Adipose tissue,systematic inflammation,and neurodegenerative diseases
Ana Paula de A.Boleti ,Pedro Henrique de O.Cardoso ,Breno Emanuel F.Frihling ,Patrícia Souza e Silva ,Luiz Filipe R.N.de Moraes ,Ludovico Migliolo,
Abstract Obesity is associated with several diseases,including mental health.Adipose tissue is distributed around the internal organs,acting in the regulation of metabolism by storing and releasing fatty acids and adipokine in the tissues.Excessive nutritional intake results in hypertrophy and proliferation of adipocytes,leading to local hypoxia in adipose tissue and changes in these adipokine releases.This leads to the recruitment of immune cells to adipose tissue and the release of pro-inflammatory cytokines.The presence of high levels of free fatty acids and inflammatory molecules interfere with intracellular insulin signaling,which can generate a neuroinflammatory process.In this review,we provide an up-to-date discussion of how excessive obesity can lead to possible cognitive dysfunction.We also address the idea that obesity-associated systemic inflammation leads to neuroinflammation in the brain,particularly the hypothalamus and hippocampus,and that this is partially responsible for these negative cognitive outcomes.In addition,we discuss some clinical models and animal studies for obesity and clarify the mechanism of action of anti-obesity drugs in the central nervous system.
Key Words:adiposity;anti-obesity drugs;hypothalamic inflammation;metabolic disease;neurodegenerative disease;neuroinflammation
Introduction
Obesity is one of the biggest public health problems in the world.The prevalence of overweight and obesity has increased dramatically in almost all developing and developed countries,reaching pandemic levels and affecting 60% to 70% of the adult population of industrialized countries,more frequently among women and in urban areas (Berger,2014;Avgerinos et al.,2019).The global prevalence of overweight and obesity has increased by 27% in adulthood and 47% in childhood over the past few decades (Ng et al.,2014).
Obesity develops when energy consumption exceeds energy expenditure for metabolic and physical activity.Consequently,there is an excessive or abnormal accumulation of fat,which exceeds genetically and epigenetically determined adipose tissue stores.Consequently,this fat is deposited and accumulated as ectopic adipose tissue,leading to an increase in the development of many disease entities.Overweight and obesity are generally defined as a body mass index (BMI) from 25–29.9 kg/mand more than 30 kg/m,respectively (Avgerinos et al.,2019).
Obesity is a risk factor for triggering some chronic diseases,such as hypertension,dyslipidemia,metabolic syndrome,type 2 diabetes mellitus,cardiovascular disease,non-alcoholic fatty liver disease,cancer,and Alzheimer’s disease (AD) (da Luz et al.,2018).Furthermore,the relationship between obesity and cognitive factors,as well as the risk of dementias,such as AD,has drawn attention.Clinical and experimental evidence indicates that obesity and/ or high-fat eating are associated with deficits in learning,memory,and executive functioning (Sabia et al.,2009;Miller and Spencer,2014) and,potentially,brain atrophy (Enzinger et al.,2005;Ward et al.,2005).Besides,evidence indicates that obesity during middle age increases the risk of dementias like AD (Anstey et al.,2011;Miller and Spencer,2014).
It is known that adipose tissue acts as an endocrine organ secreting adipokines and cytokines such as leptin,adiponectin,adipose,resistin,tumor necrosis factor-alpha (TNF-α),interleukin (IL-1β and -6),insulin-like growth factor 1,monocyte chemoattractant protein-1,and visfatin.An alteration in the normal expression of these adipokines,promoted by high levels of fatty acids,can alter the local immune response and induce obesity associated with the pathogen (de Araujo Boleti et al.,2020).In this narrative review,we provide a broad discussion about the association of excess body weight and neuroinflammation,which may be directly linked to cognitive dysfunction.In addition,we will address:1) the relationship between obesity,inflammation,and loss of cognitive function;2) the paradox of obesity and neurodegenerative diseases;3) obesity and hypothalamic inflammation and 4) mechanisms of action of potential drugs in the treatment of obesity and neural diseases.
Search Strategy and Selection Criteria
We performed a literature search of manuscripts of works published in the last 20 years in the PubMed database at the National Center Biotechnology Information (NCBI:https://www.ncbi.nlm.nih.gov) using the terms:neurodegenerative disease,metabolic disease,hypothalamic inflammation,neuroinflammation,adiposity,antiobesity drugs observed in the title,abstract and keywords of the articles.We retrieved further articles suggested by PubMed recommendations and through citation tracking.
With the research related growth to the effects of obesity on neurodegenerative diseases,we prioritized the literature that made this relationship,mainly involving hypothalamic dysfunctions and neuroinflammation.The mechanism of action of obesity-related drugs and their role in neural diseases was an important point for our review.
Inflammation and Obesity
Adipose tissue classification
In humans,several adipose tissue types are distributed throughout the body.Among these are white adipose tissue (WAT),which includes subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT),and brown adipose tissue (BAT).Both types play an important role in regulating metabolism(Figure 1
) (Luo and Liu,2016).White adipocytes from WAT,which stores energy,and it is filled with a large triglycerides drop,which makes the most of its cell volume (Figure 1A
) (Cinti,2018).WAT is divided into two regional and functional deposits– visceral white adipose tissue (vWAT) and subcutaneous white adipose tissue (sWAT)(Zorena et al.,2020).The vWAT expansion is related to insulin resistance,inflammation,dyslipidemia,obesity,type 2 diabetes mellitus,and even increased COVID-19 severity (Zhang et al.,2019;Qi et al.,2020;Favre et al.,2021).sWAT is often associated with metabolic improvement and insulin sensitivity,as it contains brown-like cells known as beige or brown inducible adipocytes,which perform mitochondrial and thermogenic functions,and burn fat (Patel and Abate,2013;Zorena et al.,2020).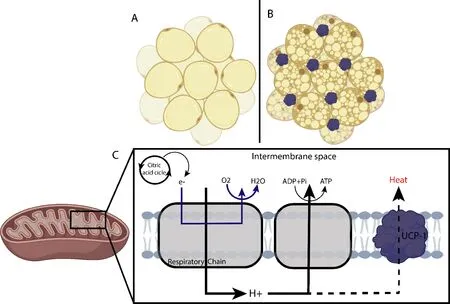
Figure 1 | Differentiation of the two main types of adipocytes and adipose tissue.
Brown adipose tissue,on the other hand,is characterized by more lipid droplets and mitochondria,giving it a brown appearance (Figure 1B
)(Zorena et al.,2020).Brown adipocytes are derived from Myf5cells and they exclusively express uncoupling protein 1 (UCP-1),which can regulate the energy conversion of energy to heat by adenosine triphosphate (ATP)uncoupling in mitochondrial respiration (Figure 1C
) (Townsend and Tseng,2012).Adipocytes maintain body temperature by producing heat without shivering.They are abundant in human neonates,gradually decreasing in adults and decreasing further in obese people (Lidell,2019).In contrast,beige adipocytes (also called Brite adipocytes) are an inducible thermogenic adipocyte form that sporadically resides in WAT deposits(Cheng et al.,2021).Similar to brown adipocytes,beige adipocytes also have abundant mitochondria that express UCP1 and multilocular lipid droplets(Qi et al.,2020).Beige adipocytes interact all the time with immune cells,and the acquisition of thermogenic characteristics requires the induction of anti-inflammatory cytokines such as interleukin-4 (IL-4) by leukocytes in subcutaneous adipose tissue (SAT) (Rao et al.,2014;Guo et al.,2021).However,as we know,tissue-resident immune cells are able to migrate to other organs,including the brain.Immune cell displacement and local cell signaling at blood-brain and blood-cerebrospinal fluid interfaces allow interorgan crosstalk,independent of circulating factors or direct access to brain parenchyma.Although peripheral macrophages have access to the brain of the chronically obese,little is known about neuroimmune interactions at blood-brain and blood-cerebrospinal fluid interfaces (Guo et al.,2021).
Adiposity and systemic inflammation
High-fat diet consumption is considered one of the main factors for obesity induction and its association with metabolic diseases in humans,resulting in changes such as hyperglycemia,hyperlipidemia,and systemic arterial hypertension (de Araujo Boleti et al.,2020).Adiposity leads to the simultaneous development of functional changes that collectively give rise to the so-called metabolic syndrome (Elks and Francis,2010).The increase in fat may result from an increase in the differentiation of preadipocytes into mature adipocytes (hyperplasia),or an increase in the mean cell size of existing adipocytes (hypertrophy).The increase in adipocytes in obesity triggers adipocyte differentiation in a process called adipogenesis,in which pro-inflammatory mediators or adipokines are generated (leptin,adiponectin,resistin,TNF-α,IL-1β,-6 and -8,insulin-like growth factor 1,monocyte chemoattractant protein-1,and visfatin) (de Araujo Boleti et al.,2020).In particular,the hypertrophic adipocyte phenotype has been associated with obesity-dependent disorders (Park et al.,2020).
Systemic inflammation originating in adipose tissue markedly alters levels of adipokines,including leptin,resistin,and adiponectin (Figure 2A
).The adipose tissue expansion,followed by adipocyte differentiation (adipogenesis)and infiltration in adipose tissue of immune cells,including macrophages,neutrophils,and T lymphocytes,has been identified as the main source of cytokines and adipokines,which are the main contributors to this systemic inflammation in obese individuals (Pavlov,2021).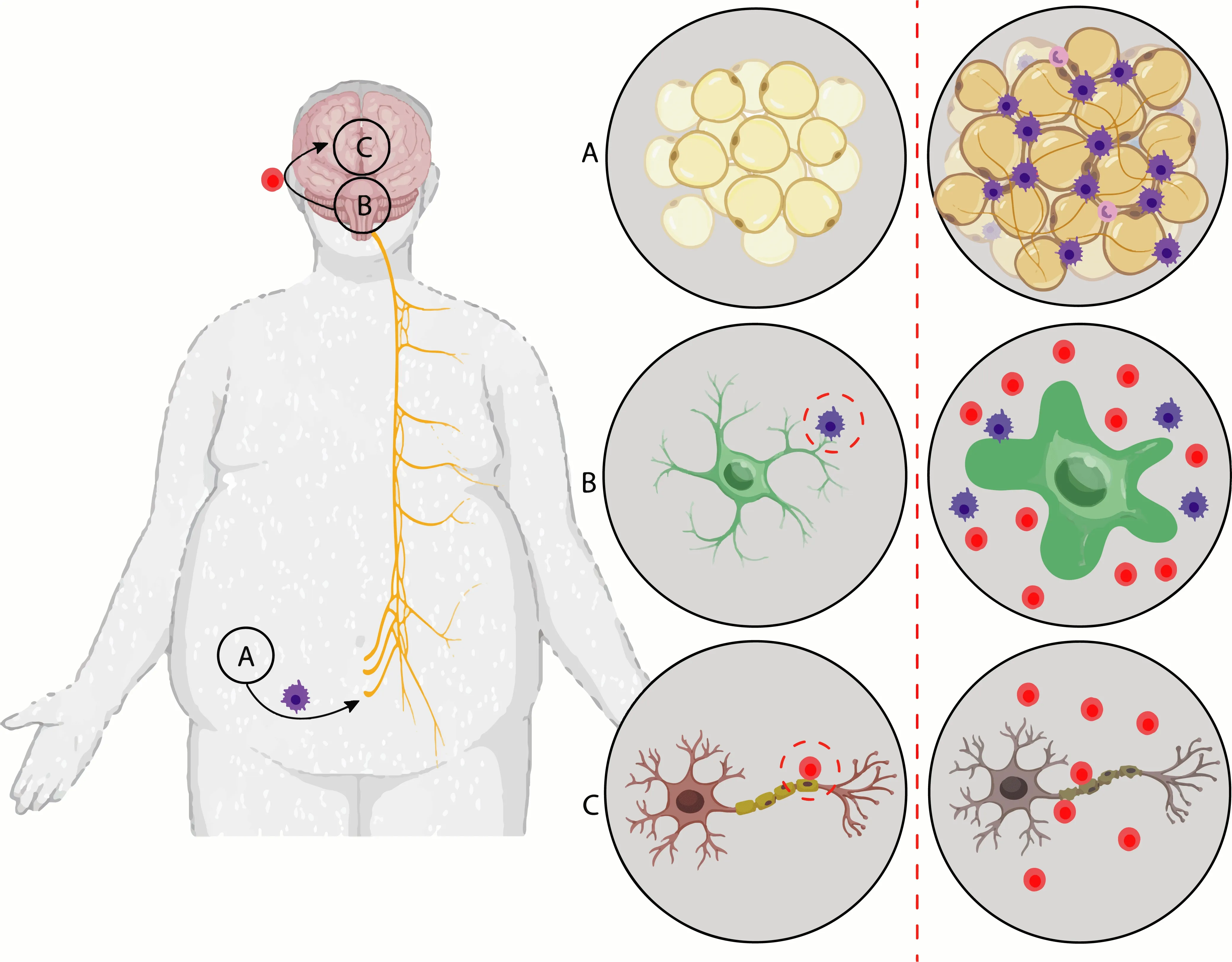
Figure 2 | Stages of the neural degeneration process from adipogenesis.
Proinflammatory cytokines circulating through the nevus vagus cross the blood-brain barrier to the brain and hypothalamus (Figure 2B
),also resulting in NF-κB activation in microglial cells in the hypothalamus and consequently leading to hypothalamic inflammation and leptin resistance (Figure 2C
) (de Araujo Boleti et al.,2020;Zorena et al.,2020).Thus,obesity-associated systemic inflammation has been identified as a risk factor for depression,white matter brain damage,and cognitive dysfunction in the elderly (Miller and Spencer,2014;de Araujo Boleti et al.,2020).High circulating levels of lipopolysaccharide were also detected in obese patients (Stoll et al.,2004).Changes in the gut microbiota (increased lipopolysaccharide-containing microbiota) as a result of high-fat content and increased body weight,and the consequent increase in intestinal permeability is associated with this“metabolic endotoxemia”,which is another major contributor to inflammation in obesity (Andre et al.,2019).Lipopolysaccharide,acting through a mechanism mediated by Toll-like receptor 4,triggers the release of TNF and other pro-inflammatory cytokines,mediating pro-inflammatory signals in the liver,skeletal muscle,and adiposetissue (Stoll et al.,2004;Andre et al.,2019).Acting through Toll-like receptor 4-mediated mechanisms in adipocytes,macrophages,and hepatocytes,free fatty acids trigger intracellular signaling,resulting in the activation of nuclear factor κB (NF-κB) and increased release of TNF and other proinflammatory cytokines (Pavlov,2021).In addition,obesity-related inflammation and insulin resistance are linked to the accumulation of fat in the liver and the development of hepatic steatosis (Kojta et al.,2020).Link between Obesity,Gut Microbiome,and Neural Disorders
Gut microbiota plays a key role in regulating brain functions,maintaining control in homeostasis in innate and adaptive immunity (Kowalski and Mulak,2019),and microbiota-gut-brain axis (Goyal et al.,2021).Normal gut functions are maintained by microorganisms and bacteria,including proinflammatory microbiomes.Evidence shows that several bacterial species,such asEscherichia coli
,Staphylococcus aureus
,andBacillus subtil
,are involved in the synthesis of acetylcholine,one of the main neurotransmitters in the brain (Cattaneo et al.,2017).Intestinal dysbiosis caused by immune system dysfunction may influence the interaction between the gut and the brain during stroke onset (Cho et al.,2021).Bad eating habits and the increase in the use of antibiotics can generate a disturbance in the functionality of the neural system,promoting the development of degenerative pathologies.It is already known that the appearance of intestinal dysbiosis is associated with the interruption of the release of dopamine,the reward hormone (Vamanu and Rai,2021).Recent studies have shown a connection between obesity,type 2 diabetes,and neurodegenerative diseases.The modulation of the microbiota associated with weight loss is among the new therapeutic strategies against neurodegenerative diseases (Ashrafian et al.,2013).
This bidirectional communication network includes the central nervous system (CNS),brain and spinal cord,autonomic nervous system,enteric nervous system,and the hypothalamic-pituitary-adrenal axis.The autonomic system,with the sympathetic and parasympathetic limbs,conducts both afferent signals,arising from the lumen and transmitted via enteric,spinal,and vagal pathways to the CNS and efferent signals from the CNS to the intestinal wall (Carabotti et al.,2015).
Although the gastrointestinal tract (GI) or enteric nervous system functions independently of the CNS,digestive activities involve parasympathetic and sympathetic control,which connect the CNS and GI.In addition,the neural fibers connecting the brain and gut allow for the relaying of sensory information to the CNS and the CNS regulation of GI function (Chi et al.,2018).After a stroke,the gut-brain axis involved in maintaining homeostasis is activated to regulate dysbiosis.The inflammatory activity generated in the gut may also be reflected in the brain microbiome,suggesting a new approach to the pathology and treatment of stroke (Vamanu and Rai,2021).
Altering the gut microbiome population can exacerbate symptoms of gut diseases and CNS diseases such as AD and Parkinson’s disease (Chiang and Lin,2019;Cho et al.,2021).Furthermore,populations of dysbiotic microbiota may exacerbate blood-brain barrier (BBB) permeability,possibly mediating the pathogenesis of AD and other degenerative CNS disorders.Bacterial secretion of amyloids and lipopolysaccharides can upregulate pro-inflammatory cytokines through the gut-brain or BBB axis.
Hypothalamic Inflammation and Neurodegenerative Diseases
The hypothalamus is an important brain region in metabolic homeostasis regulation (Morita-Takemura and Wanaka,2019).The neuronal circuitry in the arcuate nucleus within the midbasal hypothalamus plays an important regulatory role in food intake (Kälin et al.,2015;Mendes et al.,2018;Shin et al.,2019;Lee and Yau,2020).These molecules and hormones stimulate the expression of the anorectic neuropeptide pro-opiomelanocortin and inhibit the expression of agouti-related orexigenic neuropeptides and neuropeptide Y (Trotta et al.,2020).
Proinflammatory mediators from the expansion of adipose tissue reach the hypothalamus from the nevus vagus,promoting increased production of neural cytokines by activating endothelial and glial cells,particularly microglia(Lee and Yan,2020).Microglia play an important role in host defense andtissue repair by secreting inflammatory cytokines and chemokines (Kälin et al.,2015;Maldonado-Ruiz et al.,2017;Saltiel and Olefsky,2017;Lee and Yau,2020).
Pro-opiomelanocortin and neuropeptide Y/AGRP neurons are activated by cytokines,which in turn activate inflammatory signaling pathways,namely Toll-like receptor 4,myeloid differentiation factor 88,JNK,and nuclear transcription factor NF-κB,which promote the interruption of the leptin and insulin signaling pathways,thus making it difficult to detect metabolic signals and the normal regulation of energy homeostasis by these neurons (Zhang et al.,2008;Cai and Liu,2012;Benzler et al.,2015).Prolonged microglial activation can also cause hypothalamic neuronal apoptosis,especially of proopiomelanocortin neurons (Moraes et al.,2009).
Therefore,hypothalamic inflammation can be considered a key mechanism of neurodegeneration (Cai and Liu,2012),although the exact mechanism is not yet known.The phenotypic diversity of microglia is associated with the inactivation of the inflammatory response and tissue repair.Therefore,with altered leptin and adiponectin signaling in the brain,there is more likelihood of cognitive dysfunction and an increased risk of neurodegenerative diseases.
Obese individuals are at increased risk of developing age-related cognitive decline,vascular dementia,mild cognitive impairment,and AD (Panza et al.,2010).The brains of individuals with vascular dementia,cognitive impairment,and Alzheimer’s,showing elevated levels of several microgliaderived cytokines and other immunological mediators,reflect a chronic inflammatory environment in the brain (Hansen et al.,2018;Hickman et al.,2018).Insoluble amyloid β fibrils (fAβ),which constitute the extracellular plaques and neurofibrillary tangles (NFT) containing hyperphosphorylated tau protein (P-tau),where activated microglia and reactive astrocytes were found nearby,maybe the main indicators of Alzheimer’s progression (Tuppo et al.,2005;Mendes et al.,2018).In addition to these diseases,chronic neuroinflammation caused by obesity is mainly characterized by selective and coordinated inflammatory destruction of myelin,with damage to the axon in multiple sclerosis (Voet et al.,2019).
The increase in serum lipids and adipocytokines influences the inflammation of the cerebrospinal fluid (Stampanoni Bassi et al.,2020).In multiple sclerosis,proteases are released from microglia,proinflammatory cytokines,reactive oxygen species,and RNS,and they recruit reactive T lymphocytes,causing toxicity to neurons and oligodendrocyte precursors (Maldonado-Ruiz et al.,2017;Saltiel and Olefsky,2017).Axonal and myelin damage is caused by autonomous cell inhibition of the NF-κB,JNK,and ERK1/2 pathways 42.These results suggest that in multiple sclerosis,microglia cause tissue damage in neurons from obesity-related inflammation (Williams,2012;Hickman et al.,2018).
Obesity-related hypothalamic inflammation can generate changes in microglial function and mRNA profile,being associated with Huntington’s disease (Yang et al.,2017).The expression of mHTT in microglia confers an autonomous cell increase in pro-inflammatory genes.This correlates with increased expression of IL-6 and TNF (Cai and Liu,2012;Grizenkova et al.,2014;Hickman et al.,2018;Luo et al.,2019;Madore et al.,2020).
These changes are unique to microglia and are not seen in other myeloid cells.Functionally,several of the genes that are increased in mHTT microglia are involved in the detection of their environment,such asTlr2
,Cd14
,Fcgr1
,Clec4d
,Adora3
,Tlr9
,andTnfrsf1b
,suggesting an increase in extracellular stimuli.This has been associated with increased microglial neurotoxicity(Rocha et al.,2016;Yang et al.,2017;Palpagama et al.,2019).Exacerbated microglial activation can lead to motor neuron death.Microglia change their phenotype in amyotrophic lateral sclerosis disease progression(Hickman et al.,2018).Some proinflammatory microglia are seen in the spinal cord before the development of the clinical disease;they increase with disease progression and persist in end-stage disease (Ransohoff and El Khoury,2016).Microglia isolated from mSOD1 mice in early disease were neuroprotective,in contrast to microglia isolated in late-stage disease(Ransohoff and El Khoury,2016).The neurotoxicity of microglia mSOD1 is NF-κB dependent and partially mediated by IL-1β.These findings directly implicate microglia with mSOD1 in the progression of amyotrophic lateral sclerosis (Hooten et al.,2015;Chen et al.,2016;Wolf et al.,2017;Thonhoff et al.,2018;Subhramanyam et al.,2019).
Overweight people are more likely to develop these comorbidities,leaving metabolic health impaired in patients with COVID-19 (Mokry et al.,2016;McAlpine et al.,2021).Patients with neurodegenerative diseases,including dementia,Parkinson’s disease,and multiple sclerosis,constitute a significant proportion of patients hospitalized with COVID-19 (McAlpine et al.,2021).Such patients are likely to present altered mental status or worsening of their preexisting neurological symptoms.Patients with cognitive disorders and poor outcomes often have high-risk comorbidities such as obesity (Dietz and Santos-Burgoa,2020;Hussain et al.,2020;McAlpine et al.,2021).
Obesity Association with Cognitive Function and Brain
Cognitive function is one of the most important contributors to health,quality of life,and increased survival in old age.Although the main threat to cognitive function later in life is dementia,elderly people,in general,show a cognitive decline (Dahl and Hassing,2013).On average,cognitive abilities remain stable throughout adulthood and begin to decline around 65 years of age (Murman,2015).
However,there are large inter-individual differences in cognitive aging.Some people show a sharp decline;others remain reasonably constant,and some people even improve (Salthouse,2017;Mella et al.,2018).Although it is common for cognitive skills to decrease with increasing age,several factors can affect cognitive aging,from biological factors,such as genetics,to social factors,like education and watching television (Dahl and Hassing,2013).
While there is ample evidence that a relationship exists between obesity and brain health (structure and function),it is important to recognize that causality is an issue to be considered (Miller and Spencer,2014).Obesity is associated with many pathophysiological changes that have the potential to affect the brain negatively,leading to inflammation,which in turn can be both a cause and a consequence of obesity.It is also possible that reduced cognitive function,in particular executive dysfunction,may predispose individuals to obesity.Executive dysfunction is associated with obesity-related behaviors due to binge eating,depression,and reduced physical activity (Dohle et al.,2018).
There may be impacts on physical and psychological health among obese individuals.Due to difficulties in treating obesity,many other diseases can be triggered in the obese person such as depression,cognitive function loss,aging,Alzheimer and others (Naderali et al.,2009;Sellbom and Gunstad,2012).
When the obese individual presents dyslipidemia,which involves high levels of lipids in the blood,this can increase the chances of accumulation and formation of fatty deposits in the veins and arteries,thus increasing blood pressure,reducing the quality of the blood,and directly affecting organs like the liver and kidneys.Adipose tissue,especially that deposited in the abdomen,possesses a systemic effect due to the expression or inhibition of chemical markers such as prostaglandins 8-isoprostanes,the enzyme 8-oxoguanine DNA-N-glycosylase-1,superoxide dismutase,and total antioxidant capacity (Jiang et al.,2016;García-Sánchez et al.,2020).Intra-abdominal fat accumulation and a carbohydrate-rich diet are capable of contributing to a rise in noradrenaline in the peripheral tissue and of stimulating noradrenaline peripheral receptors,which activate the SNS and can cause hypertension (Kotsis et al.,2010).
Obesity affects the cerebral plasticity and brain structure,and the brain of an obese individual usually presents low cortical thickness on the upper leftand right orbitofrontal,while the ventral volume of the diencephalon is also reduced.It is suggested that alterations in the cerebellum,hippocampus,and medial orbitofrontal can be related to motor and cognitive deficits (Wang et al.,2016).
The effects of insulin on the hippocampus affect areas of the brain that play a key role in learning and memory (Scherer et al.,2021).The expression of glucose transporters such as GLUT2 in the hypothalamus and GLUT4 in specific areas of the brain such as the cerebellum,neocortex,and hippocampus suggest a role of GLUT-induced glucose uptake in neuronal activity (Bartsch and Wulff,2015).The high-fat diet (HFD) produces detrimental effects on brain functions,including decreased neurogenesis,altered BBB integrity,and changes in spinal density and synapse formation (Spinelli et al.,2019).HFD also impairs insulin signaling in the hippocampus and reduces the expression of synaptic proteins,PSD-95 (Quach et al.,2014).Obesity and type 2 diabetes mellitus have also been shown to induce insulin resistance in the hippocampus through different metabolic alterations,including alteration of the hypothalamic-pituitaryadrenal axis leading to elevated levels of glucocorticoids.Thus,glucocorticoid stimulation inhibits the translocation of GLUT4 to the plasma membrane in the rat hippocampus (Biessels et al.,2014).
Diabetes patients in the aging population are at high risk of AD,and there is a reduction in sirtuin 1 activity simultaneously with the accumulation of hyperphosphorylated tau in the AD-affected brain.Du and coworkers (2015)demonstrated through a mouse model of brain insulin resistance with an intracerebroventricular injection of streptozotocin,a reduction in cognitive function,and a decrease in the activity of the neuroprotective protein sirtuin 1 (Du et al.,2014).The neuroprotective effect of Sirt1 requires the presence of TORC1,a brain-specific modulator of CREB activity.Then,sirtuin 1,cooperates with the CREB (cyclic AMP responsive element binding protein)transcription factor promoting CREB-dependent expression of the gene related to synaptic neuroplasticity,brain-derived neurotrophic factor (Di Rosa et al.,2021).
Mice fed HFD show higher levels of reactive oxygen species,superoxide,and peroxynitrite in the brain,leading to a lower level of brain-derived neurotrophic factor and reduced cognitive performance (Di Rosa et al.,2021).Interestingly,feeding HFD to obese rodents inhibited the transport across the BBB of neuroendocrine molecules such as ghrelin and leptin,which promotes synaptic plasticity and cognitive functions.This functional impairment was accompanied by blunt activation of STAT-3,one of the main signal transduction pathways controlled by the fully functional leptin receptor isoform,ObRb (Mainardi et al.,2017).Finally,HFD has been shown to induce neuroinflammation,through the activation of microglia and astrocytes and increase of pro-inflammatory cytokines/mediators such as cyclooxygenase 2,TNF-α,IL-1-β,and IL-6 in the hippocampus of mice (Duffy et al.,2019).In summary,metabolic diseases that affect insulin signaling can impair synaptic function through a multitude of molecular mechanisms targeting neurons,astrocytes,endothelial or inflammatory cells.
Animal Models as an Alternative for In Vivo Studies for Obesity
Among the diverse studies of obesity with comorbidities,cognitive function is the most studied due to impact disease/treatment observation for tests quite and applicability for analyses of other comorbidities.Animal models are very useful in this situation because obesity can be induced,and multiple parameters can be observed,such as weight,lipid profile,and glycemic levels(Speakman,2007).Rodents are the most widely used models,especially the mouse species Mus musculus (Kleinert et al.,2018).de Farias et al.(2020)have demonstrated that obesity treatment with donepezil prevents weight gain,decreases food intake,and reduces mesenteric fat in Swiss mice.The same study shows that 5 mg/kg/d donepezil did not cause behavioral changes in the obese group in comparison with the control group (de Farias et al.,2020).
To measure these behavioral changes,the SHIRPA test (SmithKline Beecham,Harwell,Imperial College,Royal London Hospital,phenotype assessment) was used,and five parameters were analyzed:motor behavior,neuropsychiatric status,sensorial function,autonomic function,the activity of muscular tonus and muscular strength (de Farias et al.,2020).
Another lineage that is widely used because of its susceptibility to dietinduced obesity and polygenic models is the mouse lineage C57BL/6J.Lineages SWR/J and A/J are less susceptible to obesity and related complications,while FVB and 129/Sv are more susceptible to genetic manipulations of the knockout type (Kleinert et al.,2018).
Other models such asCaenorhabditis elegans
(nematode),Drosophila melanogaster
(fruit fly),andDanio rerio
(tropical teleost fish) are also used as an alternative model for anti-obesity studies.These biological models have their entire genomic library of RNAi available,a relatively short life cycle,low cost,conserved biochemistry,and obesity-like examples,but with different physiology and anatomy from mammals (Kleinert et al.,2018).There are several other possible animal models,such as non-human primates,large animals (dogs and pigs),mice,and non-mammals.Each of them presents its advantages and disadvantages,but non-mammals cannot replace obesity studies with mammals,especially humans,which are essential for food intake research,the relationship between cerebral control and metabolic flows,body fat distribution,glycose systemic metabolism,and other vital aspects in understanding obesity (Kleinert et al.,2018).
Molecular markers have been quantified in adipose tissue of animal models.Pro-inflammatory cytokines like IL-6,TNF-α,and IL-1β,triggered by the inflammasome and that are unduly produced by macrophage infiltration in adipose tissue,still have their role in obesity despite the adipocyte,leading to the beginning of inflammation (Rodríguez-Hernández et al.,2013).
Other factors that can modulate the impact of neuroinflammation on cognitive function and emotional changes related to obesity and that have been observed in C57BL/6J with diet-induced obesity are the hypothalamicpituitary-adrenal axis,leptin,insulin,and intestinal microbiota.These factors are possibly linked to the development of comorbidities and the emergence of new neuropsychiatric symptoms from an inflammatory process in obesogenic animals (Castanon et al.,2015).
Obese patients’ systemic inflammation characteristics are also linked to cognitive dysfunctions related to aging,with many studies of animal models such as db/db,C57BL6/J,and Germ-free mice.Evidence that peripheral inflammation triggers a central inflammation in the brain of male db/db mice(C57BLKS/J-leprdb/leprdb) and db/+(C57BLKS/Jleprdb/+),which generates a synaptic plasticity alteration;this,in turn,contributes to neurodegeneration and the beginning of brain atrophy (Dinel et al.,2014;Solas et al.,2017).
Studies demonstrate that part of the brain in animal models induced to obesity is affected in different stages of obesity.The pre-frontal cortex and perirhinal cortex are compromised at the beginning of obesity,while the hippocampus is shown to be affected in more advanced stages.Together with the loss of synapses in affected areas,changes in the microglia cells morphology and hormonal changes are the possible mechanisms of impact of obesity in the brain (Bocarsly et al.,2015).
Clinical Studies with Obese Patients
Clinical trials are part of obesity treatment clinical research and are at the heart of all medical advances.The clinical trial explores new ways to prevent,detect or treat disease.Scientists are researching to learn more about overweight and obesity,including studies on the role of dietary patterns in obesity development and treatment;new behavioral,medication,device,and surgical approaches;and other research areas that can tell us more about why people develop obesity or respond to treatment.They provide new information about how the patients’ condition is influenced and about possible mechanisms of action to combat obesity and its effects on the human body (Kakkar and Dahiya,2015;Thompson et al.,2017;Peterli et al.,2018;Staiano et al.,2018;Xu et al.,2018).
Due to the high number of comorbidities related to obesity,clinical trials with obese patients provide an opportunity to test possible treatments regarding not only obesity but also other common conditions associated with obesity.These include cancer,diabetes,hepatic steatosis,cardiovascular diseases,binge eating disorder,and many others (Hofsø et al.,2010;Alisi et al.,2014;Libman et al.,2015;McElroy et al.,2015;Neuhouser et al.,2015).
Obesity by itself is capable of generating changes in the patient’s cognitive function,as do many other comorbidities related to obesity.It is therefore unsurprising that there are varied studies on the impact of obesity on cognitive function (Alosco et al.,2014;Prickett et al.,2015;Brown et al.,2016).
Clinical trials can vary a lot,mostly due to the number of patients,length of the study,age,comorbidities,and other factors that depend on the target and scope of the research.A good example is a clinical trial,the Baltimore Longitudinal Study of Aging,which was carried out with 1700 patients.This study began in 1958,with visits every 2 or 3 years,dealing with individuals ranging from 19 to 93 years old,from different demographic groups in the United States of America,specifically in Baltimore and Washington.This clinical trial seeks to understand the relationship between measurements of waist circumference and other parameters and a decline in cognitive function decline (Gunstad et al.,2010).
The Baltimore Longitudinal Study of Aging shows that the rise in BMI,or the increasing waist circumference or waist-to-hip ratio (WHR) by the accumulation of fat in the abdominal region,is associated with loss of cognitive function,evidenced in tests such as the Mini-Mental State Examination and Blessed IMC that evaluate the cognitive function and information,memory and cognition,respectively (Gunstad et al.,2010;Sellbom and Gunstad,2012).
The attention and executive function analyzed by this test showed that participants with an increase in BMI and waist circumference,because of the deposition of adipose tissue especially in the abdominal area,present a changed performance in Trail Making Test A,a test that measures the capacity of visual attention and task changes.High WHR is related to slower performance with the increase of age (Gunstad et al.,2010).
The cognitive function was evaluated in diverse tests,such as Prospective Memory,which demonstrated that the waist circumference and BMI of the obese were linked to poorer results than normal.High waist circumference and high WHR were related to worse performances in the Benton Visual Retention Test,and results became worse as time passed and obesity increased (Gunstad et al.,2010).
The individuals were analyzed using tests in language fluency,with two tests applied to demonstrate the relationship in parameters observed in the obese body and its performance in each test.The experiment showed that obese individuals with high WHR presented lower results in Category Fluency,while a high waist circumference was linked to inferior performances in Letter Fluency;a high BMI was related to more unsatisfactory results in both tests(Gunstad et al.,2010).
Alosco and coworkers (2014) performed the follow-up for 30 days before bariatric surgery of 50 patients between 20 and 70 years old.After the surgery,the patients were observed for an additional 12,52,104,156 weeks.After this period,data collection began,such as body mass index (BMI),and the cognitive function performance,memory,and language skills were evaluated (Alosco et al.,2014).Inclusion and exclusion factors were used in clinical trials,aiming for consistent and reliable results (Alosco et al.,2014).
Inclusion factors are used when looking for some specific characteristics of the patients,so that if a study is examining the effect of a diet or medicine on obese or post-surgery patients,it should use only obese or post-surgery patients,respectively.Exclusion factors are used to prevent other factors from disturbing the observed results.A classic example of inclusion and exclusion factors are patients that have already had a heart attack,present mental illness,or are drug addicts who can interfere with the results of tests to evaluate cognitive function (Gunstad et al.,2010;Alosco et al.,2014).
Animal models can be used on a large scale,thus presenting more data,a possible mechanism of action,and molecular pathways,and paving the way for clinical trials.However,these data are still unclear,and more information on the impact of inflammation on the nervous system is needed,probably by means of clinical trials and studies on humans (Gunstad et al.,2010;Agusti et al.,2018).
Anti-Obesity Drugs and Mechanism of Action in the Central Nervous System
Three main pathways in the human body are directly related to obesity,the net connections between the nervous system,digestion,and metabolism,and its cause may vary according to the dysfunction in complex communication and biochemical signaling path that regulates since food intake until its nutrients and metabolism absorption (Pilitsi et al.,2019).
When lifestyle changes do not have an expected effect or when there are diseases that predispose the patient,the drug’s use is necessary to complement the obesity treatment.Chemical and organic compounds that can act as modulators of one of these three pathways can contribute to weight loss,acting as anti-obesity drugs (Table 1
) (Narayanaswami and Dwoskin,2017).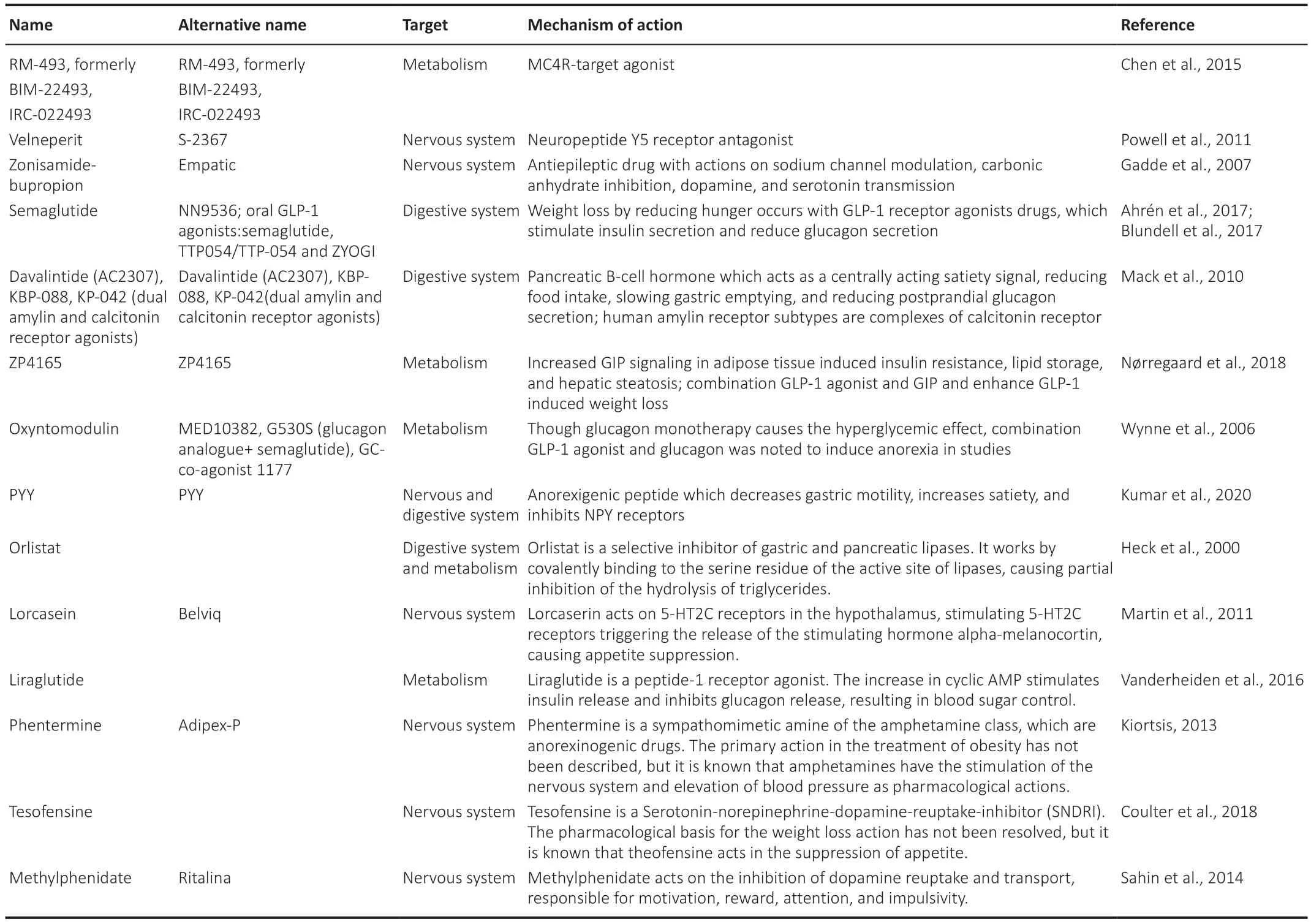
Table 1 | Drugs most used in the treatment of obesity,with emphasis on the target and mechanism of action of these compounds
Anti-obesity drugs include compounds that act as much as the gastrointestinal tract (GIT),modulating by inhibiting nutrient absorption and those that act on the central nervous system.Compounds that are capable of acting as inhibitors of the catalytic action that degrade macromolecules from food or that may interfere with the GIT tract cells functionality responsible for the absorption of the nutrient can be included in this class (Chen,2016).In this group are the gastric lipase inhibitors (Bryson et al.,2009;Pilitsi et al.,2019)and the glucose absorption inhibitors (Rebello and Greenway,2020).
Gastric lipases play a fundamental role during the degradation food process,enabling the large lipid chains biocatalysis in monomers allowing the lipids absorption by the GI tract (Bialecka-Florjanczyk et al.,2018).Dietary triacylglycerol is cleaved through the action of the enzymes,called lipases,which catalyze its substrate to monoacylglycerol and free fatty acids,which can be absorbed by the intestinal epithelium and re-esterified in dietary triacylglycerol,to later be transported through chylomicrons throughout the lymphatic system (Hussain et al.,2020).
The enzyme diacylglycerol acyltransferase 1 (DGAT1) is a target for antiobesity drugs,as it plays a fundamental role in fat absorption,studies have shown that this enzyme is expressed in large quantities in the adipose tissue and small intestine and that its deletion or inhibition in rodents resulted in body weight and adiposity reduction,allowing an increase in the glucagon like peptide and pancreatic peptide YY secretion (Cao et al.,2008;Zhao et al.,2008;Rebello and Greenway,2020).
Orlistat has its action in the digestive system,specifically GIT,preventing the lipids absorption as an enzymatic action of gastric lipases inhibitor,data from randomized experiments with patients in a state of obesity (100 kg) where applications of doses of 60 and 120 mg were performed,resulting in weight loss of patients in up to 30% fewer calories than they had at the beginning of the experiments (Heck et al.,2000).In addition to this medication,other lipase inhibitors can prevent the large lipid chains degradation,such as Cetilistat,which has the same effect as Orlistat,however,it causes up to 30%fewer side effects at concentrations of 60,120,and 240 mg/kg (Kopelman et al.,2007).
Another drug that can affect the GIT and that was initially developed for the treatment of diabetes type II,liraglutide is a synthetic analog of glucagonlike peptide-1 (GLP-1) (Vanderheiden et al.,2016).Its mechanism of action is based on the attachment of a fatty acid to the GLP-1 molecule,allowing reversible binding to albumin in the subcutaneous tissue (Malm-Erjefält etal.,2010).This binding to albumin results in slower degradation of GLP-1,being released over time.This effect results in increased insulin secretion and reduced glucagon secretion and,in weight control,may result in increased satiety and reduced food consumption (Russell-Jones,2009;Isaacs et al.,2016).
In addition to lipid absorption inhibitors,recent studies address a different type of mechanism of action,also related to the debate treatment,glucose absorption or reabsorption inhibition (Rebello and Greenway,2020).These inhibitors target the body’s proteins called selective sodium-glucose cotransporter 1 and 2,which play a fundamental role in the glucose levels bloodstream modulation,and glycosylated hemoglobin formation (Wilding et al.,2018).These transporters are responsible for glucose absorption in the TGI and in the kidneys glucose reabsorption (Abdul-Ghani et al.,2013).Among the glucose absorption inhibitors we can mention licogliflozin and sotagliflozin,studies using these drugs in the obesity treatment resulted in a reduction in body weight of 7.5% when compared to placebo,during a treatment performed with 150 mg/kg for 2 weeks (Garg and Strumph,2018;He et al.,2019).
The main action route in the treatment of anti-obesity is through mechanisms that act on the central nervous system.Based on their mechanisms,these drugs promote a reduction in food intake,in some cases stimulating brain regions related to the feeling of satiety,correlated with changes in eating and appetite behavior,like the hypothalamus,brainstem,peripheral nervous system,and cortical limbic (Adan et al.,2008;Heymsfield and Wadden,2017).
Central nervous system receptors are the main targets of anti-obesity drugs in this class,like adrenalin,serotonin,dopamine,and GABA receptors (Adan et al.,2008).An example is Lorcaserin,an anorectic drug active at 5-HT2C receptors in the hypothalamus.,can act as a serotonin antagonist,interacting with the neuroreceptor 5HT-2C,in regions such as the parietal and visual cortex in the limbic system and decreasing activity in these brain areas,which can cause a behavioral effect related to the decrease in the need for food intake (Martin et al.,2011;Pilitsi et al.,2019).
A drug initially used in the treatment of epileptic seizures,topiramate,is now used as an adjunct therapy for weight management (off-label) (Sari et al.,2021).Its mechanism of action is not fully known,but it is known that the drug exerts actions on voltage-dependent sodium channels,GABA,and glutamate receptors (Shank et al.,2000).The ways of action of topiramate are not directly related to weight loss,however,the action of the drug can reduce food intake and,in rodents,it had effects on lipoprotein lipase (adipose tissue and muscle) (Richard et al.,2000;Husum et al.,2003).
Another anti-obesity medication that also acts on the hypothalamus is Phentermine,experiments performed demonstrated 6.1% weight loss after a randomized treatment with 15 mg for 28 weeks,which has a structure similar to amphetamines.Phentermine is a sympathomimetic amine that acts by releasing norepinephrine from presynaptic vesicles in the hypothalamus.This increase in the concentration of norepinephrine in the synaptic cleft results in the stimulation of the adrenergic receptors.Phentermine may inhibit neuropeptide Y,being the main hunger-inducing signaling pathway.This combined effect produces a continuous fight-or-flight response,as this state is related to the immediate need for energy (Kiortsis,2013).
Some of the anti-obesity drugs are used or were used initially to treat diseases that affect the nervous system.As nerve cells deteriorate,the neurotransmitters (dopamine and serotonin) amount is reduced,which is one of the characteristics of biochemical diseases such as Parkinson’s disease and Alzheimer’s disease (Meder et al.,2019).In the obesity case,low extracellular serotonin levels are related to the increased desire to eat sweets and carbohydrates.Tesofensine is an example of a drug that can be used in the nervous system and obesity diseases,being able to inhibit the dopamine,serotonin,and norepinephrine recapture in the synaptic cleft.After this inhibition,an increase in these neurotransmitters’ extracellular levels is observed,leading to a normality situation (Lam and Heisler,2007).
The alteration of dopaminergic levels in the body is one of the main mechanisms described for drugs of this class.Methylphenidate,for example,prevents dopamine transport and reuptake,increasing synaptic levels of dopamine and norepinephrine in areas of the brain (prefrontal cortex,hippocampus,and precuneus) responsible for motivation,reward,attention,and impulsivity.Dopaminergic neurotransmission participates in food reward,so a low amount of dopamine increases food consumption.The side effects of methylphenidate were anorexia and weight loss while it was used in attention deficit hyperactivity disorder (Birn et al.,2019).
Finally,recent studies indicate the relationship between obesity and dysfunctions in the patient’s metabolism,among some of the most common dysfunctions involving the energy-storage molecules over-storage (Lynes et al.,2019).These compounds are usually lipids that are stored in adipose tissue,but when in excess,accumulations can also be found in the bloodstream or other organs such as the liver (Milić et al.,2014).
Thereat,metabolism can promote a regulation between each person’s energy and the energy expenditure availability,through a biochemical,anabolic,and catabolic reactions series that result in the storage or energy release (Heindel et al.,2017).It consists of biochemical agents complex communication that acts as chemical reactions mediators throughout the human organism,these cascade reactions can result in different biological functions,including thermogenesis and chemical energy production such as adenosine triphosphate (Ježek et al.,2019).
These reactions can be regulated by the sympathetic nervous system itself,through stimuli related to the ambient temperature perception by activating the temperature regulation system (Morrison,2018;Blaszkiewicz et al.,2019) or directly in the target cells independently from the nervous system or together,as by the biochemical compounds release that act as signalers in adipocytes,which can be considered as endocrine and paracrine hormones,called lipokines (Lynes et al.,2019).
Lipoxins are biochemical mediators derived from lipids and fatty acids,they are produced by enzymes that regulate these cellular activity mediators,however,these compounds are so specific that the same derivative can result in an inverse signaling process (Lynes et al.,2019).As is the case with palmitic acid which moderately inhibits protein kinase B phosphorylation,while omega-7 palmitoleate an unsaturated variation can result in glucose uptake (Cao et al.,2008;Lynes et al.,2019).Another example may be the arachidonate-derived lipokines that activate thermogenesis,while arachidonic acid itself can reduce mitochondrial activity (Fleckenstein-Elsen et al.,2016;Lynes et al.,2019).
Among the lipokines,Oleoylethanolamine is present,it increases the expression of UCP1 in beige adipocytes;prostaglandins increases the UCP1 expression in brown adipocytes;12,13-diHOME can regulate the fuel absorption and transport in the fatty acids form in brown tissue adipocytes and supports thermogenic function (Madsen et al.,2010).
The endocannabinoid system consists mainly of CBR1 and CBR2 receptors,and its main ligands are arachidonoyl ethanolamide and 2-araquidonoyl glycerol (Pertwee,2010;Boleti et al.,2022).This system performs as one of its functionalities the control of the storage and consumption of chemical energy in organs related to the nervous system and areas responsible for the regulation of hunger and appetite,such as the hypothalamus and prefrontal cortex,consequently,this results in lipogenesis and storage of lipids and use of energy by brown tissue adipocytes and muscles,correlating the endocannabinoid system and the regulation of energy homeostasis (Rossi et al.,2018;Ruiz de Azua and Lutz,2019).
Recent studies related to phytocannabinoids derived from theCannabis sativa
plant show that mainly cannabidiol affects reducing body weight,allowing an increase in lipolysis and thermogenesis,as well as acting as a lipogenesisreducing agent in adipose tissue (Bielawiec et al.,2020).Cannabidiol can act in the liver,promoting a positive influence on the reduction of intracellular lipids inin vitro
models of hepatosteatosis,with evidence of increased lipolysis and mitochondrial activity through fatty acid oxidation.Allowing an increase in the expression of the catalytic subunit of adenosine-monophosphate-activated protein kinase a2 and extracellular signal-regulated kinase 1/2 along with signal transducers and activators of transcription in hepatocytes (Silvestri et al.,2015;Bielawiec et al.,2020).Conclusion
Increased visceral adiposity is a risk factor for the development of a wide range of neurological conditions,as well as obesity and metabolic disorders.Although the cellular mechanisms of obesity-induced neuronal and cognitive dysfunction need to be fully elucidated,it is clear that obesity,insulin resistance,and dyslipidemia converge in an inflammatory process,neurological dysfunction,and neurodegeneration.These associations could be related to dietary-induced alterations in the intestinal microbiota that,in turn,may contribute to neuro-inflammation and dysregulation of the neuroendocrine system,associated with obesity and mental impairments.Accumulating evidence suggests that atypical inflammatory insults disturb hypothalamic regulation,resulting in metabolic imbalance and aging progression,establishing a common causality for these two pathophysiological statuses.Studies have causally linked these changes to the activation of key proinflammatory pathways,especially NF-κB signaling within the hypothalamus,which leads to hypothalamic neuronal dysregulation,astrogliosis,microgliosis,and loss of adult hypothalamic neural stem/progenitor cells.Experimental models have been used to investigate the effectiveness of compounds altering the gut microbiota,in both attenuating obesity and associated mental disorders.In addition,most of the drugs used in the treatment of obesity were initially developed to treat neurological diseases and,because of that,the central nervous system is the site of action for most of these drugs.Thus,it is important to emphasize that diet and a sedentary lifestyle can be determining factors in the process of adipogenesis and,consequently,may trigger neurological diseases.
Author contributions:
Manuscript writing and data analysis:APdAB;data search and manuscript editing:LM;data collection:PHdOC,BEFF,PSeS,LFRNdM.All authors approved the final version of the manuscript.
Conflicts of interest:
The authors declare no conflicts of interest.
Availability of data and materials:
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement:
This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others
to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:
Dong Kong,Boston Children’s Hospital and Harvard Medical School,USA.
Additional file:
Open peer review report 1.
- 中国神经再生研究(英文版)的其它文章
- Neuroaxonal and cellular damage/protection by prostanoid receptor ligands,fatty acid derivatives and associated enzyme inhibitors
- Extracellular vesicles in Alzheimer’s disease:from pathology to therapeutic approaches
- Molecular approaches for spinal cord injury treatment
- Sex-biased autophagy as a potential mechanism mediating sex differences in ischemic stroke outcome
- Interleukin-1:an important target for perinatal neuroprotection?
- Neurotrophic fragments as therapeutic alternatives to ameliorate brain aging

