salina, 2012; Shevchenko and Geraskina, 2019). In our opinion, the similarity and highly competitive nature of the ecological niches of the two species(Table 4,SI Figs.1 and 2)determined the low probability of the occurrence of fir-spruce co-dominated forests. Probably, Abies nordmanniana displaced Picea orientalis from territories suitable for both species.Gokturk and Tıras¸ (2020)also reported that in the mixed stands of Ovacik Forests of Artvin, Turkey, Picea orientalis tended toward a clumped distribution, avoiding a tree-wise mixture with Abies nordmanniana and Pinus sylvestris. Accordingly, in such mixed communities,Picea orientalis was not competitive and could only thrive in groups.Anthropogenic effects and the ability of fir to recover faster after felling and fires could also limit the distribution of fir-spruce forests in the Caucasus (Shevchenko and Geraskina, 2019). In addition, according to our data (Table 4, SI Figs. 1 and 2), as well as Litvinskaya and Salina(2012)report,the difference in the real ranges of Abies nordmanniana and Picea orientalis was also due to the fact that fir forests prefer wetter habitats.Thus,in BA Models,embergerQ still retained a significant effect on the distribution of pure fir forests.
The distribution of pure spruce forests in the Caucasus was also limited by the presence of pure beech forests in habitats suitable for both species(Table 4,SI Fig.2).Fagus orientalis is the most widespread shade tolerant deciduous species in the Caucasus. Its potential range covered the area of coniferous-broad leaved forests of the North-Western Caucasus(Shevchenko and Geraskina,2019)and the potential range of dark coniferous forests throughout the Caucasus (SI Figs. 9b, 11b and 12b).The range of embergerQ values in habitats suitable for beech forests was wider than those for spruce and fir forests. At the same time, Fagus orientalis preferred rather gentle slopes located lower in altitude. This was probably why the upper TRI values for pure spruce forests in BA Model shifted to the range of highly rugged areas(Tables 4 and SI Fig.2).In our study,despite the similarity of ecological niches,Fagus orientalis was not a competitive species for Abies nordmanniana. This result is consistent with a previous study of fir-beech co-dominated forests in the Northwest of Turkey (Coban, 2020), which showed that shade tolerance of both species provided a high degree of their spatial mingling.In the Western Caucasus, beech and fir also formed stable mixed stands in the final stages of forest development(Litvinskaya and Salina,2012;Gornov et al.,2018).
The present study revealed that the most important ecological predictor in the potential distribution of Fagus orientalis was Carpinus betulus(Table 4,SI Fig.4).According to Sikkema et al.(2016)and Gornov et al.(2018), the hornbeam is a fast-growing tree species with long-distance seed distribution that prefers sunny habitats, but at the same time, it is one of the most shade-tolerant native trees in Europe. In mixed forests,this species can be a dangerous invader (Sikkema et al., 2016). Jensen et al. (2008) showed that in central and northern Europe, there was a significant negative correlation at the local scale between relative areas of Fagus orientalis and Carpinus betulus due to the competitive relationship between the two species.This finding was also supported by Yakhyayev et al. (2021), who reported that in the northern regions of Azerbaijan,complex cuttings in the secondary hornbeam stands were an effective measure for regenerating the natural beech stands. Carpinus betulus was observed in pure groups in the fir-beech co-dominated forest of northwestern Turkey, where it was not competitive compared to both shade-tolerant species(Coban,2020).However,Carpinus betulus replaced beech forests at the felling sites,which caused an increase in the area of hornbeam forests in the Central Caucasus by 6%in the first decade of the 21st century(Tembotova et al.,2012).
At the same time, the ecological niche of Carpinus betulus was most similar to the ecological niche of Pinus sylvestris, which was probably largely due to the relative drought resistance of both species(Table 4,SI Figs.3 and 5).In turn,there were no strong competitors for Pinus sylvestris among the studied species. Significant influence on the species distribution was retained by TRI,the lower values of which shifted to the range of moderately rugged areas.Like spruce forests,according to BA Model,pure pine forests were concentrated on steeper slopes at higher altitudes.Based on the studies by Coban(2020)and Gokturk and Tıras¸ (2020),we assumed that light-demanding Pinus sylvestris was able to avoid competition due to concentration in the upper layer of stands and exclusion from suppression by shade-tolerant species.Thus,Coban(2020)showed that Pinus sylvestris demonstrated random distribution and spatial association with other species in fir-beech forests of northwestern Turkey.The author also concluded that the pioneer character of this species allowed its establishment early in the succession stage (Coban, 2020).The ecological plasticity of Pinus sylvestris and its ability to occupy habitats unsuitable for other species(Table 4,SI Figs. 3 and 10a) were also important in reducing competition with other species.
The similarity of ecological niches of both birch species was due to their similar requirements for relief conditions, temperature, and water regimes.These species often form mixed stands of the upper forest belt in the Caucasus Mountains, below which there is a belt of pure pine or birch-pine forests.Accordingly, the influence of the biotic factor caused the displacement of pure birch forests upward and to steeper slopes(Table 4,SI Figs.6 and 7).
4.3. BAM Model
According to Peterson et al. (2011), the movement factor (M set of environmental conditions)represented the geographic regions accessible for the species for a certain period.Analysis of this factor,along with sets of biotic and abiotic environmental conditions, made it possible to establish the “occupied distributional area” (Sober′on and Peterson,2005;Peterson et al.,2011).In our study,we aimed to approximate the final maps of the forest-forming species ranges to their real distribution in the Caucasus with the possibility of practical application.Therefore,we defined the geographic regions accessible for the species as the distances from the sites with the most optimal conditions,where the probability of species occurrence was higher than 0.5.We considered these distances as an indicator of species mobility.Birch forests were the most“mobile”in the Caucasus (0–20 km of accessible areas from optimal habitats), followed by fir,beech,and pine forests(0–10 km),and spruce forests(0–6 km). Areas suitable for hornbeam forests were the most compact (only 0–1 km from optimal habitats).
We revealed a significant effect of movement factor on the potential distribution of the main forest-forming species in the Caucasus,with the exception of Betula litwinowii and B. pendula. BAM Models predicted a relatively low contribution of movement factor to the distribution of pure birch forests.Competition from each other and Pinus sylvestris,as well as mountain terrain and water regime, mainly determined the modern ranges of both species in the Caucasus. The acceptable area for Betula pendula exceeded that for B.litwinowii(Table 5,SI Figs.13c and 14c)due to lesser dependence on the habitat humidity and the slope steepness.At the same time,the area of optimal habitats for Betula litwinowii exceeded that for B. pendula because of the large occupied territories in the relatively humid highlands of the Western and Central Caucasus.Geographic expression of the occupied distributional area of Betula litwinowii and B. pendula was the upper forest belt in the highlands throughout the Caucasus(Fig.3, SI Fig.15).
Movement factor significantly limited the areas of suitable habitats of widespread forest-forming species in the Caucasus(Pinus sylvestris,Fagus orientalis, and Carpinus betulus) (Table 5, SI Figs. 8c, 9c and 10c). According to A Models, the geographic expression of fundamental ecological niches of these species covered more than 40 thousand km2throughout the Caucasus, but the influence of biotic and movement factors reduced this area by 2–2.7 times. Pinus sylvestris with a wide ecological range in main environment gradients,spread from nearly level to highly rugged areas with warm summer continental, hemiboreal,oceanic, or hot summer continental climates. Among studied forestforming species, there were no strong competitors for Pinus sylvestris.However,the complex influence of biotic and movement factors shifted the distribution of pure pine forests to more local areas in the highlands of the Greater and Lesser Caucasus(Fig.3,SI Fig.15).
Although Fagus orientalis preferred more humid bioclimatic conditions than Carpinus betulus, the potential ranges of these species largely overlapped throughout the Caucasus(Fig.3,SI Fig.15)and there was a competitive relationship between them. Carpinus betulus mainly limited the distribution of Fagus orientalis only in disturbed beech forests (e.g.felling sites) due to its rapid growth and renewal. Nevertheless, the competition from Carpinus betulus, and, to a lesser extent, the species mobility(0–10 km from optimal habitats),limited the real distribution of Fagus orientalis to more compact areas within the boundaries of its potential distribution (foothills, low and middle mountains of the Greater and Lesser Caucasus). To the greatest extent, the movement factor influenced the distribution of Carpinus betulus in the Caucasus. Initially,small optimal area and low mobility of the species (only 0–1 km)significantly limited the geographic expression of the realized niche of Carpinus betulus to relatively small suitable and optimal sites from the foothills to the middle mountains of the Western Greater Caucasus and the Lesser Caucasus.
Due to the dependence of Abies nordmanniana and Picea orientalis on factors of water regime, their predicted ranges in the Caucasus were initially relatively small (Table 5) and almost completely coincided(Fig. 3, SI Fig. 15). At the same time, competition from other forestforming species (Fagus orientalis, Pinus sylvestris, Abies nordmanniana)and relatively low mobility of Picea orientalis (0–6 km) limited its“occupied distributional area”to the small territories in the highlands of the potential range (SI Fig. 12c). Thus, the highlands of the Western Caucasus and the Georgian part of the Central Greater Caucasus,as well as the highlands of the western Lesser Caucasus and the borders of Russia and Azerbaijan can be recommended for conservation and restoration of Picea orientalis in the Caucasus. Abies nordmanniana is able to displace Picea orientalis from territories suitable for both species, especially in disturbed ecosystems. Therefore, the real distribution of Abies nordmanniana in the Caucasus was mainly determined by habitat humidity and species mobility (0–10 km). Areas suitable and optimal for pure fir forests, which can be recommended for conservation and restoration of Abies nordmanniana, were compacted to the middle mountains and highlands throughout the Western Greater Caucasus,Georgian part of the Central Greater Caucasus, and the west of the Lesser Caucasus (SI Fig.11c).
5. Conclusions
In our study, the potential spatial distribution of the main forestforming species in the Caucasus depended on competitors, then on species dispersal capability and abiotic variables. The main abiotic factors were topographic conditions (TRI) and water regime (embergerQ, PETDriestQuarter). Competitive factors reduced the area of geographic expression of fundamental ecological niches of species by 1.2–1.7 times.Picea orientalis was the main competitor to Abies nordmanniana,while the main competitor of Fagus orientalis was Carpinus betulus, the main competitor of Betula pendula was B.litwinowii,and Pinus sylvestris was also a competitor to both birch species.The similarity and highly competitive nature of ecological niches led to the displacement of Picea orientalis by Abies nordmanniana from territories suitable for both species, and to a reduction in the area of pure spruce forests.The difference in the ranges of Abies nordmanniana and Picea orientalis was also due to the fact that fir forests preferred wetter habitats (an indirect indicator of the differentiation of species niches). Carpinus betulus replaced Fagus orientalis at felling sites, but in undisturbed forests, it is less competitive compared to more shade-tolerant beech stands. Carpinus betulus occupied territories with drier bioclimatic conditions than Fagus orientalis.Both birch species occurred in the upper forest belt in the Caucasus Mountains, however,Betula litwinowii preferred wetter habitats in more rugged areas. The competition from Pinus sylvestris caused the displacement of pure birch forests upward and to steeper slopes.
Species mobility reduced the areas of geographic expression of realized ecological niches by 1.2–1.5 times for Abies nordmanniana (species mobility 0–10 km from optimal habitats), Picea orientalis (0–6 km), and Pinus sylvestris(0–10 km)and by 1.7–1.8 times for Fagus orientalis(0–10 km)and Carpinus betulus(0–1 km),but almost did not affect the potential distribution of Betula litwinowii and B.pendula(0–20 km).
Distribution maps modeled on abiotic,biotic,and movement factors were the“occupied distributional areas”of the forest-forming species and can be used to develop strategies for the protection and restoration of mountain forests of the Caucasus.
Funding
This work was supported by the state assignment “Patterns of the Spatiotemporal Dynamics of Meadow and Forest Ecosystems in Mountainous Areas(Russian Western and Central Caucasus)”,No.075-00347-19-00.
Authors’ contributions
The idea of research belongs to PR, FT, and VCh. PR developed the distribution models and maps.YS,MM,and AA made a literature review and data processing.Statistical treatment,analysis of the results,and the writing of the paper were made by PR,FT,and VCh.The authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Part of the datasets used and/or analyzed during the current study is publicly available in the Global Biodiversity Information Facility(GBIF)on the above-mentioned DOI. Part of the datasets is available from the corresponding author on reasonable request.
Acknowledgements
Not applicable.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://do i.org/10.1016/j.fecs.2022.100019.
- Forest Ecosystems的其它文章
- Removing harvest residues from hardwood stands affects tree growth,wood density and stem wood nutrient concentration in European beech (Fagus sylvatica) and oak (Quercus spp.)
- Modeling of cold-temperate tree Pinus koraiensis (Pinaceae) distribution in the Asia-Pacific region: Climate change impact
- Carbon stocks in a highly fragmented landscape with seasonally dry tropical forest in the Neotropics
- Intraspecific variation in shoot flammability in Dracophyllum rosmarinifolium is not predicted by habitat environmental conditions
- Current climate overrides past climate change in explaining multi-site beta diversity of Lauraceae species in China
- Impact of nitrogen input from biosolids application on carbon sequestration in a Pinus radiata forest

