Sex-biased autophagy as a potential mechanism mediating sex differences in ischemic stroke outcome
Brian Noh,Louise D.McCullough,Jose F.Moruno-Manchon
Abstract Stroke is the second leading cause of death and a major cause of disability worldwide,and biological sex is an important determining factor in stroke incidence and pathology.From childhood through adulthood,men have a higher incidence of stroke compared with women.Abundant research has confirmed the beneficial effects of estrogen in experimental ischemic stroke but genetic factors such as the X-chromosome complement can also play an important role in determining sex differences in stroke.Autophagy is a self-degrading cellular process orchestrated by multiple core proteins,which leads to the engulfment of cytoplasmic material and degradation of cargo after autophagy vesicles fuse with lysosomes or endosomes.The levels and the activity of components of these signaling pathways and of autophagy-related proteins can be altered during ischemic insults.Ischemic stroke activates autophagy,however,whether inhibiting autophagy after stroke is beneficial in the brain is still under a debate.Autophagy is a potential mechanism that may contribute to differences in stroke progression between the sexes.Furthermore,the effects of manipulating autophagy may also differ between the sexes.Mechanisms that regulate autophagy in a sex-dependent manner in ischemic stroke remain unexplored.In this review,we summarize clinical and pre-clinical evidence for sex differences in stroke.We briefly introduce the autophagy process and summarize the effects of gonadal hormones in autophagy in the brain and discuss X-linked genes that could potentially regulate brain autophagy.Finally,we review pre-clinical studies that address the mechanisms that could mediate sex differences in brain autophagy after stroke.
Key Words:autophagy;brain;estrogen;gonadal hormones;neurodegeneration;neuron;stroke;X-chromosome
Introduction
Growing evidence supports the idea that the onset,progression,prevention,and treatment of diseases are influenced by sex and gender (Mauvais-Jarvis et al.,2020).Considering these factors,gender medicine,as a part of precision medicine,aims to design therapies that include the use of a specific drug,in a specific dose,for each individual (Shang et al.,2021).
Biologic and epidemiologic differences importantly contribute to sex differences in stroke incidence,prevalence,severity,and case fatality (Zhang et al.,2021b).Identifying risk factors that lead to sex differences in stroke is challenging,as these factors may be dependent on other variables,such as age.Exploring different biological factors that affect the incidence,severity,and recovery from ischemic stroke may help elucidate the mechanisms that determine sex differences in ischemic stroke.One of the most studied factors is gonadal hormones,but genetic factors including the sex chromosome complement (XXvs
.XY) may also play an important role in mediating sex differences in stroke.Ischemic stroke triggers multiple pathological events,including oxidative stress,adenosine triphosphate (ATP) deprivation,mitochondrial dysfunction,cellular ion overload,and cytotoxic edema,all of which contribute to neuronal death.Ischemic stroke also activates autophagy,which influences ischemic stroke outcomes (Wang et al.,2021).Autophagy is a self-degrading cellular process that is tightly regulated by multiple signaling pathways regulated by internal and external stimuli.Macroautophagy,the best-studied autophagy mechanism,is orchestrated by numerous core proteins to engulf cytoplasmic material and degrade cargo after autophagy vesicles eventually fuse with lysosomes (Klionsky et al.,2021;Figure 1
).The levels and the activity of these signaling pathways and of autophagy-related proteins are altered during ischemic insults (Wang et al.,2021).Gonadal hormones regulate several signaling pathways associated with autophagy and several autophagy-related genes (ATG) are located on the X-chromosome (Congdon,2018;Azcoitia et al.,2019),suggesting that autophagy may be regulated by sex-related factors.
Figure 1 | Estrogen and X-linked genes contribute to autophagy in the brain.
Recent reviews have comprehensively summarized clinical and pre-clinical studies on the role of brain autophagy in stroke (Ajoolabady et al.,2021;Wang et al.,2021).However,none have specifically addressed sex-biased autophagy as a contributor to differences in stroke outcome between the sexes.Most preclinical studies in the stroke field still do not include both sexes in theirin vitro
andin vivo
analyses.Identifying the cellular mechanisms that govern these differences is required to reach the ultimate goal of providing precision medicine for patients.In this review,we highlight the evidence for sex differences in stroke in human and in animal models,and how these differences can be explained by gonadal hormones and the sex chromosome complement.We discuss the contribution of gonadal hormones and the X-chromosome as biological factors in brain autophagy.We also discuss the limited studies that investigate sex differences in autophagy in preclinical stroke models.Search Strategy
Publications included in this narrative review were retrieved from the PubMed database by using the following sets of search terms:(1)“sex differences”[title/abstract],brain[title/abstract],stroke[title/abstract],and (2) stroke[title],brain[title/abstract],autophagy[title].Retrieval time:up to November 2021.
Sex Differences in Stroke
Stroke is a leading cause of mortality in the United States,and sex is a determining factor in the incidence and pathology of stroke (Bushnell et al.,2018).Age-specific incidence rates are substantially lower in females than males in younger and middle-aged groups (Bushnell et al.,2018).However,age reverses the“female protected”phenotype.Incidence rates in females are similar or even higher than those in males in the oldest age groups (Howard et al.,2019;Madsen et al.,2020).Currently,more women (4.1 million) live with stroke-related disability than men (3.1 million),and women are more likely to die than men (6.2%vs
.4.3%) after a stroke (Benjamin et al.,2017).In pre-clinical studies,young female mice and rats subjected to transient ischemia induced by middle cerebral artery occlusion (MCAO) have reduced brain injury compared to young males (Acaz-Fonseca et al.,2020;Patrizz et al.,2021).Similarly,in spontaneously hypertensive stroke-prone rats (a model relevant to human lacunar stroke,a common type of ischemic stroke),young male rats had their first stroke 3 weeks after a high-salt diet while females were event-free for 6 weeks.Further,females had significantly longer survivaltime,and brain damage progressed at a slower rate (detectable with magnetic resonance imaging) in females compared with males (Ballerio et al.,2007).This protection was lost after ovariectomy in MCAO models and restored with estrogen supplementation (Acaz-Fonseca et al.,2020;Patrizz et al.,2021).As observed in humans,age plays a critical role in the response to experimental stroke.Manwani et al.(2013) found that infarct size was affected by aging in mice subjected to experimental MCAO.Mice were subjected to focal transient cerebral ischemia for 60 minutes followed by reperfusion.Young (5–6 months old) males had larger infarcts compared to young females;however,in middle-aged (14–15 months old) male mice,the infarct volume was smaller compared with young males,but middle-aged females had larger infarcts than young females.The infarct volume did not differ between sexes in aged (20–22 months old) mice (Manwani et al.,2013).Clinical evidence also supports the hypothesis that aging worsens stroke outcomes disproportionally in women compared to men.
After menopause,the decline in gonadal hormones is associated with an increased risk of stroke,suggesting that women lose the protection that estradiol confers,which may explain the shift in the stroke incidence in women with aging.Differences in life expectancy between sexes and other factors also contribute to the reversal of the trend (Bushnell et al.,2018).
Considerable research has confirmed the beneficial effects of estrogen in stroke in preclinical models (Sohrabji et al.,2019).Importantly,the timing in the initiation of hormone therapy appears to be critical for determining its beneficial effects.Oral administration of estradiol within 6 years after menopause mitigated the increase of carotid-artery intima-media thickness seen with aging,a measure of atherosclerotic burden.However,the beneficial effect of estradiol was absent when the hormone was administered more than 10 years after menopause (Hodis et al.,2016).A similar effect was observed in experimental models.Chronic estrogen replacement therapy initiated in late middle-aged (16 months old) mice reduced infarct volume,led to improved neurological outcomes,and decreased neuroinflammation in both sexes,but more significantly in aged females (Liu et al.,2012).Female mice treated with estradiol for 3 months prior to an induced stroke had smaller infarcts compared to animals treated acutely for 2 weeks prior to stroke(Liu et al.,2012) mirroring clinical findings that earlier treatment increases benefit.Sexually mature rats subjected to MCAO and supplemented with estradiol showed reduced infarct size compared with stroke rats treated with placebo (Selvamani and Sohrabji,2010).However,estradiol supplementation increased the infarct size in reproductively senescent rats compared with control ovariectomized mature rats (Selvamani and Sohrabji,2010).Thus,early administration of female hormones in post-menopausal women may reduce stroke severity;the timing of initiation of hormone therapy matters.
In contrast to the beneficial effects of estrogen,clinical and preclinical studies have demonstrated that the male gonadal hormone testosterone contributes to enhanced vulnerability to cerebral ischemia (Uchida et al.,2009).Therefore,gonadal hormones play a critical role in ischemic vulnerability.However,not all sex differences in stroke are associated with sex hormones.Evidence demonstrates a sex bias in stroke incidence in pre-pubertal children,where the levels of gonadal hormones are equivalent between sexes;boys have a higher incidence of stroke and poorer outcomes (Dunbar et al.,2020).Indeed,using a mouse model in which the effects of sex chromosome complement can be analyzed independently of gonadal sex (the four-coregenotype (FCG) model) found that both sex chromosomes and gonadal hormones contribute to sex differences (Cambiasso et al.,2017;Vousden et al.,2018).
The FCG mouse model has been used to evaluate the role of the chromosomal sex (XX or XY) in the response to stroke.In the FCG model,the gene Sry,the mammalian testes determining-gene,is deleted from the Y-chromosome and inserted on autosomal chromosome 3 (A).This leads to a male that inherits Y,but still has a copy of theSry
gene on chromosome 3.Thus,mice that inherit chromosome 3 with a copy of theSry
gene (represented as A)will develop the secondary sex characteristics of male mice,such as testis development and typical male-like behavior (Qi et al.,2021).Genetic crosses between a wild-type female,XX(A),and the phenotypically normal male,XY(A),will produce four distinct genotypes:phenotypically female with Y-chromosome,XY(A);wild-type female,XX(A);phenotypically normal male,XY(A);and a phenotypically male with two X-chromosomes,XX(A)M(Figure 2
).Studies with this model helped determine that ischemic sensitivity is driven by the gonads in young mice.Young gonadal male mice,XX(A) and XY(A),that had testis (as they inherited theSry
),had higher larger infarct volumes after MCAO,compared with animals with ovaries and estrogen,XX(A) and XY(A).However,the protection was lost with gonadectomy,meaning all infarcts were equalized (Manwani et al.,2015).Thus,ischemic sensitivity is primarily driven by gonadal hormones in young mice.However,the X-chromosome complement plays a critical role in ischemic sensitivity in aging mice.18–20-month-old mice with an XX-chromosome complement,XX(A) and XX(A),regardless of gonads,had larger strokes than animals with only one X-chromosome,XY(A) and XY(A) (McCullough et al.,2016).Therefore,gonadal hormones drive the“female protected”phenotype seen in young mice,but in older reproductively senescent animals,the X-complement(XX) leads to ischemic sensitivity.Sex-biased differences in ischemic sensitivity driven by the X-chromosome may be present during the lifespan,but these sex differences may be held in check by estrogen.We subsequently found that two genes that escape from X-inactivation (Kdm5c
andKdm6a
),which regulate inflammatory interferon regulatory factors,were upregulated in the brain from mice with two X-chromosomes (XX(A) and XX(A)),compared with brains from XY (XY(A) and XY(A)) mice (Qi et al.,2021).This indicates that the X-chromosome contributes to sex differences in stroke sensitivity of aged mice.Thus,with aging,detrimental genes on the second X-chromosome are revealed.Autophagy Overview
Autophagy is a self-degrading mechanism that mediates the degradation of intracellular material,such as protein aggregates,damaged organelles,and microorganisms.Basically,autophagy (1) recycles molecules to rebuild new ones,(2) recovers energy by breaking intramolecular bonds,(3) allows cells to adapt to rapid changes in the cellular environment,and (4) protects cells by eliminating pathogens and toxic aggregates (Klionsky et al.,2021).
Three types of autophagy are classified by the manner in which the cargo is delivered into the lysosomes:macroautophagy,microautophagy,and chaperone-mediated autophagy.Chaperone-mediated autophagy specifically degrades proteins containing a KFERQ-like motif.Microautophagy non-specifically degrades cytoplasmic material by lysosome-mediated phagocytosis.Macroautophagy is the most well-studied autophagy type and is characterized by using double-membrane structures (phagophores)isolated from different intracellular lipid membrane sources (endoplasmic reticulum,Golgi apparatus,mitochondria,and plasma membrane) (Pavel and Rubinsztein,2017).During macroautophagy (hereafter called autophagy),a phagophore elongates while engulfing cytoplasmic material,and intermediate filaments and microfilaments mediate phagophore closure to form a doublemembrane vesicle (autophagosome) that later fuses with endosomes(amphisome) and/or lysosomes (autolysosome).The hydrolytic content of lysosomes/endosomes releases into the double-membrane vesicle and degrades the cargo (Loeffler,2019;Figure 1
).Many neurodegenerative disorders exhibit autophagy impairment,which may interfere with the degradation of protein aggregates,toxic compounds,and damaged organelles.Differentiated neurons are especially vulnerable to protein aggregate accumulation as they cannot dilute harmful cellular material by cell division,and neurons importantly depend on autophagy for clearing these substrates (Kulkarni et al.,2018).Adequate autophagy regulation helps to remove damaged mitochondria that cause cellular stress and provides energy in energy-depleting conditions.Thus,autophagy is beneficial for many aspects of brain function.However,autophagy also has deleterious effects on the brain.Overactivation of autophagy can induce apoptosis.For example,in the ischemic stroke field,several studies have shown that inhibiting autophagy reduces brain damage (Liu et al.,2016;Zhang et al.,2019),and the activation of autophagy-related signaling pathways induces oxidative stress and apoptosis (Li and McCullough,2010).Therefore autophagy can play a dual role in the ischemic stroke brain.Identifying the mechanisms that regulate the activation and progression of autophagy is critical to the development of therapeutic approaches to mitigate brain damage after stroke.
Summary of autophagy core proteins
Over 30 ATG’s participate in the machinery of autophagy.The essential autophagy core proteins can be classified into four functional groups:(1)UNC-51-like kinase 1 (ULK1),ATG13,and ATG17 form the initiation complex;(2) phosphatidylinositol 3-kinase (PI3K),Beclin1,ATG14,and AMBRA1 form the autophagy activating class III PI3K complex to form the phagophore;and (3) the ATG12 conjugation system (ATG12,ATG5,ATG16L1,and ATG10)in conjunction with (4) the microtubule-associated protein 1A/1B-light chain 3 (LC3) conjugation system (LC3,ATG3,ATG7,and ATG4B) lipidate and conjugate LC3 to phosphatidylethanolamine (LC3-II) to the membrane of the autophagosome and hence promote autophagosome maturation (Ajoolabady et al.,2021;Table 1
).LC3-II in combination with Sequestosome 1 (SQSTM1 or p62) is commonly used as autophagy markers.LC3-II is found in the outer and inner membrane of autophagy vesicles autophagosomes,and p62 is a marker of autophagy cargo.Thus,increased levels of LC3-II (suggesting a higher number of autophagosomes) and reduced levels of p62 (suggesting enhanced autophagy cargo degradation) are generally interpreted as increased autophagy flux.Autophagy flux is the progression of the entire process of autophagy since autophagosome formation progresses until fusion with lysosomes and cargo degradation.However,it is important to consider that inner-membrane LC3-II is degraded after autophagosome-lysosome fusion(autolysosome).Thus,in conditions that interrupt fusion and/or lysosomal activity,we may expect enhanced levels of both markers LC3-II and p62.Otherwise,autophagy studies should not be limited to the use of only these two markers.The use of multiple autophagy markers and several techniques is strongly recommended to avoid ambiguous conclusions (Klionsky et al.,2021).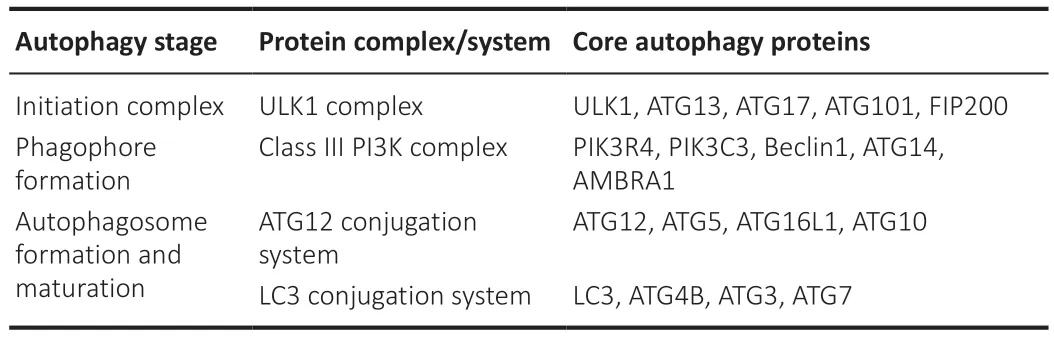
Table 1 | Different protein complexes in each autophagy stage contribute to the progression of autophagy
Summary of autophagy-related signaling pathways
Multiple signaling pathways that respond to extracellular and intracellular stimuli are coordinated to regulate autophagy.There have been several excellent recent reviews on the mechanisms that govern brain autophagy during the ischemic stroke (Ajoolabady et al.,2021;Wang et al.,2021).Here,we briefly introduce the pathways that might have specific relevance for understanding potential sex differences in autophagy in stroke.
mTOR is a serine/threonine kinase that inhibits autophagy initiation by phosphorylating ULK1.mTOR plays a central role not only in autophagy but also in cell growth,protein synthesis,cell cycle,and apoptosis (Kim and Guan,2015).The activity of mTOR is regulated by the PI3K family.PI3Ks are classified into three classes (class I,II,and III).While class I PI3Ks are negative regulators of autophagy by activating mTOR via phosphorylation,class III PI3Ks promote autophagy progression by producing the phospholipid phosphatidylinositol 3-phosphate (Yu et al.,2015).
5′-AMP-activated protein kinase (AMPK) is a serine/threonine kinase that is activated when the ATP/AMP ratio decreases,which occurs after ischemic stroke.AMPK inhibits mTOR and consequently activates autophagy (Zhang and Miao,2018).Indeed,pharmacological and genetic studies have demonstrated that activating AMPK exacerbates stroke outcome,and that AMPK inhibition can provide neuroprotection in stroke models (Li and McCullough,2010).
Nuclear factor-kappa B (NF-κB) is a transcription factor that is involved in autophagy by regulating the expression of mTOR in ischemic mice (Li et al.,2013).Thus,the upregulation of NF-κB leads to autophagy repression.
B-cell lymphoma-2 (Bcl-2) binds to and inhibits Beclin-1,which is required for autophagy initiation.When Bcl-2 is phosphorylated,Beclin-1 is released from the complex Beclin-1/Bcl-2 and joins other autophagy-related proteins to form the phagophore assembly site.Thus,a phagophore expands,which engulfs cytoplasmic material until its extremes fuse to form an autophagosome(Menon and Dhamija,2018).
In the sections below,we summarize the evidence for sex-dependent regulation of these pathways.
Sex-Biased Regulation of Autophagy in the Brain
Role of sex steroid hormones in brain autophagy
Autophagy is importantly regulated by sex steroid hormones,such androgens,estrogens,and progesterone via their receptors,androgen receptor (AR),estrogen receptors α and β (ERα,ERβ),and progesterone receptors,respectively.These receptors localize to the gonads,but they are also found in the human and rodent brains.Androgens,estrogens,and progesterone influence different stages of the autophagy machinery via their receptors by the regulation of the expression of multiple ATG’s.AR,ERα,and ERβ act on phagophore induction and phagophore expansion by mediating the expression ofAtg3
,Atg4b
,Atg5
,Atg7
,Lc3b
,andSqstm1
/p62
(Congdon,2018).AR is also important for regulating autophagosome maturation as ERα contributes to elevating LC3-II levels (Li et al.,2015).In lysosome biogenesis,AR can upregulate the expression of the gene encoding the transcription factor EB,Tfeb
,which controls the expression of lysosomal genes (Blessing et al.,2017).Furthermore,ERα is suggested to induce the lysosomal proteinase cathepsin D (Augereau et al.,1994;Figure 1
).Thus,steroid hormones contribute not only to the formation of autophagy vesicles but also to maintaining lysosomal integrity for efficient cargo degradation during autophagy.Sex differences exist in the expression of these sex steroid hormone receptors,which is also brain region-specific.The expression ofAr
is higher in the hypothalamus of men than in women (Fernandez-Guasti et al.,2000).Female rats express higher levels of ERα,ERβ,and G protein-coupled estrogen receptor than males in the trigeminal ganglia (Warfvinge et al.,2020).Furthermore,the expression of these receptors varies with the cellular context in a sex-dependent manner.For example,whileErα
and G proteincoupled estrogen receptor genes are upregulated,Ar
is downregulated in male rats after stroke.However,female rats did not show significant differences in the expression of these sex steroid hormone receptors after MCAO,compared with sham rats (Acaz-Fonseca et al.,2020).Thus,these differences in the expression of gonadal hormone receptors in the brain between sexes may determine sex differences in the regulation of autophagy.Estradiol activates autophagy in the brain
Estradiol acts on multiple signaling pathways associated with brain autophagy(Azcoitia et al.,2019;Xiang et al.,2019).Estradiol activates the PI3K/AKT signaling pathway in the brain.PI3K activity induces the phosphorylation and activation of AKT,which phosphorylates and inhibits the serine/threonine kinase glycogen synthase kinase 3β (GSK3β) (Saraceno et al.,2018).Pharmacological inhibition of GSK3β increases levels of LC3-II and decreases levels of p62 (Wang et al.,2019).Alternatively,class III PI3K/AKT activation by estradiol can also inhibit mTORC1 (Perez-Alvarez et al.,2018),a master nutrient sensor that inhibits autophagy.Estradiol can activate AMPK (Guo et al.,2017a),an energy sensor that is activated with ATP depletion and acts as the antithesis of mTORC1 by activating autophagy.Furthermore,estradiol inhibits the activity of the transcription factor NF-κB (Cook et al.,2018;Yun et al.,2018).Enhanced activity of the transcription factor NF-κB is associated with reduced autophagy during neural progenitor cell differentiation(FitzPatrick et al.,2018) and in the cortex of a barrel cortex ischemic stroke mouse model (Li et al.,2013).This data suggests that estradiol activates autophagy via inhibiting NF-κB.However,the activation of NF-κB exacerbates autophagy in a traumatic brain injury rat model (Liu et al.,2020).The signaling pathways linked to brain autophagy positively regulated by estradiol are summarized inFigure 3
.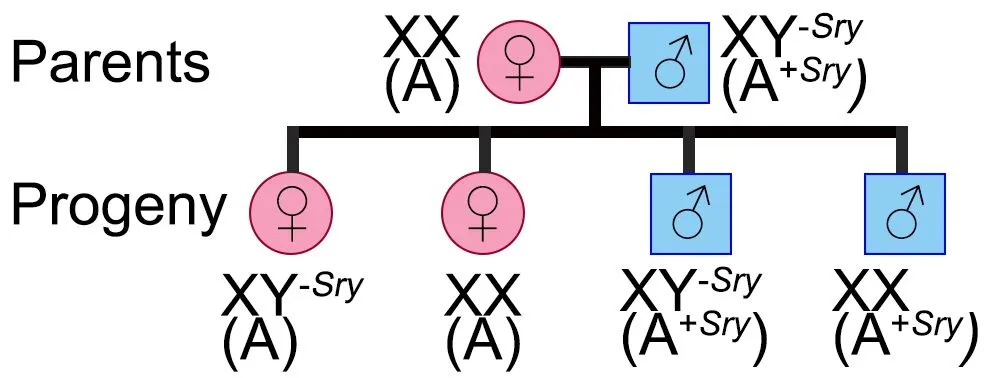
Figure 2 | The Four Core Genotype mouse model.
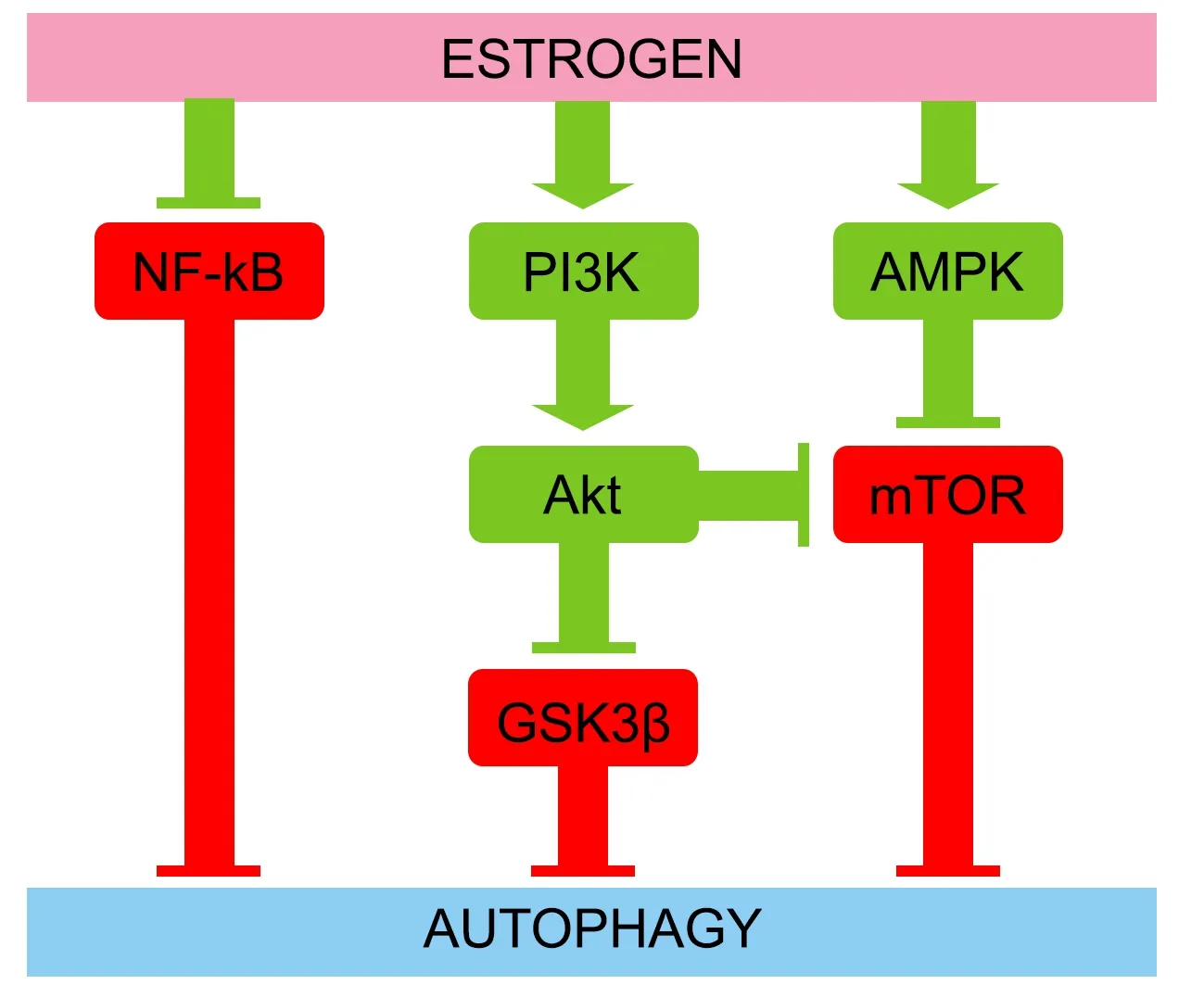
Figure 3 | Estrogen induces autophagy through several signaling pathways.
Estradiol inhibits autophagy in the brain
In ischemic stroke mouse models,estradiol supplementation may also have an inhibitory effect on autophagy in the brain by downregulating hypoxia inducible factor 1α (HIF-1α),which is a transcription factor that induces autophagy (Hsieh et al.,2015).Estradiol can also inhibit autophagy by increasing levels of p62 and by reducing levels of LC3-II,ATG5 (a component of the ATG12 conjugation system),and Beclin1 (involved in phagophore formation),in the hippocampus after stroke (Li et al.,2017).Thus,in which conditions estradiol can act as an autophagy stimulator or inhibitor in stroke models need to be identified for the proper application of therapeutic strategies.
Effects of other gonadal hormones in autophagy in the brain
Progesterone first appeared as a potential neuroprotective steroid by upregulating autophagy in an amyotrophic lateral sclerosis mouse model(Kim et al.,2013).In cultured astrocytes,progesterone activates autophagy and prevents the accumulation of neurotoxic aggregates (Kim et al.,2012).In ischemic brains of male rats and inex vivo
microglia derived from adult male rats treated with the toxin lipopolysaccharide,progesterone administration enhanced autophagy and reduced inflammasome activation (Espinosa-Garcia et al.,2020).These studies highlight the beneficial effects of progesterone in the brain.Few studies have investigated the effects of testosterone on autophagy in the brain,and existing data from rat cardiac tissue indicate that testosterone supplementation improved heart function by upregulating the expression ofBcl-2
,which binds to Beclin1 and interrupts autophagy initiation (Fu et al.,2017).However,testosterone can be aromatized into estradiol by aromatase (Shay et al.,2018).Thus,the inhibitory effects of testosterone in autophagy could turn out to autophagy stimulation induced by aromatizationderived estradiol.Androstenedione is another androgen that can upregulate autophagy via reactive oxidative species in osteosarcoma (Liu et al.,2014).However,there is not much data about the role of androstenedione in autophagy in the brain.In conclusion,estrogen is a master regulator of autophagy in the brain by altering the expression of multiple ATG’s and by modulating different signaling pathways.Overall,estrogen is an activator of autophagy;however,under certain circumstances,estrogen can inhibit autophagy (Hsieh et al.,2015;Li et al.,2017).Additional research is necessary to determine the sex-biased effects of the different gonadal hormones in autophagy in ischemic stroke.Potential sex-specific signaling pathways seen in both health and disease need to be elucidated.
Role of the X-chromosome in autophagy in the brain
Several genes involved in the machinery and signaling pathways that regulate autophagy in the brain localize to the X-chromosome.So far,no ATG’s linked to the Y-chromosome have been found,suggesting that the sex differences in autophagy mediated by the sex chromosomes are likely due to the X-chromosome.Known genes associated with autophagy in the brain and localize to the X-chromosome are listed (Table 2
).Abnormalities in these genes have deleterious consequences for brain function.Given that males only have one copy of the X-chromosome,alterations in X-linked genes are associated with worse symptoms in males compared to females.Generally,in neuropathology associated with X-linked genes,heterozygous females have less severe phenotypes,or no clinical phenotype,compared with hemizygous males.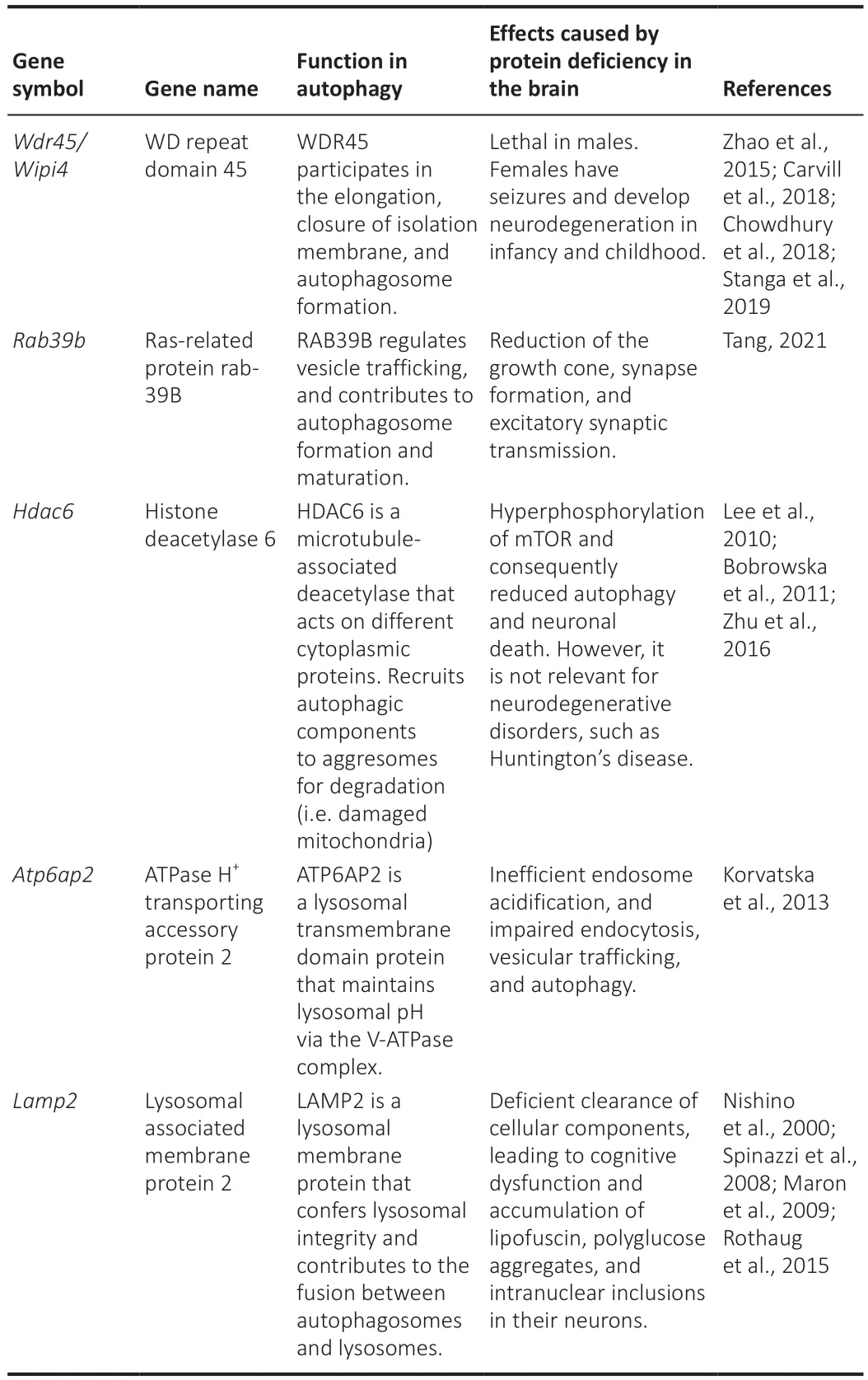
Table 2 | Several autophagy-related genes localize in the X-chromosome
The X-chromosome plays an important role in autophagy in the brain as it has genes that encode proteins that regulate:the formation and maturation of autophagosomes (Wdr45
,Rab39b
);vesicle trafficking (Rab39b
);autophagy cargo recruitment (Hdac6
);and the maintenance of lysosomal integrity (Atp6ap2
andLamp2
) (Figure 1
).Deficiency in any of these genes negatively affects the progression of autophagy in the brain and leads to neurodegeneration and brain dysfunction.No sex differences have been reported yet in the expression of the X-linked genes associated with autophagy in the brain,except forWdr45
,as WDR45 deficiency is lethal only for males (Carvill et al.,2018).This is likely due to the X-chromosome inactivation (XCI) that occurs in females to compensate for double X-chromosome dosage (Marks et al.,2015). Thus,males and females may have equivalent expression levels of X-linked genes.However,the XCI mechanism becomes less effective with aging,and some X-linked genes may escape from XCI (skewing) (Shvetsova et al.,2019).Reduced XCI also occurs more frequently in the brain compared to other tissues (Nguyen and Disteche,2006),which could lead to altered expression of key autophagy genes located on the X-chromosome during reproductive senescence.Changes in gene expression could alter the autophagic response to ischemia.Thus,identifying escapee genes associated with autophagy in the brain could lead to novel treatment strategies.In conclusion,differential expression of X-linked genes associated with autophagy may occur during aging and contribute to sex differences in brain autophagy.Future research may focus on identifying these autophagy-related X-linked genes that escape from XCI.
Autophagy in Ischemic Stroke
The clinical research in autophagy in stroke faces a challenging methodological situation:currently,there is a lack of methods that can measure the dynamic cellular process of autophagy in the most relevant human tissue,the brain,which is often inaccessible.Thus,blood samples are often used as a surrogate to determine changes that occur with stroke in the brain.Autophagy-associated circular RNAs,which are a noncoding RNA isoform with the potential to regulate neurological diseases,are differentially expressed in the plasma of large-artery ischemic stroke patients.Levels of these are positively correlated with neurological deficits and infarct volumes (Li et al.,2021).Gene microarrays analyses of blood samples from ischemic stroke patients revealed that several autophagy-associated genes (AKT
,ATG4B
,HDAC6
,andLAMP1
) are upregulated in stroke patients compared with controls (Guo et al.,2017b).Patients with a variant in the promoter of the autophagy-inducer lncRNA MALAT1 have a higher risk of ischemic stroke (Wang et al.,2020c).Thus,clinical data suggest that autophagy is altered in stroke patients.There is a general assumption that autophagy is beneficial under basal conditions,and that upregulating autophagy improves brain function in neurodegenerative disorders and in the aged brains,which shows reduced autophagy (Loeffler,2019).For example,rapamycin,an inhibitor of mTOR that plays a central role in energy homeostasis and autophagy inhibition,confers protection to the brain after ischemic stroke in clinical and pre-clinical studies(reviewed in (Hadley et al.,2019).More specifically,restoring autophagy by enhancing NAD:NADH levels or upregulating Beclin1 improved mitochondrial function and reduced stroke development in stroke-prone spontaneously hypertensive rats (Forte et al.,2020).However,enhanced autophagy can be deleterious under stressful conditions such as stroke,which may lead to consider autophagy inhibition as a strategy to reduce infarct volume.Recent reviews have highlighted the evidence on the role of autophagy in ischemic stroke (Ajoolabady et al.,2021;Wang et al.,2021).During an ischemic insult,a plethora of intertwined pathways is coordinated to activate autophagy(Wang et al.,2020a).For example,there is a positive correlation between LC3-II levels (enhanced number of autophagosomes) and cortical infarct volume after stroke in MCAO rat models (Acaz-Fonseca et al.,2020).However,as we discuss below,the activation of autophagy by ischemia may occur in a sex-dependent manner.Autophagy can lead to cytoprotective (adaptive autophagy) or detrimental (maladaptive autophagy) consequences depending on the cellular context (Wang et al.,2020a;Ajoolabady et al.,2021).Thus,inhibiting autophagy may not have similar effects in males and females.
Manipulating autophagy may be a feasible approach to prevent or mitigate brain damage after ischemic stroke.However,before the development or implementation of therapeutic strategies for stroke via inhibition or promotion of autophagy,it is critical to first elucidate the effects of manipulating autophagy in both sexes.
Sex Differences in Autophagy in Stroke
Autophagy is importantly regulated by sex hormones,and several ATG’s locate on the X-chromosome.Thus,it is reasonable to think that sex differences exist in autophagy and that these differences contribute to sex differences in stroke outcomes.
Sex differences in autophagy in stroke animal models
Acaz-Fonseca et al.(2020) found that autophagy is upregulated by an ischemic stroke in a sex-dependent manner by examining the levels of two ATG’s,uncoupling protein 2 (Ucp-2
) andHif-1α
.HIF-1α is a transcription factor that induces autophagy (Lu et al.,2018),and UCP-2 is a mitochondrial protein involved in the regulation of autophagy (Mao et al.,2021).Authors found that these genes were upregulated in males after stroke compared with females,which showed no changes in these markers.The upregulation ofUcp-2
andHif-1α
expression was associated with enhanced levels of LC3-II after stroke only in male rats.Consistent with this work,we found that autophagy was enhanced in the brain of mouse males after stroke,compared with sham males;both Beclin1 and LC3-II were upregulated,and p62 was reduced.However,changes in autophagy in females after stroke were less evident;LC3-II and ATG7,which contribute to LC3 conjugation and autophagosome maturation,were upregulated,but Beclin1 was downregulated and p62 levels remained unaltered.This suggests that stroke-induced autophagy may be muted in females (Patrizz et al.,2021).A study using a neonatal hypoxia-ischemia (HI) rat model compared the levels of Beclin1 between sham and HI in each sex and found no differences.This suggests that the early steps of autophagy were not stimulated by HI.However,they found that HI males had reduced levels of LC3-II than sham males,suggesting a reduction of autophagosomes/autolysomes after HI.Contrarily,LC3-II levels were increased (increased autophagy vesicles) in HI femalesvs
.sham females (Weis et al.,2014).Considering that LC3-II is degraded when autophagosomes fuse with lysosomes,we may hypothesize that the autophagy flux is stimulated in males after HI,but blocked in females.However,analyses of additional markers,such as p62,would help for a better interpretation of this data.Another study found postnatal day 8 male rats eliminate damaged mitochondria (likely by mitochondrial autophagy,known as mitophagy) more efficiently than female rat pups after HI (Demarest et al.,2016).HI males showed reduced mitochondrial pool and mitochondrial size compared with HI females.Thus,the studies above suggest that the autophagy process is upregulated in males compared to females after ischemia.Enhanced levels of LC3-II correlate with increased cortical infarct volume after stroke in MCAO rat models (Acaz-Fonseca et al.,2020),and that inhibition of autophagy may reduce brain injury (Tower et al.,2020).Given that strokeinduced autophagy appears to be more relevant in male mice than in females,the inhibition of autophagy could lead to neuroprotection to a greater extent in males.In a focal model of stroke we found that inhibiting autophagy with 3-methyladenine (3-MA),which blocks the signaling pathway that induces phagophore formation and autophagosome nucleation (class III PI3K signaling pathway),reduced infarct volume by 6-fold in young (8–12 weeks old) male mice subjected to MCAO. In contrast,3-MA exacerbated ischemic damage in MCAO female mice (Patrizz et al.,2021).To determine if this was secondary to gonadal hormones,female mice were ovariectomized and treated with 3-MA or vehicle after stroke.Ovariectomized females treated with 3-MA had reduced infarct volume compared with ovariectomized females treated with vehicle,suggesting that gonadal hormones mediate the deleterious effects of autophagy in stroke,independent of sex.3-MA also had beneficial effects in young (3-months old) ovariectomized females by preventing the increase of pro-inflammatory cytokines induced by stroke (Li et al.,2017).However,despite these studies demonstrate the inhibitory effect of 3-MA on autophagy,others found that 3-MA may stimulate autophagy in ischemic stroke samples (Zhang et al.,2021a).Thus,it is necessary to specifically target autophagy components to inhibit autophagy.In a cardiac arrest and global ischemia using 16–18-day-old postnatal rats (with equivalent estradiol levels between sexes),intracisternal administration of siRNA against ATG7 led to an improvement in motor function in female rat pups but had no benefit in male pups (Au et al.,2015).Moreover,the treatment led to a reduction of degeneration of cerebellar Purkinje neurons in females,but not in males.This seems to contradict evidence from 3-MA studies.Note that using alternative genetic tools may lead to different outcomes compared with using nonspecific approaches to inhibit autophagy.In addition,ATG7-independent autophagy has been demonstrated in different cell lines,such as MEF and HEK293T cells (Nishida et al.,2009;Ra et al.,2016).Whether there are differences in ATG7-dependent autophagy pathways between sexes has not been elucidated.
Sex differences in autophagy in cultured brain cells
At the cellular level,cultured neurons from embryonic male rat pups exhibit enhanced autophagy compared with female neurons.Inin vitro
assays,male neurons showed higher levels of LC3-II (Du et al.,2009;Patrizz et al.,2021)and ATG7 (Patrizz et al.,2021),and reduced levels of the autophagy cargo p62(Patrizz et al.,2021),compared with female neurons,suggesting that basal autophagy is enhanced in malevs.
female neurons.After oxygen and glucose deprivation (OGD),male neurons showed an increase in LC3-II levels,and a more dramatic reduction in p62 levels,compared with female neurons,which supports that the activation of autophagy is greater in male neurons than in female cells after OGD (Du et al.,2009).Thus,ATG7 may act as a limiting factor in both basal autophagy and autophagy activation in female neurons.It is evident that neurons play a major role in brain function,but they only represent approximately 50% of all cells in the human brain.The remaining cells are primarily glial cells (Wang et al.,2020b),which also regulate neuronal autophagy (Kulkarni et al.,2018).It is reasonable to think that sexbiased mechanisms could govern autophagy in the brain via non-neuronal cell types,such as microglia,astrocytes,and endothelial cells.However,no studies have explored sex differences in autophagy in these brain cell types.Sex hormones affect the fate of brain cells in ischemia models.For example,estradiol prevents microglia activation after MCAO (Perez-Alvarez et al.,2012;Zhang et al.,2018) and in cultured microglia exposed to hypoxia (Slowik et al.,2018).In co-cultures of astrocytes and neurons,activation of ERβ in astrocytes by estradiol leads to neuronal protection after OGD (Ma et al.,2016).Furthermore,estradiol plays a beneficial role in the brain by preventing BBB disruption and leakage during and after ischemic injury (Sohrabji,2015),and prevents tight junction impairment in cultured brain-derived endothelial cells after OGD (Na et al.,2015).However,whether the effects of estradiol in glia and endothelial cells are mediated by autophagy needs to be examined.It is imperative to determine if sex hormones and X-linked autophagy genes regulate autophagy in specific cell types in the brain.
Conclusions
The incidence,prevalence,and outcomes after ischemic stroke are strongly influenced by sex.Age,gonadal hormone exposure,and the sex chromosome complement are important factors in the sex differences seen in stroke.Elderly women have poorer functional outcomes,and more women live with a disability after stroke compared to men.This is in part due to the older age of women when they have their first stroke.Pre-clinical studies have found that both gonadal hormones and the X-chromosome complement play important roles in the sex-biased outcomes seen after stroke,but their contribution differs throughout the lifespan.Autophagy may be a mechanism that contributes to these differences.In the brain,autophagy is importantly regulated by estrogen.However,despite our knowledge of multiple X-linked autophagy genes,there is no evidence yet that these genes contribute to sex differences in brain autophagy after ischemic stroke.
In males,ischemic stroke activates autophagy.Given that autophagy inhibition reduces volume infarction after stroke in males,we could hypothesize that maladaptive autophagy is more importantly upregulated in male mice than adaptive autophagy.However,while females show reduced brain damage after ischemic stroke compared with males,autophagy stimulation after stroke in female brains is less evident than in males.Thus,inhibiting autophagy benefited males,but not females,an effect that is mediated by gonadal hormones.This could be explained if stroke induces maladaptive autophagy in males.However,in females,the stroke did not stimulate autophagy and the brain tissue from females may retain only basal autophagy levels.Using autophagy inhibitors as 3-MA in females may then block adaptive autophagy,which is beneficial and neuroprotective.Thus,blocking basal autophagy in females may expose brain cells to energy deficits making them more vulnerable to ischemia (Figure 4
).Research in sex differences in autophagy in stroke is still in its infancy,as the mechanisms and signaling pathways that regulate the different steps of autophagy in a sex-dependent manner have not been identified.Furthermore,published studies have important limitations in using multiple autophagy markers for an accurate interpretation of data.Future studies on this topic should follow appropriate guidelines for monitoring autophagy (Klionsky et al.,2021).Using existing tools such as the FCG mouse model,transgenic mice with ATG deletions,and cell and animal models with autophagy-flux reporters will help to identify the mechanisms that regulate autophagy after stroke in a sex-dependent manner,but both sexes need to be evaluated.This knowledge could contribute to the development of therapeutic targets for both men and women.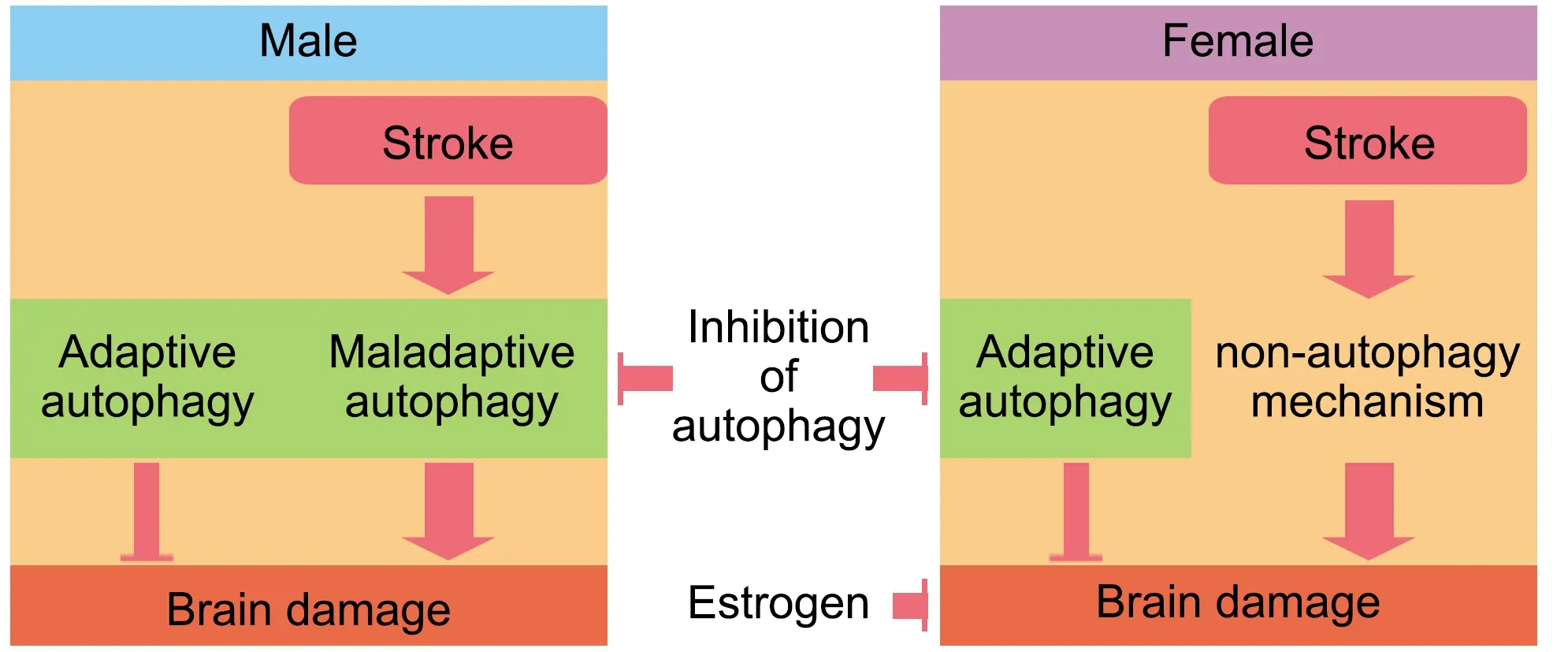
Figure 4 | Stroke may upregulate autophagy more in males than in females,which may have alternative mechanisms to respond to the high cellular stress during and after an ischemic stroke.
Author contributions:
JFMM wrote the manuscript.BN and LDM edited the manuscript and advised about the content.All authors approved the final version of the manuscript.
Conflicts of interest:
The authors declare no conflicts of interest.
Open access statement:
This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Neuroaxonal and cellular damage/protection by prostanoid receptor ligands,fatty acid derivatives and associated enzyme inhibitors
- Extracellular vesicles in Alzheimer’s disease:from pathology to therapeutic approaches
- Molecular approaches for spinal cord injury treatment
- Adipose tissue,systematic inflammation,and neurodegenerative diseases
- Interleukin-1:an important target for perinatal neuroprotection?
- Neurotrophic fragments as therapeutic alternatives to ameliorate brain aging

