Electrodeposition of chitosan/graphene oxide conduit to enhance peripheral nerve regeneration
Ya-Nan Zhao ,Ping Wu ,Zi-Yuan Zhao ,Fei-Xiang ChenAo XiaoZhi-Yi YueXin-Wei HanYong Zheng,Yun Chen
Abstract Currently available commercial nerve guidance conduits have been applied in the repair of peripheral nerve defects.However,a conduit exhibiting good biocompatibility remains to be developed.In this work,a series of chitosan/graphene oxide (GO) films with concentrations of GO varying from 0–1 wt%(collectively referred to as CHGF-n) were prepared by an electrodeposition technique.The effects of CHGF-n on proliferation and adhesion abilities of Schwann cells were evaluated.The results showed that Schwann cells exhibited elongated spindle shapes and upregulated expression of nerve regeneration-related factors such as Krox20 (a key myelination factor),Zeb2 (essential for Schwann cell differentiation,myelination,and nerve repair),and transforming growth factor β (a cytokine with regenerative functions).In addition,a nerve guidance conduit with a GO content of 0.25% (CHGFC-0.25) was implanted to repair a 10-mm sciatic nerve defect in rats.The results indicated improvements in sciatic functional index,electrophysiology,and sciatic nerve and gastrocnemius muscle histology compared with the CHGFC-0 group,and similar outcomes to the autograft group.In conclusion,we provide a candidate method for the repair of peripheral nerve defects using free-standing chitosan/GO nerve conduits produced by electrodeposition.
Key Words:chitosan;electrodeposition;free-standing;graphene oxide;nerve conduit;nerve factors;Schwann cells;tissue engineerin
Introduction
Peripheral nerve injury (PNI) caused by trauma,burn,or surgical intervention is a global clinical problem that leads to complete or partial loss of motor,sensory,and autonomic functions,therefore drastically affecting the quality of life of patients (Mobini et al.,2019;Hussain et al.,2020;Chen et al.,2021;Zaminy et al.,2021).Regeneration following PNI is accompanied by a multiplex biological process in which Schwann cells (SCs) play an important role(Zaming et al.,2020a;Li et al.,2021).Mechanistically,SCs provide a suitable microenvironment for the inventory and regeneration of nerve cells,furnish vital nutrition,support cell adhesion,and guide directional nerve cell migration(Wang et al.,2021a,c;Cai et al.,2022).SCs inherently participate in Wallerian degeneration,whereby they contribute to phagocytosis of myelin and axon debris,axon regeneration,deposition of a supportive extracellular matrix,neurotrophin release,and macrophage recruitment to clear debris after PNI(Navarro et al.,2007;Novikova et al.,2008;Deumens et al.,2010;Carriel et al.,2017).Thus,implantation of a nerve guidance conduit (NGC) at the site of injury needs to support SC growth to benefit functional recovery of the injured nerve.
Artificial NGCs developed by tissue engineering from materials including polylactide-caprolactone,polylactic acid,chitosan (CH),collagen,graphene oxide (GO),and cellulose have been widely used for PNI repair (Siemionow et al.,2010;Wu et al.,2020).Among these components,CH is a cationic polysaccharide broadly applied in the field of peripheral nerve regeneration for its biocompatibility,biodegradability,and antimicrobial activity (Lau et al.,2018;Wu et al.,2021;Liu et al.,2022).The oxidized form of graphene,GO,has abundant oxygen-containing groups,such as hydroxyl (-OH) and carbonyl(-C=O) groups,a high specific surface area,good mechanical properties,flexibility,and excellent electrical conductivity (Reina et al.,2014;Gardin et al.,2016).In addition,GO is biocompatible at low concentrations (Nishida et al.,2014;Nagarajan et al.,2016) and acts as an alternative for reinforcing material (Han et al.,2011;Ouyang et al.,2015;Sayyar et al.,2015).Importantly,GO has also been shown to promote the proliferation of SCs,hippocampal cells,PC12 cells,and stem cells (Li et al.,2011b,2016;Gardin et al.,2016;Liu et al.,2017),
CH-based NGCs can be prepared by chemical crosslinking or mold-forming techniques.The basic function of the NGC is to support the adhesion,proliferation,and migration of nerve and glial cells (Vijayavenkataraman,2020).To improve thein vivo
performance of CH-based NGCs,a series of material modification strategies were developed.Rao et al.(2020) designed an aligned-CH hydrogel NGC grafted with bioactive peptides,which yielded great application effects for repair of long-distance sciatic nerve defects.Chen et al.(2021) showed that depletion of interleukin 17F in the nerve regeneration microenvironment could improve the repair effect of CH-based NGCs.In addition,numerous physical and biological parameters were shown to be beneficial for nerve regeneration (Grinsell and Keating,2014;Li et al.,2020).In this work,we hypothesized that incorporation of GO into CH-based NGCs could enhance their biocompatibility.No previous report has described a CH/GO composite NGC,likely because of a lack of an appropriate material processing technology.Electrodeposition is an attractive material processing technique characterized by programmable assembly in response to device-imposed stimuli (Xi et al.,2009;Sun et al.,2015).Because of its self-assembling and pH-responsive properties,CH can be used to build a film on a conducting electrode using electrodeposition methods (Wang et al.,2005).Compared with conventional techniques,electrodeposition is a more facile and precise method to form stable CH films with controllable thickness,stiffness,and other properties on the electrode surface (Liu et al.,2014).Specifically,the oxygencontaining functional groups of GO greatly enhance its surface ability to bind other materials (Shen et al.,2012;Li et al.,2016).Thus,GO could be homogeneously dispersed into CH solution and subsequently co-deposited along with CH.
This work aimed to fabricate a series of CH/GO composite NGCs using a novel electrodeposition technique,and subsequently evaluate their chemical composition,surface morphology,andin vitro
biocompatibility.Our findings suggest that the obtained CH/GO composite NGCs have relatively good performancein vivo
for PNI repair.Methods
Materials
Electrodeposition of chitosan/graphene oxide composites
CH solution (CS,1 wt%) was prepared by liquefying CH in a 0.25 M HCl solution under vigorous stirring,and then adjusting the pH value to 5.5 using 0.25 M NaOH solution.GO (0.2 wt%) was dispersed into deionized water under continuous stirring.The GO suspension was added to the CS solution and sonicated for 5 minutes to obtain a homogeneous solution.Next,the prepared CS/GO solution was transferred into a beaker for electrodeposition.A titanium plate (0.1 × 40 × 20 mm) served as the cathode and a platinum wire served as the anode.Electrodeposition was performed by immersing the titanium plate and 1.5 cm of the platinum wire into the CS/GO solution.A programmable DC power supply (Keithley,Shanghai,China) was employed to apply 5–6 V to these two electrodes for 1 hour,resulting in films with a thickness of~3 mm.Following washing of films with distilled water to clear any unbound GO particles,they were dried for further studies.The resultant films were named CHGF-0/0.25/0.5/1 or collectively CHGF-n,where CH stands for chitosan,G for graphene oxide,F for film,and n for the concentration of GO (wt%) in the mixed solution.Table 1
shows the sample names and compositions of various CHGF-n preparations.To further prepare CH/GO NGCs with GO contents of 0 and 0.25 wt%,we replaced the titanium plate of the cathode with a stainless-steel needle.The resultant conduits were named CHGFC-0 and CHGFC-0.25.
Table 1 | Sample codes and compositions of films and conduits
Characterization of CHGF
Morphological analysis of CHGF was evaluated with a digital camera (Coolpix P6000,Nikon,Tokyo,Japan) and scanning electron microscope (SEM;TESCAN,Shanghai,China).For SEM analysis,gold was employed to sputter-coat the dried films,which were then observed with an accelerating voltage of 20 kV(Hu et al.,2019b,2020).To evaluate sample cross-sections,samples were immersed in liquid nitrogen for 10 minutes,fractured,and then broken offto obtain a fresh section.A fourier transform infrared (FTIR) spectrometer(Nicolet,Waltham,MA,USA) was employed to record FTIR spectra in the wavelength range of 4000–500 cm.Afterwards,an automated laser Raman microscope (Horiba,Villeneuve-d’Ascq,France) with an excitation wavelength of 633 nm was employed to characterize the chemical composition of CHGF.A WAXD diffractometer (Bruker,Waltham,MA,USA) in the 2θ range of 5°–40°with a 4°/min scan speed was employed to obtain X-ray powder diffraction(XRD) patterns.
The tensile strength of CHGF was determined with a universal testing machine(SANS,Dongguan,China) at 8 mm/min rate of elongation (Wang et al.,2020).CHGF samples were cut into 10 × 1 cmpieces and immersed in phosphatebuffered saline (PBS) for 2 hours.Finally,careful blotting of the wet samples was performed with filter paper to remove the surface solution,and samples were immediately tested.Six samples were evaluated to determine the mean value.
Biocompatibility evaluations 3-[4,5-Dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide assay
The cytotoxicity of CHGF-n preparations was tested by 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) assay.SCs were cultured with α-MEM containing 10% fetal bovine serum and 100 U/mL penicillinstreptomycin under 37°C and 5% COconditions.CHGF extracts were prepared by adding sterilized CHGF-n to α-MEM (0.2 g sample in 1 mL culture medium) and incubating at 37°C for 72 hours (Wang et al.,2021b).
SC viability and proliferation was detected using an MTT assay.Initially,cells were seeded in 96-well plates coated with laminin (2 × 10cells/well) for incubation at 37°C for 24 hours.Next,extracts (50 µL) were added to the plate and cells were allowed to grow in 5% COat 37°C.Culture medium with cells but no extract was employed as a negative control,while culture medium without extract or cells was utilized as a blank control.Cells were allowed to grow for 24,48,or 72 hours and then 20 µL of MTT solution(Beyotime,Suzhou,China) was mixed into wells and incubated for 4 hours at 37°C.Subsequently,200 µL of DMSO was added.Swirling of the dissolved solution was performed for approximately 10 minutes with a shaker to obtain a homogenous solution.The absorbance of each well was measured with a microplate reader (Shimadzu UV-1601,Shanghai,China) at a 490-nm wavelength.The following formula was employed to compute cell viability:

In which A,A,and Adenote the absorption values of the sample,negative control,and blank control,respectively.
Then she sat down and cried, but her tears fell just on the spot where a rose bush had sunk, and when her warm tears watered the earth, the bush came up in full bloom just as it had been before
Cell contact experiments
To explore the potential utilization of CHGF as an NGC,SCs were directly cultured on the samples to observe their behaviors.Prior to the seeding stage,CHGF was sterilized and washed three times with distilled water.For cell morphology observation,SCs were seeded at a density of 5 × 10cells per well on the surface of CHGF and incubated for 72 hours in 5% COat 37°C.Tissue culture plastic coated with laminin was used as a control.After incubation,cells on the films were fixed with 3.7% paraformaldehyde solution overnight at room temperature.The following day,fixed cells on the surfaces of CHGF samples were dehydrated in a graded series of ethanol solutions(Zhao et al.,2018b).Finally,samples were coated with gold under vacuum for observation by SEM.SCs on the surface of CHGF were further identified by calcein acetoxymethyl ester (calcein AM) staining (50 µg;Calcein AM Assay Kit,ab 141420;Abcam,Cambridge,UK).Briefly,SCs on samples were stained with AM dye for 30 minutes at 37°C.Finally,samples were rinsed three times with PBS and observed by fluorescence microscopy (Olympus,Tokyo,Japan).
Quantitative reverse transcription-polymerase chain reaction analysis
mRNA expression ofKrox20
(a key myelination factor),transforming growth factor β (TGF-β
,a cytokine with regenerative functions),andZeb2
(essential for Schwann cell differentiation,myelination,and nerve repair) was evaluated in SCs cultured with CHGF-n extracts.After SCs were planted onto tissue culture plastic or CHGF for 72 hours,mRNA expression levels ofKrox20
,Zeb2
,andTGF-β
in SCs were determined with quantitative reverse transcriptionpolymerase chain reaction (qRT-PCR).First,total cellular RNA was extracted from SCs with TRIzolreagent (Sigma) according to the manufacturer’s instructions.Next,cDNA was transcribed from total cellular RNA with a cDNA Synthesis Kit (Thermo Fisher Scientific,Waltham,MA,USA).Next,Maxima SYBR Green or ROX qPCR Master Mix was employed to perform qRT-PCR.The temperature gradient of amplification was as follows:95°C for 15 minutes;and 40 cycles of 15 seconds at 94°C,30 seconds at 60°C,and 30 seconds at 72°C.Relative expression levels were calculated using the comparative 2method.Primers used in this study are listed inTable 2
.
Table 2 | Primer sequences for quantitative reverse transcription-polymerase chain reaction
Animals and surgical procedures
All animal experiments were performed in accordance with Guiding Opinions on the Treatment of Laboratory Animals issued by the Ministry of Science and Technology of the People’s Republic of China,and approved by the Animal Care and Welfare Committee of the Wuhan University School of Medicine(2018116) on October 22,2018.Forty-two female Sprague-Dawley rats (200–220 g) were provided by Wuhan University Laboratory Animal Center (Wuhan,Hubei Province,China),and then randomly divided into three groups:autograft,CHGFC-0,and CHGFC-0.25.For surgical procedures,animals were anesthetized by an intraperitoneal administration of 1% pentobarbital sodium (150 mg/kg) (Sigma).Next,the sciatic nerve in the right hind limb was exposed and a 10-mm nerve defect was created and repaired with autograft or a CHGFC conduit.For the autograft group,the excised autograftwas rotated by 180° and sutured to the proximal and distal nerve stumps.In CHGFC-0 and CHGFC-0.25 groups,conduits with GO contents of 0% and 0.25%were sutured to the proximal and distal nerve stumps of the injured nerve.After transplantation,the musculature and skin were sutured.All procedures were performed under aseptic conditions and penicillin (3 mg/kg) was administered postoperatively for 3 days.After 90 days,rats were euthanized and evaluated for nerve regeneration.
Sciatic functional index
To explore functional recovery after surgery,sciatic functional index (SFI) was evaluated at 90 days.Black ink was applied to the hind paws of rats,which were subsequently placed in a 15 × 100 cm corridor covered with a sheet of white paper.Three parameters including print length (PL:longest distance from toe to heel),intermediate toe spread (IT:distance from the second to fourth toes),and toe spread (TS:distance from first to fifth toes) were obtained from the paw prints.Data for normal (N) and experimental (E) hind legs were utilized.The formula SFI=(–38.3 × (EPL– NPL)/NPL)+(13.3 × (EIT–NIT)/NIT)+(109.5 × (ETS– NTS)/NTS)– 8.8 was employed to compute SFI.SFI values range between–100 and 0,with–100 denoting complete dysfunction of nerves and 0 denoting good function.
Electrophysiological test
Electrophysiological studies were also used to evaluate functional recovery of the regenerated nerve.At 90 days after implantation,rats were anesthetized and the sciatic nerve on the operative side was re-exposed.A stimulation electrode was applied to the proximal nerve trunk of the rat,and compound muscle action potentials (CMAPs) of the gastrocnemius belly on the operative side were recorded.The stimulating mode was set to pulse mode with a 1-Hz frequency,10-mV stimulus intensity,and 1-ms duration.
Histological detection of nerve regeneration
At 90 days after implantation,the distal nerve ends of each group were obtained and fixed in 2.5% glutaraldehyde overnight.After washing with PBS,samples were post-fixed in 1% osmium tetraoxide (Sigma) for 1 hour,cleaned,dehydrated,and embedded in Epon 812 epoxy resin (Sigma).Next,the epoxy resin was cut into ultrathin sections of 50 nm thickness and stained with lead citrate and uranyl acetate.Finally,stained sections were examined by transmission electron microscopy (FEI,Thermo Fisher Scientific).Image-Pro Plus 6.0 (Media Cybernetics,Silver Spring,MD,USA) was employed to obtain the area of myelinated axons,thickness of myelin sheaths,and number of myelin sheaths.
Gastrocnemius muscle measurement and histological assessment
Morphology and function of the gastrocnemius muscle are reliable parameters to examine nerve regeneration (Xue et al.,2017).At 90 days after implantation,the bilateral gastrocnemius muscles were dissected and weighed.The muscle recovery ratio was calculated as the right-side weight divided by the left-side weight.Next,gastrocnemius muscles were stored in 4% paraformaldehyde until staining with a Masson’s Trichrome Staining Kit(Beyotime) to visualize collagen contents.Images of five randomly selected fields in each sample were acquired with a light microscope (Olympus).Image-Pro Plus software was employed to determine the cross-sectional areas of muscle and collagen fibers.The percentage area of collagen fibers was calculated as the collagen fiber area divided by the sum of collagen and muscle fiber areas.
Statistical analysis
No blinded method was applied in the collection or analysis of the results.Data are expressed as the mean ± standard deviation.Statistical comparisons were performed by one-way analysis of variance (ANOVA) followed by the least significant difference test using SPSS 20.0 (IBM,Armonk,NY,USA).P
<0.05 was considered statistically significant.Results
Fabrication of CHGF
The increased pH gradient generated close to the electrode causes CH electrodeposition on the cathode (Zhao et al.,2014).GO sheets are chemically reduced in aqueous solutions,leading to their irreversible agglomeration(Stankovich et al.,2007).Thus,it is reasonable to speculate that the GO sheets could directly attach to the electrode surface (Chen et al.,2011).When a voltage is applied,protons around the electrode surface are consumed,leading to a relatively high localized pH.As a result,the positively charged CS chains and negatively charged GO wrap around to the cathode and are co-deposited from bulk solution together.Conclusively,the electrodeposition method is a simple and alternative strategy to fabricate CHGF on conductive substrates.
Characterization of CHGF
Optical images of CHGF-n are shown inFigure 1A
.It was observed that CHGF-0 was transparent,while CHGF-0.25,CHGF-0.5,and CHGF-1 appeared light brown,dark brown,and black,respectively.These results confirmed that GO was successfully electrodeposited together with CH.Next,the microstructure of CHGF-n was observed by SEM.As shown inFigure 1B
,CHGF-0 exhibited a rough surface morphology.Compared with CHGF-0,CHGF-0.25 exhibited the typical crumpled and wrinkled texture associated with the presence of flexible and ultrathin graphene flakes (Liu et al.,2013).These flakes were evenly distributed among the film surface,providing physical cues for cell adhesion growth.In addition,several studies documented that GO could be used as chemical cue to enhance nerve cell growth and neurite extension (Liu et al.,2017).However,the surface changed as the GO content increased,with CHGF-0.5 exhibiting flake-like structures and CHGF-1 exhibiting a relatively smooth surface,potentially caused by GO accumulation.Figure 1C
displays SEM images of CHGF-n cross-sections.The cross-section structure of CHGF-0 was observed to be smooth.When the addition of GO,uniform flakelike structures appeared on the cross-section of CHGF-0.25.This flake-like structure was replace by a more obvious multilayered structure in CHGF-0.5.However,the multilayered structure appeared slightly deteriorated in CHGF-1,likely owing to the excessive GO added to CS during the electrodeposition process.Conclusively,GO could be evenly dispersed within the CH film and the structure of deposited CHGF-n was influenced by the proportion of GO.Figure 2A
displays the FTIR spectra of CH,GO,and CHGF-n preparations.Bands at 3368,1735,1621,1398,and 1051 cmof GO were attributed to the stretching vibrations of hydroxyl groups,stretching vibrations of carboxylic acid,skeletal vibrations of unoxidized graphitic domains,O-H deformations of the C-OH groups,and C=O stretching vibrations,respectively (Zhang et al.,2017).The FTIR spectrum of CH showed bands at 1655 cmand 1581 cmthat were assigned to amide I and amide II,respectively.CHGF-n exhibited characteristic bands of CH at about 1655 and 1581 cm,verifying the presence of CH in CHGF-n.However,characteristic bands of GO were not observed in the FTIR spectra of CHGF-0.25,CHGF-0.5,or CHGF-1 because the amount of GO was too small.Thus,Raman spectra were further employed to confirm GO incorporation.The Raman spectra of CHGF-n preparations are shown inFigure 2C
.CHGF-0.25,CHGF-0.5,and CHGF-1 exhibited an obvious diamondoid band at 1290 cmand graphitic band at 1600 cmthat were attributed to the two vibration modes of graphene (Ferrari,2007).In contrast,CHGF-0 did not exhibit diamondoid or graphitic bands.Figure 2B
indicates the XRD pattern of CHGF.In the XRD profile of GO,the strong diffraction peak at 11.2° was linked to the presence of oxygen-abundant groups on both sides of the nanosheets,along with the water molecules trapped between nanosheets (Rajabzadeh et al.,2014).CH displayed two sharp peaks at 9.8°and 19.9°,consistent with previous reports.Peaks of CHGF-n observed at 9°–11° and 19°–20° were slightly weaker compared with those of GO and CS,indicating a minor decrease in crystallinity.Collectively,these results indicate that the resultant CHGF was fabricated on the electrode by a green and precise electrodeposition method.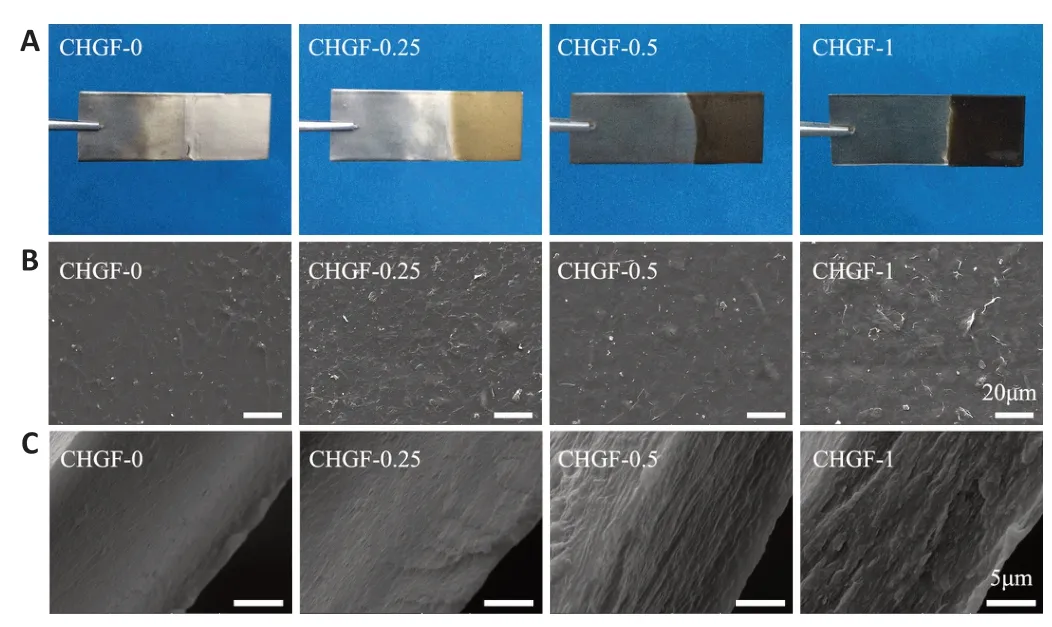
Figure 1 | Morphological observations of prepared CHGF composite nerve conduits.
Figure 2D
displays the tensile strength of CHGF-n preparations.Compared with CHGF-0,the film tensile strength increased with GO concentration to reach 0.58 ± 0.25 MPa for CHGF-0.5.This demonstrates that GO addition could enhance the mechanical properties of CHGF,consistent with previous research (Shen et al.,2012).Nevertheless,incremental increases in GO contents of the film reduced its tensile strength,probably because of deterioration of the multilayered structure,as indicated inFigure 1C
.These results illustrate that the addition of GO enhanced the mechanical properties of neat CHGF-0,resulting in the peeling off of films from the titanium plate as a free-standing chitosan/graphene oxide matrix to construct CHGFC.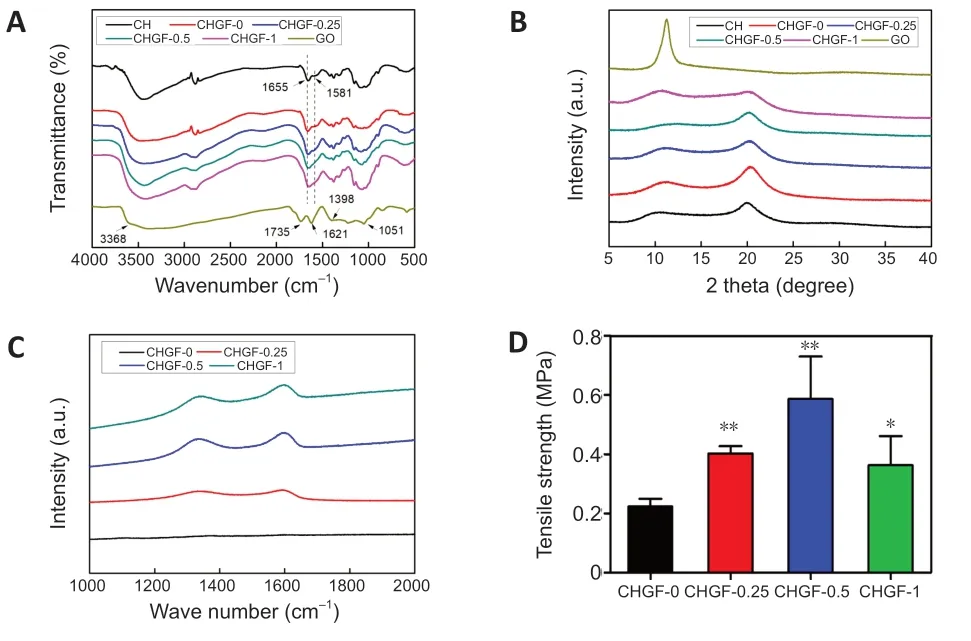
Figure 2 | Physicochemical properties of chitosan/GO composite nerve conduits.
Effects of CHGF on SC growth and behaviors in vitro
As shown inFigure 3A
,the activity of SCs on CHGF-0.25 was highest at 24 hours,but decreased on CHGF-0.5 and CHGF-1,consistent with the notion that GO has dose-cytotoxicity.At 48 hours,the cell activity of each group was higher compared with that at 24 hours,and cell activity on CHGF-0.25 remained the highest.At 72 hours,cell activities on CHGF-0,CHGF-0.5,and CHGF-1 began to decrease.However,CHGF-0.25 retained a remarkable increase of cell viability compared with the control group.These results clearly suggest that cell viability depended on GO content and cell culture duration,and the excellent cytocompatibility of CHGF-0.25 makes it a promising material to construct CHGFC for peripheral tissue engineering applications.Figure 3C
shows SEM images of SCs on CHGF-n.Cells cultured on the CHGF-0,CHGF-0.5,and CHGF-1 were round and clustered together,which may be caused by the hydrophobic surface,microstructure,and chemical composition of these three samples.Cells cultured on CHGF-0.25 were well spread out and exhibited long spindle shapes,possibly due to its hydrophilic characteristics.SCs were further characterized by immunofluorescence staining.As shown inFigure 3B
,in contrast to cells on other samples,cells cultured on CHGF-0.25 had spindle shapes.Therefore,samples with a GO content of 0.25% were identified as the favorable cell carrier to promote the spread and growth of SCs.As shown inFigure 3D–F
,mRNA expression levels ofKrox20
,TGF-β
,andZeb2
in all CHGF-n groups were remarkably higher compared with the control group.Additionally,mRNA expression levels were relatively increased in the CHGF-0.25 group.Conclusively,CHGF-n,especially CHGF-0.25,promoted expression of nerve-related factors such as Krox20,Zeb2 and TGF-β,thus ensuring the regeneration of damaged nerves.Collectively,the results described above show that CHGF-0.25 had the most suitable surface structure,mechanical properties,and cell compatibility for SC growth,and the best performance for nerve-related factor secretion.Thus,the conduit referred to as CHGFC-0.25(based on CHGF-0.25) was selected for animal experiments to evaluate nerve repair effects.CHGFC-0 and autograft groups were used as controls.Animal experiments for CHGFC Motor function of rats
There was a remarkable improvement in motor function of the CHGFC-0.25 group compared with the CHGFC-0 group (Figure 4A
).SFI values of the CHGFC-0.25 group were similar to those of the autograft group,but SFI values of the CHGFC-0 group were significantly lower compared with autograft and CHGFC-0.25 groups (P
<0.01 andP
<0.05,respectively;Figure 4B
).CMAP amplitudes on the surgical side of the CHGFC-0 and CHGFC-0.25 groups were significantly lower compared with the autograft group (P
<0.01 andP
<0.05,respectively;Figure 4D
).Morphology of regenerated nerves
As shown inFigure 5A
,regenerated myelinated fibers were scattered in clusters (with the exception of an occasional unmyelinated fiber) and myelinated axons were coated with a clear myelin sheath in the autograft group.Structures of myelinated fibers in the CHGFC-0.25 group were similar to those of the autograft group.In contrast,myelinated fibers were seldom observed in the CHGFC-0 group.Areas of myelinated axons,thicknesses of myelin sheaths,and numbers of myelin sheath layers were significantly decreased in the CHGFC-0 group compared with the autograft group (P
<0.05 orP
<0.01;Figure 5B–D
),while thicknesses of myelin sheaths and numbers of myelin sheath layers were significantly increased the CHGFC-25 group compared with the CHGFC-0 group (P
<0.01 andP
<0.05).Regenerated nerves in the CHGFC-0.25 group showed better recovery compared with the CHGFC-0 group but remained inferior to the autograft group.
Figure 3 | CH/GO composite nerve conduits exhibited excellent biocompatibility towards SCs in vitro.

Figure 4 | CHGFC-0.25 improved the motor function of rats with peripheral nerve injury 90 days after transplantation.
Morphology and function of target muscles
As displayed inFigure 6A
andB
,gastrocnemius muscle dystrophy on the operative side of the CHGFC-0 group was more obvious compared with that of the autograft group.Results shown inFigure 6C–E
indicate that muscle recovery of the CHGFC-0 group was worse compared with the autograft group,while muscle recovery of the CHGFC-0.25 group was similar to that of the autograft group.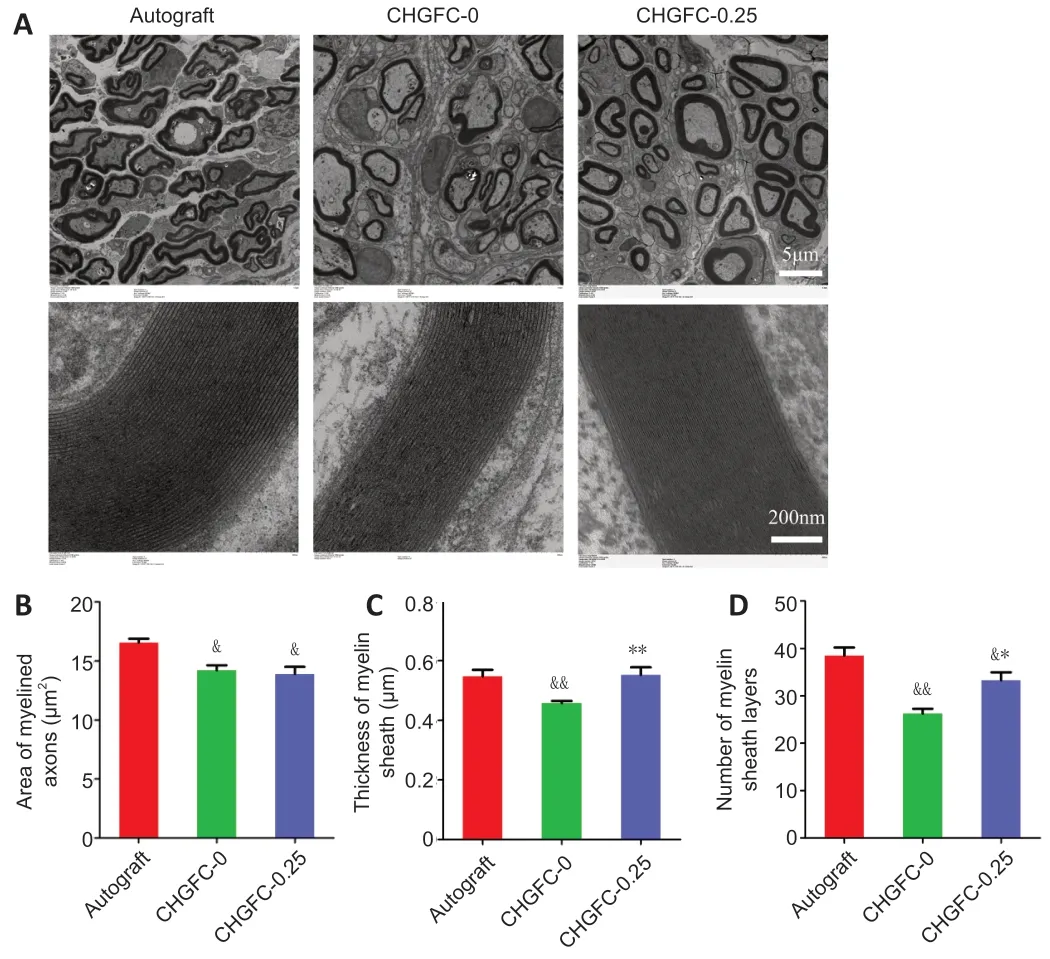
Figure 5 | Histology of sciatic nerve 90 days after transplantation.

Figure 6 | CHGFC-0.25 promoted gastrocnemius muscle regeneration.
Discussion
CH is an ideal biomedical polymer that can be processed by mold-forming and other techniques (Hu et al.,2019a).In our previous works,we successfully developed a variety of CH-based NGCs.On this basis,thein vivo
application effect of these CH-based NGCs was further improved by physicochemical and biological methods.For example,multi-channel NGCs were prepared to promote the proliferation and directional migration of nerves cells,effectively shortening the time required for nerve recanalization (Zhao et al.,2018a).Electrodeposition,a simple and efficient material processing technology,has been widely used for surface modification of metal devices.Our group and cooperative teams successfully introduced electrodeposition technology into the field of biomaterials,and successively developed a series of chitin/CH biomaterials with good economic benefits (Zhao et al.,2018a;Liu et al.,2021).Preliminary progress has been made in the development of electrodeposited CH-based NGCs.Notably,this work revealed for the first time that GO could effectively improve the biocompatibility and bioactivity of CH-based NGCs for glial cells,and thus improve the application effectin vivo
.GO is an inorganic non-metallic material with desirable conductivity and biodegradability.Owning to its high performance,publications describing GO in the field of biomedical materials have consistently increased annually (Shende and Pathan,2021).Our results showed that the introduction of GO into CH significantly changed the chemical composition,micro-structure,mechanical strength,and biocompatibility of the obtained composite NGC.Thus,inclusion of GO might be a key reason for the promotive effects observed for NGCs.In recent years,the biological mechanism of GO has been revealed.We assumed that GO could be swallowed by glial cells during degradationin vivo
,whereby it participates in intracellular signal transduction to synergistically promote nerve regeneration.In conclusion,a GO-modified CH-based NGC prepared by electrodeposition technique promoted the treatment effect for PNI,which was attributed to synergistic effects between the NGC and glial cells.Above all,the application of GO can be refined into a general strategy to upgrade existing NGC materials.Limitations
Even animals with permanently sectioned sciatic nerves tend to develop locomotor strategies that partially compensate for the loss in function induced by nerve damage.Thus,an additional experimental group with impeded reinnervation (a negative control group) is necessary to evaluate the magnitude of treatment responses.
Conclusions
In this study,we constructed a series of CHGFs and evaluated their physicochemical and biological properties.In vitro
experiments showed that GO was successfully inserted in CHGF to enhance its mechanical properties,thus promoting CHGF to peel off from the mold to form free-standing films.CHGF-0.25 significantly enhanced SC growth,extension,and secretion of nerve-related factors such as Krox20,Zeb2,and TGF-β.In vivo
results showed that CHGFC-0.25 could guide the regeneration of injured nerves,thus representing a candidate strategy for peripheral nerve repair.CHGFC prepared by electrodeposition is green,simple,accurate,nontoxic,and harmless,and provides a new direction for preparation of NGCs.Acknowledgments:
The authors are grateful to the Experimental Teaching Center of Basic Medical Sciences,Wuhan University for technical support.
Author contributions:
YNZ,PW and YC designed the study and drafted the manuscript.ZYZ,FXC and AX conducted surgery and behavioral tests.ZYY and YZ carried out histology and image analysis.XWH and YZ helped to draft the manuscript.YC performed manuscript editing and review.All authors read and approved the final manuscript for publication.
Conflicts of interest:
The all authors declare no competing financial interest.
Open access statement:
This is an open access journal,and
articles are distributed under the terms of the Creative Commons Attribution Non Commercial-Share Alike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Neuroaxonal and cellular damage/protection by prostanoid receptor ligands,fatty acid derivatives and associated enzyme inhibitors
- Extracellular vesicles in Alzheimer’s disease:from pathology to therapeutic approaches
- Molecular approaches for spinal cord injury treatment
- Sex-biased autophagy as a potential mechanism mediating sex differences in ischemic stroke outcome
- Adipose tissue,systematic inflammation,and neurodegenerative diseases
- Interleukin-1:an important target for perinatal neuroprotection?

