Interleukin-1:an important target for perinatal neuroprotection?
Sharmony B.Kelly ,Elys Green ,Rod W.Hunt ,Claudia A.Nold-PetryAlistair J.Gunn,Marcel F.Nold,,Robert Galinsky,
Abstract Perinatal inflammation is a significant risk factor for lifelong neurodevelopmental impairments such as cerebral palsy.Extensive clinical and preclinical evidence links the severity and pattern of perinatal inflammation to impaired maturation of white and grey matters and reduced brain growth.Multiple pathways are involved in the pathogenesis of perinatal inflammation.However,studies of human and experimental perinatal encephalopathy have demonstrated a strong causative link between perinatal encephalopathy and excessive production of the pro-inflammatory effector cytokine interleukin-1.In this review,we summarize clinical and preclinical evidence that underpins interleukin-1 as a critical factor in initiating and perpatuating systemic and central nervous system inflammation and subsequent perinatal brain injury.We also highlight the important role of endogenous interleukin-1 receptor antagonist in mitigating interleukin-1-driven neuroinflammation and tissue damage,and summarize outcomes from clinical and mechanistic animal studies that establish the commercially available interleukin-1 receptor antagonist,anakinra,as a safe and effective therapeutic intervention.We reflect on the evidence supporting clinical translation of interleukin-1 receptor antagonist for infants at the greatest risk of perinatal inflammation and impaired neurodevelopment,and suggest a path to advance interleukin-1 receptor antagonist along the translational path for perinatal neuroprotection.
Key Words:brain;inflammation;interleukin-1 receptor antagonist;interleukin-1;interleukin-1β;neonatal encephalopathy;neuroprotection;preterm brain injury
Perinatal Inflammation Underpins Neurodevelopmental Impairments
Perinatal inflammation is a significant risk factor for neonatal mortality and morbidity,including neurodevelopmental impairments,such as cerebral palsy,which can have a devastating lifelong impact (Wu and Colford,2000;Honeycutt et al.,2004;Fleischmann et al.,2021).Indeed,perinatal inflammation is associated with a several-fold increase in the risk of cerebral palsy in preterm and near-term/term infants (odds ratio:2.5–9.3) (Grether and Nelson,1997;Wu et al.,2003;Soraisham et al.,2013).The cumulative lifetime economic cost of cerebral palsy in the USA was estimated to be over USD11.5 billion in 2003 (Honeycutt et al.,2004).More recent evidence indicates that the cost of disability associated with perinatal brain injury continues to rise,and that prevention of such injury and therefore disability would significantly reduce the socio-economic burden on affected individuals,their families,and society (Shih et al.,2018).No established effective therapy for inflammation-induced brain injury is available and this deficit is a major unmet medical need (Galinsky et al.,2020).Developing more effective therapeutic interventions requires improving our understanding of the underlying pathophysiological mechanisms that lead to impaired neurodevelopment in infants exposed to perinatal inflammation.
There are multiple triggers of perinatal inflammation;however,there is compelling evidence that chronic inflammation caused by perinatal infection,hypoxia-ischemia,pulmonary volutrauma and barotrauma,and oxygen toxicity during postnatal respiratory support can independently or synergistically cause inflammation in the fetus and neonate (Bui et al.,2017;Galinsky et al.,2018).In recent cohort studies,long-term neurodevelopmental disturbances were associated with chronic inflammation and diffuse injury in the white matter tracts in both term and preterm infants (Leviton et al.,1999;Wu and Colford,2000;Wu et al.,2003;O’Shea et al.,2012,2013;O’Muircheartaigh et al.,2020).As previously reviewed,when unbridled,both systemic and central nervous system inflammation is strongly implicated in the disturbances to neuronal and oligodendrocyte development and reductions in brain growth that lead to reduced white and grey matter volumes and long-term behavioral and intellectual disabilities seen in preterm and term infants (Hagberg et al.,2015;Galinsky et al.,2018).
Search Strategy
PubMed and Ovid MEDLINE databases were searched between September 20,2021 and November 20,2021 with no limitations set to the literature search in this narrative review.All years were chosen in the search.Search terms were“preterm brain injury OR neonatal encephalopathy OR neonatal inflammation AND IL-1 receptor antagonist OR anakinra OR kineret”.Other sources used to identify studies included relevant original manuscripts and reviews.
Preterm and Term Encephalopathy:Outcomes and Available Interventions
Encephalopathy in preterm and near-term/term neonates continues to be associated with a high risk of lifelong neurodevelopmental impairment.For example,in a large cohort of preterm infants born between 1997 and 2011 in France,survival without moderate to severe neuromotor or sensory disabilities increased from 46% to 62% in infants born at 25–26 weeks of gestational age but remained unchanged at 22–24 and 32–34 weeks of gestation (Pierrat et al.,2017).Similarly,the Australian cerebral palsy register found no significant change in the overall risk of cerebral palsy from 1993–2006;however,there was a trend for a reduced risk of cerebral palsy after extremely preterm birth (births <28 weeks of gestation) (Smithers-Sheedy et al.,2016).By contrast,in a population-based study of 8-year-old children who were born preterm in the Australian state of Victoria,rates of major neurosensory disability were similar for cohorts born in 1991–1992,1997,and 2005.Of concern,academic performance was worse in 2005 than in previous cohorts,after controlling for other factors (Cheong et al.,2017).Meta analyses of large randomized controlled trials have shown that maternal treatment with magnesium sulfate (MgSO) for threatened preterm labor at <30 weeks of gestation is associated with a reduced risk of cerebral palsy and gross motor dysfunction after premature birth (Conde-Agudelo and Romero,2009).Whilst follow-up to school age is yet to find a long-term clinical benefit(Chollat et al.,2014;Doyle et al.,2014),antenatal MgSOis currently the only treatment available for preterm neuroprotection.Antenatal corticosteroids and neonatal caffeine administration have been linked to a reduced risk of preterm brain injury (Ment et al.,1995;Schmidt et al.,2007);however,the pathways by which this reduction occurs remain unclear.
Although mild therapeutic hypothermia,via whole body or head cooling,for moderate to severe neonatal encephalopathy in near-term (>35 weeks of gestation) and term infants is now well established to improve survival without disability,current hypothermia protocols are only partially protective,such that approximately 30% to nearly half of infants still die or survive with disability despite cooling (Jacobs et al.,2013;Shankaran et al.,2017).For example,in a cohort of infants who underwent therapeutic hypothermia from 2000 to 2003 for moderate to severe encephalopathy,neurodevelopmental assessment at 6 to 7 years of age showed that over 25% of children had subnormal IQ scores.A subnormal IQ was more prevalent in survivors with cerebral palsy (96%) compared to children without cerebral palsy (40%)(Pappas et al.,2015).Furthermore,20% of children with a normal IQ and 28%of children with a subnormal IQ score received special educational support or were held back ≥ 1-grade level.A recent multicenter cohort study of mild encephalopathy at term showed that cognitive performance at 2 years of age was lower compared to the control group.Neuroprotective interventions for mild encephalopathy are yet to be developed.
Collectively,these data indicate that while progress in perinatal neuroprotection has been made,there is still an urgent unmet need to develop more effective therapies for preventing or minimizing injury to the preterm and near-term/term brain.
Interleukin-1:a Promising Target for Perinatal Neuroprotection
There are two IL-1 cytokine agonist isotypes,IL-1α and IL-1β,which both signal via the heterodimeric receptor pair IL-1R1:IL-1R3,augmenting inflammation through activation of downstream effectors such as mitogenactivated protein kinases,nuclear factor kappa B,and activator protein-1.IL-1β is bioactive at femtomolar concentrations (Dinarello et al.,2012;Dinarello,2018);hence,to curb excessive activation of this potent cytokine,the endogenous IL-1 receptor antagonist (IL-1Ra),which competitively blocks binding of the IL-1 ligands to their receptor,is often co-induced with IL-1α and IL-1β.Another antagonist of IL-1 signaling is IL-1R2,which functions as a decoy receptor that sequesters IL-1 without transducing pro-inflammatory signals (Dinarello et al.,2012;Dinarello,2018;Figure 1
).IL-1 function is furthermore controlled by the requirement of inflammasome activation to render IL-1 bioactivation.Activation of pattern recognition receptors triggers the assembly of the multi-protein inflammasome complex,which converts pro-caspase-1 into active caspase-1,and in turn,cleaves the pro-IL-1β protein.This cleavage generates mature,bioactive IL-1β that is ready for release from the cell (Dinarello,2018;Figure 1
).IL-1 protein abundance is usually minimal in the brain under steady-state conditions;however,it is rapidly induced in cases of pathological inflammation and tissue injury (Rothwell,2003).In the brain,IL-1 is produced by astrocytes,microglia,lymphocytes,and infiltrating myeloid cells and is also capable of penetrating the blood-brain barrier from systemic circulation (Prasad et al.,2021).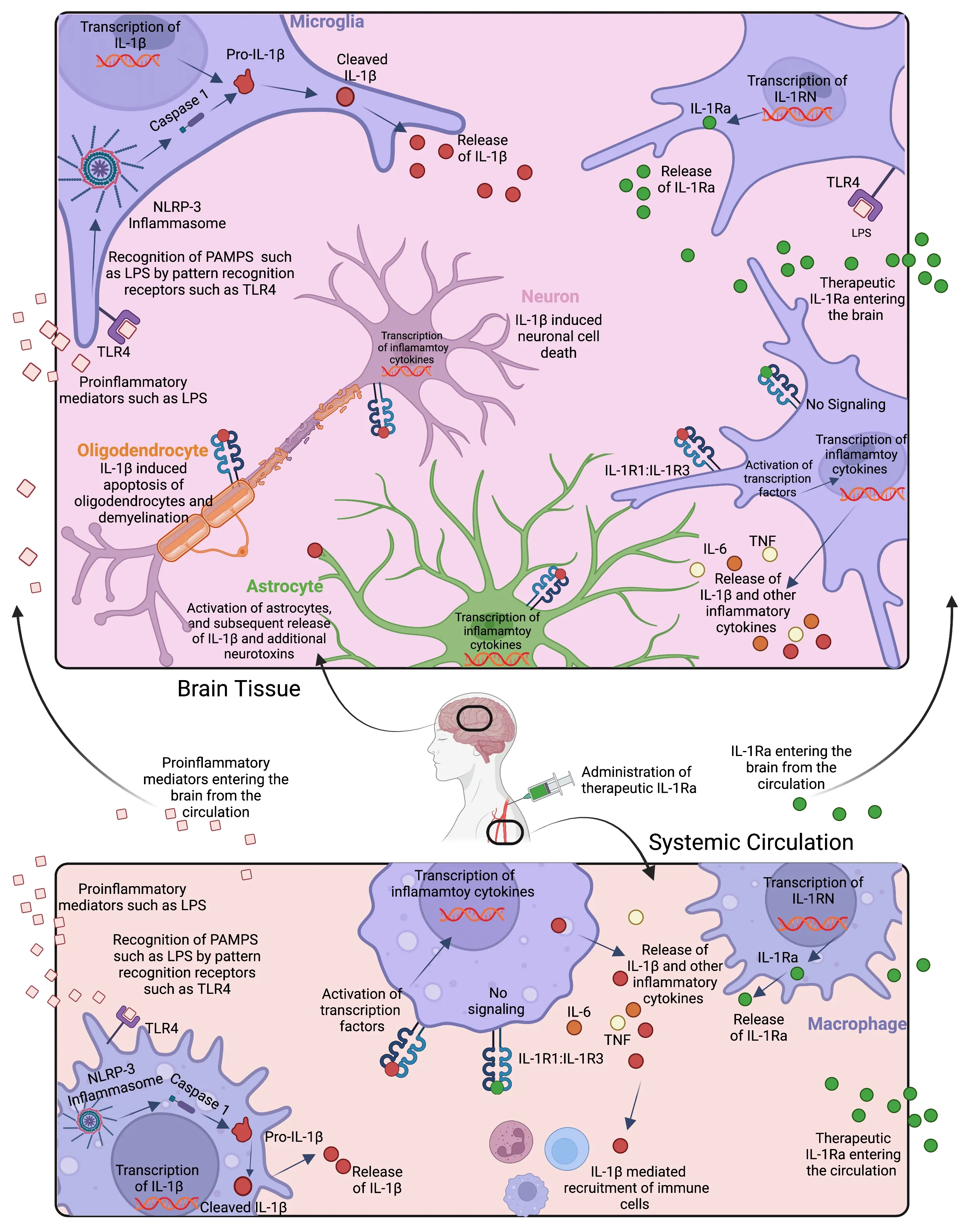
Figure 1 | Innate immune stimulant-induced activation and signaling of IL-1β in brain(top) and circulation (bottom),and effects of endogenous and exogenous IL-1Ra.
IL-1β is the main isoform implicated in neural injury (Rothwell,2003).For example,in term/near term newborns,elevated systemic and cerebrospinal IL-1β on the first days of life were associated with impaired cerebral metabolism and developmental delay at 2 years of age (Bartha et al.,2004;Bajnok et al.,2017).Similarly,in extremely preterm infants chronically elevated circulating IL-1β within the first 2 weeks after birth were associated with impaired neurodevelopment at 2 years of age (O’Shea et al.,2012).Furthermore,polymorphisms in theIL1B
gene that result in increased production of the IL-1β protein are associated with an increased risk of intraventricular hemorrhage and periventricular leukomalacia (Baier,2006).In post-mortem brain tissue sections from preterm infants,accumulation of IL-1β was primarily localized to areas of white matter inflammation and injury.Furthermore,in areas of white matter injury,IL-1Ra was significantly less augmented than IL-1β;and this imbalance in the ratio of pro-versus
anti-inflammatory IL-1 family cytokines was more pronounced in very preterm infants (born <32 weeks)compared to near-term infants that developed white matter injury (Girard et al.,2010a).Consistent with the evidence linking IL-1β to perinatal brain injury in human cohort studies,mechanistic work in small and large animal models of perinatal brain injury has found a strong link between elevated systemic and cerebral IL-1β and white matter injury (Girard et al.,2008,2010b,2012;Favrais et al.,2011;Rocha-Ferreira et al.,2017;Kelly et al.,2021).Blocking the endogenous production of IL-1Ra exacerbates ischemic injury in adult rodents (Loddick et al.,1997;Pinteaux et al.,2006).Mice deficient in IL-1Ra show increased neurodegeneration and learning and memory deficits after exposure to soman-induced neuroinflammation (Ferrara-Bowens et al.,2017).Further,polymorphisms in the gene encoding IL-1Ra,IL1RN
,that reduce the production of IL-1Ra lead to an increase in the IL-1ɑ/β:IL-1Ra ratio,resulting in increased pro-inflammatory signaling,and an increased risk of stillbirth (Gerber et al.,2005).Collectively,these data strongly support the hypothesis that IL-1 inhibition is a promising therapeutic approach for attenuating neural injury caused by perinatal inflammation.In a study designed to test this hypothesis,we used a clinically relevant large animal model to determine whether IL-1 inhibition,using the commercially available recombinant IL-1Ra called anakinra,started 1 hour after the induction of progressive lipopolysaccharide (LPS)-induced systemic inflammation,could mitigate neuroinflammation and brain injury in late preterm (0.85 gestation) fetal sheep.At this age,brain development in sheep is broadly equivalent to the late preterm human infant (Barlow,1969).Progressively increasing doses of LPS were used in this study to reflect the progressive inflammation typical of perinatal infection (Küster et al.,1998;Oh et al.,2019).We showed that IL-1 inhibition during progressive LPS-induced inflammation was associated with reduced microgliosis and apoptosis,and improved pre-oligodendrocyte survival in the intragyral and periventricular white matter,which are among the large white matter tracts that are highly susceptible to neuroinflammation and injury.The reduction in neuroinflammation and pre-oligodendrocyte cell death was associated with reduced circulating pro-inflammatory cytokines (IL-1β,tumor necrosis factor,and IL-6) and improved recovery of electrophysiological brain activity and fetal movement (assessed using electroencephalography and electromyography,respectively (Kelly et al.,2021)).Critically,these data demonstrate that IL-1,and particularly IL-1β,plays a key role in the pathophysiology of white matter inflammation and injury,that is secondary to systemic inflammation,and that systemic administration of IL-1Ra improves histological and functional outcomes in a clinically relevant large animal model of perinatal inflammation.These data are complemented by neonatal rodent studies that have reported IL-1Ra-induced reductions in placental inflammation,gliosis and microvascular degradation,and improved oligodendrocyte survival,myelination,and neurobehavioral outcomes after exposure to inflammatory and or hypoxic insults (Girard et al.,2010b,2012;Nadeau-Vallée et al.,2017;Pierre et al.,2019).
Interleukin-1 Receptor Antagonist:an Established Therapeutic Agent
The commercially available IL-1Ra anakinra is a recombinant non-glycosylated form of the human IL-1Ra,and it has been in clinical use for over 20 years(Food and Drug Administration approval in 2001) in a range of auto-immune conditions,as well as sepsis and other hyper-inflammatory syndromes to reduce inflammation-related morbidity.IL-1Ra has a molecular weight of 17 kDa and has been shown to penetrate the blood-brain barrier in human and preclinical animal studies (Galea et al.,2011;Sadowska et al.,2015).After intravenous administration,IL-1Ra has been reported to cross the bloodbrain barrier and achieve therapeutic concentrations in the brain within approximately 45 minutes (Galea et al.,2011).Thus,we may reasonably speculate that IL-1Ra exerts its neuroprotective actions through modulating both the central nervous system and systemic inflammation (Figure 1).Experience in an estimated 200,000 patients,including infants and children,has established an excellent safety record for IL-1Ra.Endogenous cytokine expression is important for normal brain development;hence,at least in theory,high dose anti-inflammatory therapy could affect the otherwise normal brain (Deverman and Patterson,2009).Reassuringly,no effect on organ growth and development has been observed (Pascual et al.,2005;Dinarello et al.,2012;Rudloff et al.,2017).Therefore,the US FDA assigned anakinra to pregnancy category B,the second most favorable of 5 categories,signifying that,while there are limited controlled data in human pregnancy,there is no evidence of fetal harm in animal models.Case reports of babies born to mothers on anakinra noted no abnormalities (Berger et al.,2009;Fischer-Betz et al.,2011),despite anakinra crossing the placenta (McDuffie et al.,2001).Importantly,anakinra gained FDA approval in 2013 for the treatment of neonatal-onset multisystem inflammatory disease,and deficiency of IL-1Ra in 2020 and has since been used successfully and safely in young infants and children.
Considerations for Clinical Translation:What Is Still Missing?
IL-1Ra is now established as a safe and effective treatment for neonatal-onset multisystem inflammatory disease,deficiency of IL-1Ra,and rheumatoid arthritis in young infants and children.In addition to mitigating systemic and central nervous system inflammation and related brain injury and improving neurological function in small and large animal trials,IL-1Ra has been shown to reduce neonatal pulmonary hypertension,bronchopulmonary dysplasia,and retinopathy of prematurity in preclinical animal studies (Nold et al.,2013;Zhou et al.,2016;Rudloff et al.,2017;Beaudry-Richard et al.,2018;Bui et al.,2019;Sayah et al.,2020).Collectively,these data suggest IL-1 is implicated in the pathophysiology of multiple inflammatory driven neonatal diseases and that IL-1Ra has the potential to provide multiorgan protection.However,there are no safety data in human preterm or term infants with neonatal encephalopathy,who are often at risk of long-term neurodevelopmental impairment.Thus,before progressing to studies of IL-1Ra for perinatal neuroprotection,it is advisable to conduct pilot trials to establish safety in these at-risk populations.Thus,we strongly suggest that it is now time for a phase 1 safety trial of IL-1Ra for infants at greatest risk of inflammationinduced brain injury,such as extremely preterm infants.
Another consideration in assessing the therapeutic potential of IL-1Ra for perinatal encephalopathy is when to treat.Indeed most of the preclinical trials demonstrating histological and or functional benefits administered IL-1Ra before (Girard et al.,2010b;Leitner et al.,2014) or shortly (1 hour)after (Kelly et al.,2021) inducing inflammation. In a study of neonatal rats(neurodevelopmentally equivalent to extremely preterm humans) exposed to LPS and/or neonatal hypoxia-ischemia,delaying IL-1Ra treatment by approximately 48–72 hours after LPS-induced fetal inflammation was associated with reduced gliosis and improvements in cognition,numbers of neuronal stem cells and myelination (Girard et al.,2012).It is important to note that IL-1Ra was administered repeatedly,every 12 hours for 9 days after birth,suggesting that delayed and prolonged IL-1Ra administration could be an effective therapeutic approach (Girard et al.,2012).By contrast,in a neonatal mouse model of bronchopulmonary dysplasia induced by antenatal LPS and 28 days of postnatal hyperoxia,both delayed (treatment started on postnatal day 6) and very high-dose IL-1Ra (100 mg/kg,equating to approximately 10× the dose clinically used in humans after correction for interspecies application) were less effective than early (treatment started on postnatal day 1) and standard-dose IL-1Ra (10 mg/kg) at mitigating pulmonary inflammation and tissue injury.Notably,neither intervention was associated with abnormalities in cerebral growth or morphology (Rudloff et al.,2017).Thus,based on the available evidence,early intervention appears to be the most promising strategy.Nevertheless,these data suggest that after establishing safety and efficacy in human neonates,there is scope to further evaluate the therapeutic window and therapeutic range of IL-1Ra for reducing inflammation and injury in future preclinical studies,similar to the strategy by which therapeutic hypothermia was developed (Gunn et al.,2017).
As previously reviewed,inflammation is fundamental to the pathogenesis of neonatal encephalopathy at term (Cho et al.,2020).Identifying adjunct therapies to further improve outcomes for neonatal encephalopathy in nearterm/term infants is a key priority area for improving outcomes in neonatal encephalopathy.In a term-equivalent rat model of neonatal encephalopathy,IL-1Ra administered during mild therapeutic hypothermia was associated with accumulation of cerebral IL-1Ra and a paradoxical upregulation of IL-1 signaling in the brain which underpinned a reduction in the neuroprotective efficacy IL-1Ra (Chevin et al.,2018).Indeed,in a piglet model of neonatal encephalopathy,a delayed rise in circulating cytokines,including IL-1β,was observed following therapeutic hypothermia and was associated with white matter and basal ganglia injury (Rocha-Ferreira et al.,2017).Collectively,these data suggest that the timing of IL-1Ra treatment in the setting of therapeutic hypothermia requires further preclinical evaluation.
In conclusion,there is compelling evidence to support the premise that IL-1 is a major culprit in the manifestation of perinatal inflammation and subsequent brain injury,and blocking IL-1 signaling using IL-1Ra could be a safe and effective therapeutic approach.Thus,determining whether IL-1Ra is a safe intervention for extremely preterm infants who are at greatest risk of perinatal inflammation and long-term neurodevelopmental impairments represents a vital next step in progressing IL-1Ra down the translational path for perinatal neuroprotection.
Author contributions:
RG served as a guarantor.All authors participated in study concept,design,definition of intellectual content,literature search,manuscript preparation,editing,and review,and approved the final version of the manuscript.
Conflicts of interest:
The authors declare no conflicts of interest.
Open access statement:
This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Neuroaxonal and cellular damage/protection by prostanoid receptor ligands,fatty acid derivatives and associated enzyme inhibitors
- Extracellular vesicles in Alzheimer’s disease:from pathology to therapeutic approaches
- Molecular approaches for spinal cord injury treatment
- Sex-biased autophagy as a potential mechanism mediating sex differences in ischemic stroke outcome
- Adipose tissue,systematic inflammation,and neurodegenerative diseases
- Neurotrophic fragments as therapeutic alternatives to ameliorate brain aging

