Neuroaxonal and cellular damage/protection by prostanoid receptor ligands,fatty acid derivatives and associated enzyme inhibitors
Najam A.Sharif
Abstract Cellular and mitochondrial membrane phospholipids provide the substrate for synthesis and release of prostaglandins in response to certain chemical,mechanical,noxious and other stimuli.Prostaglandin D2,prostaglandin E2,prostaglandin F2α,prostaglandin I2 and thromboxane-A2 interact with five major receptors (and their sub-types) to elicit specific downstream cellular and tissue actions.In general,prostaglandins have been associated with pain,inflammation,and edema when they are present at high local concentrations and involved on a chronic basis.However,in acute settings,certain endogenous and exogenous prostaglandins have beneficial effects ranging from mediating muscle contraction/relaxation,providing cellular protection,regulating sleep,and enhancing blood flow,to lowering intraocular pressure to prevent the development of glaucoma,a blinding disease.Several classes of prostaglandins are implicated (or are considered beneficial) in certain central nervous system dysfunctions (e.g.,Alzheimer’s,Parkinson’s,and Huntington’s diseases;amyotrophic lateral sclerosis and multiple sclerosis;stroke,traumatic brain injuries and pain) and in ocular disorders(e.g.,ocular hypertension and glaucoma;allergy and inflammation;edematous retinal disorders).This review endeavors to address the physiological/pathological roles of prostaglandins in the central nervous system and ocular function in health and disease,and provides insights towards the therapeutic utility of some prostaglandin agonists and antagonists,polyunsaturated fatty acids,and cyclooxygenase inhibitors.
Key Words:AL-8810;axon;brain;central nervous system;cyclooxygenase inhibitors;neuron;neuroprotection;ocular;polyunsaturated fatty acids;prostaglandins
Introduction
Prostaglandins
Arachidonic acid formed by the action of phospholipase A2 on cell membrane phospholipids is the major substrate for future conversion by lipoxygenases to create leukotrienes,and for cyclooxygenases (COX-1[constitutive] and COX-2 [inducible by inflammatory stimuli]) to form prostaglandins (PGs)via PGG2 and PGH2 (Coleman et al.,1994).The five distinct bioactive lipid molecules created by COX-1 and COX-2 include PGD,PGE,PGF,PGI,and thromboxane-A2 (TXA) (Coleman et al.,1994;Figure 1
).The biological actions of the major prostanoids are mediated by separate receptors whose names originate from these PGs and are known as DP,EP,FP,IP,and TP receptors,respectively (Coleman et al.,1994).Some of these major receptors have sub-types such as DP,DP,EP,EP,EP,EP,TP,and TPreceptors(Figure 1
).Some additional derivatives of PGD2,including PGJ2 and deoxy-PGJ2 for instance,have various biological functions either through activating DP receptors or via cross-talk with other receptors/pathways such as transient receptor potential cation channels to cause inflammation and pain (Jang et al.,2020).Some specific inhibitors of COX-1 and COX-2 have been developed as therapeutics to treat pain and other conditions and these will be discussed later (Patrignani and Patrono,2015).Prostaglandin receptors,signal transduction and general actions
The heptahelical guanine-nucleotide-coupled-receptors associated with these PGs are embedded in the plasma membranes of the majority of the mammalian cells,and transduce either an elevation (DP-,EP2-,EP4-,and IP-receptors) or reduction (EP3-receptors) of cAMP (Narumiya et al.,1999;Woodward et al.,2011).Activation of the remaining PG receptors (EP,FP-and TXA-receptors) by their respective agonist ligands results in the production of intracellular inositol phosphates (IPs) that in turn raise intracellular Ca([Ca]) by releasing it from the endoplasmic reticulum of the cells (Narumiya et al.,1999;Woodward et al.,2011),and diacyl glycerol that activates various enzymes (Figure 1
).These changes in [Ca],diacyl glycerol,and cAMP then evoke downstream signal transduction such as phosphorylation and activation of various kinases,culminating in the final biological activity of the eicosanoid(e.g.,enzyme or hormone release,muscle contraction or relaxation,platelet aggregation,pain induction,and lowering of intraocular pressure) (Coleman et al.,1994;Smyth et al.,2009).Some receptor-selective agonists and antagonists have also been developed and introduced into medical treatments of some diseases that will be discussed below.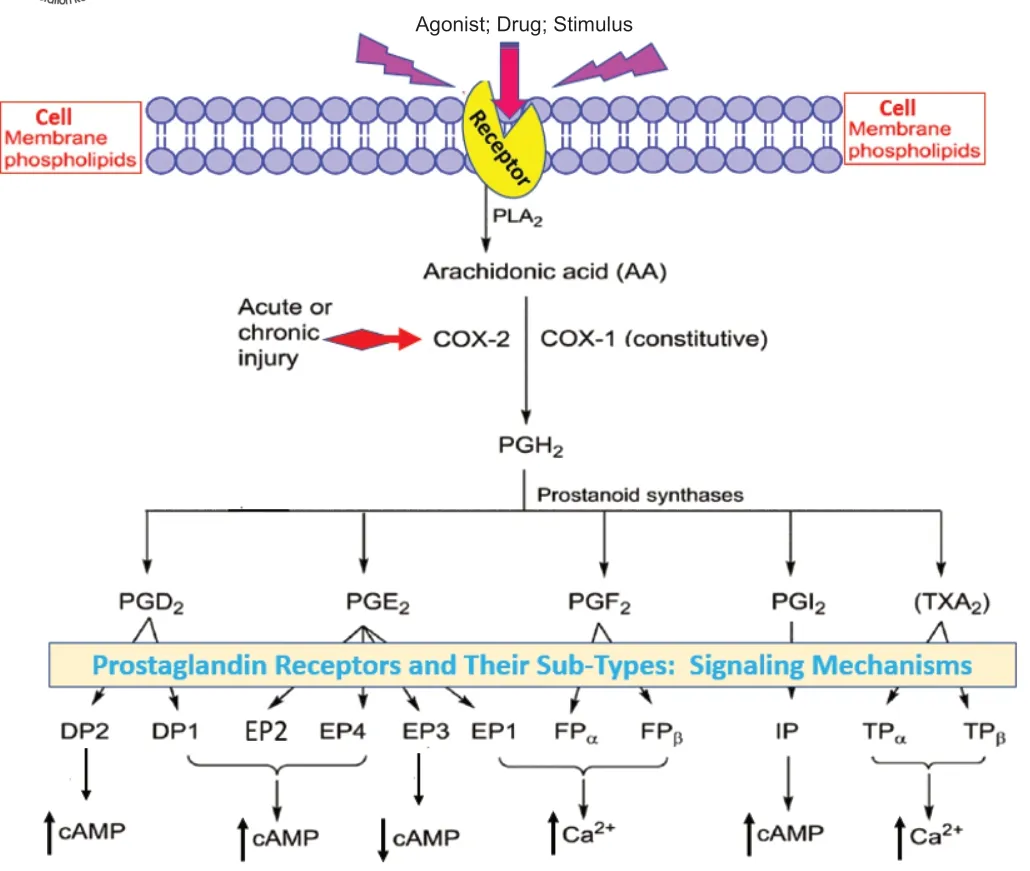
Figure 1 | The generation of various prostanoids and their receptor activation and signaling profiles.
Preformed or newly synthesized PGs mediate numerous biological functions such as causing cytokine release,enhancing fluid hydrodynamics,contracting or relaxing smooth muscles,initiating hormone and growth factor secretion,eliciting pain,causing fever,modulating gene expression as destructive agents,or enhancing cellular survival.Some of these actions are paradoxical and reflect the pro-or anti-inflammatory properties.PGs can exhibit different actions depending on their acute or chronic release,their concentrations,cell types involved,and site(s) of action.Regarding inflammation,for instance,PGs in the acute phase are usually protective,while in chronic situations,they become destructive,especially when their concentration remains elevated for a protracted time frame.This“Jekyll and Hyde”nature of PGs is reflected by activities of cytokines where certain forms are pro-inflammatory(e.g.,interleukin-1 (IL-1),IL-6 and IL-8),whereas others are generally antiinflammatory (e.g.,IL-10,IL-17,and IL-37) (Akdis et al.,2016).Interestingly,in many circumstances,PGs regulate the synthesis and secretion of certain cytokines and thus there is a complex relationship between these cellular mediators due to feed-forward and feedback mechanisms that involve the immune system.
As often happens in nature,body tissues and cells self-regulate to maintain homeostasis.Thisying
-yang
nature of mediators of communication and inflammation and healing is also evident in the eicosanoid field.Thus,whilst endogenous PGs are generally nonspecific in their receptor selectivity (Table
1
),as compared to synthetic PGs (e.g.,FP-receptor agonists;Table 2
),and cause inflammation,docosahexaenoic acid (DHA),an omega-3 fatty acid that is a primary structural component of the human brain,skin,and retina,is anti-inflammatory.DHA comprises 40% of the polyunsaturated fatty acids (PUFAs)in the brain and 60% of the PUFAs in the retina and is derived mainly from food sources and some are endogenously synthesized from α-linolenic acid(Figure 2
).The neuronal plasma membrane contains up to 50% DHA,which modulates the carrier-mediated transport of choline,glycine,and taurine,the function of delayed rectifier potassium channels,and the response of rhodopsin contained in the synaptic vesicles.Phosphatidylserine,which has a high DHA content,has roles in neuronal signaling and neurotransmitter synthesis,and DHA deficiency is associated with cognitive decline (Cedarholm et al.,2013).DHA levels are reduced in the brain of severely depressed people(McNamara et al.,2013).The conversion of DHA to the anti-inflammatory lipids,neuroprotection D1,and D-and E-resolvins (Figure 2
),is the basis of DHA’s beneficial properties in neuronal functions and in preventing or reducing oxidative stress in many diseases (Kim and Spector,2018).
Figure 2 | The PUFA-derived cytoprotective/neuroprotective agents and their biological actions.
Database Search Strategy
This narrative review article was constructed using the information gathered,assembled,and harmonized from publications revealed using PubMed of the National Institute of Health (NIH),National Library of Medicine (PubMed.org),and Google.Searches were performed till June 2021.The search strategy and selection criteria utilized keywords and combinations thereof such as prostanoids and brain;prostanoids and eye or ocular;prostanoids and disease;prostanoids and protection;prostaglandins and neuroprotection;prostaglandins and neurodegeneration;inflammation and prostanoids.No limit was placed on the year of publication or authorship.During the assembly of various references for each section of the review article,particular attention was paid to using relatively recent review articles on given topics where possible,with emphasis on the last decade.Sometimes,it was necessary to cite original ground-breaking research discovery publications to give the credit to the original authors and thus older citations were used.However,pertinent materials and information reported over the last 30 years were accounted for in formulating opinions and being as factual as possible.
Prostanoids in Neurological Functions:Do They Help or Hinder?
Lipids perform many structural and functional roles within the central nervous system (CNS),both within the grey matter (neuronal elements)and the white matter (axonal elements).Therefore,it is to be expected that any malfunction associated with neuronal or axonal lipid metabolism or structural defects involving membranes of the cell exterior and/or intracellular organelles would have major deleterious consequences.Accordingly,in pathological situations,the cellular machinery would mobilize its resources to combat the disease processes by modulating lipid metabolism and altering gene expression to augment reparative and survival counter-measures.Below are presented a range of roles of PGs and their downstream signaling pathways involved in CNS and ophthalmic diseases and the potential utility of synthetic PG receptor agonists and antagonists to ameliorate these conditions.Due to the multiplicity of PGs and their receptors,the interplay between them sometimes yields conflicting conclusions indicating the complexity of their effects (Yagami et al.,2016).To better understand the roles that various PG receptors may play in CNS,their relative presence and distribution need to be understood.Accordingly,using thin-layer chromatography,Holmes and Horton (1968) showed the presence of endogenous PGD,PGE,and PGFin dog brain cortex,hippocampus,caudate nucleus,hypothalamus,cerebellum,medulla,and pons,cortical white matter,and spinal cord.Additionally,in situ
hybridization (Suzuki-Yamaoto et al.,1994;Zhang et al.,1995;Candelario-Jalil et al.,2005),autoradiography (Yamashita et al.,1983;Matsumura et al.,1992,1995;Olda et al.,1997) and immunohistochemistry (Nakamura et al.,2000) revealed presence of the genes and their products on PG receptors in numerous mammalian CNS tissues (Table 3
).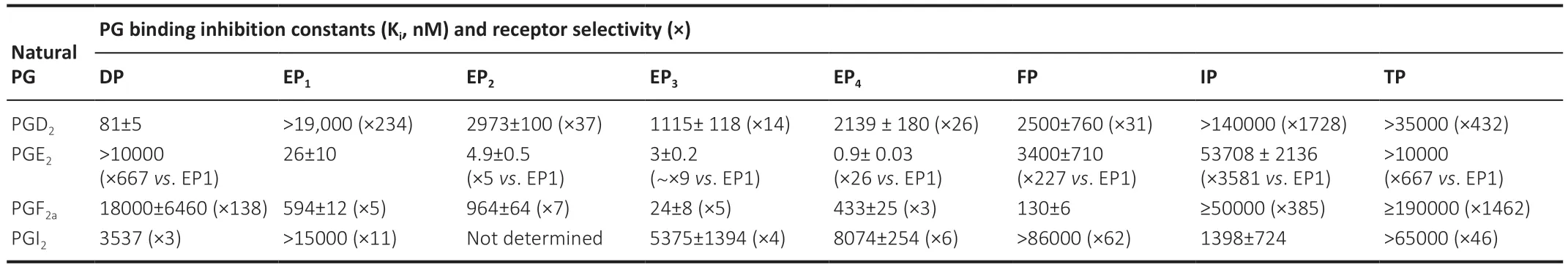
Table 1 | Relative affinities and receptor selectivities of natural prostaglandins for PG receptors and some receptor subtypes
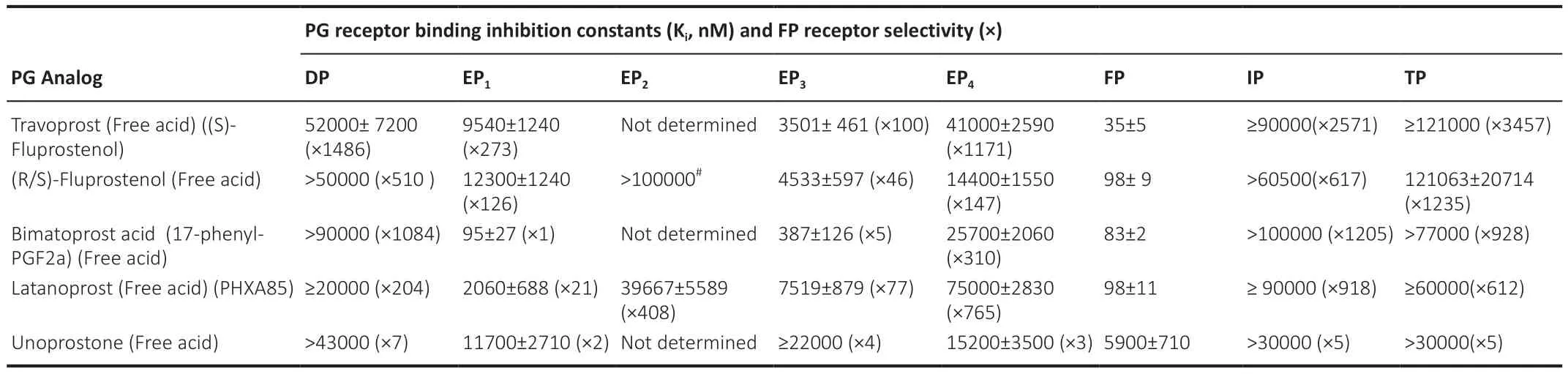
Table 2 | Relative affinities of synthetic FP-receptor agonist prostaglandins for various PG receptors and some receptor subtypes,and their relative selectivities against non-FP-receptors

Table 3 | Central nervous system localization and distribution of prostanoid receptors determined by various techniques
Alzheimer’s Disease
Alzheimer’s disease (AD) and the associated dementia/memory loss is one of the most frightening neurological diseases afflicting humans on a global scale (at least 44 million patients worldwide,with~6 million in the US).Despite decades of research,the exact etiology of AD has remained elusive,and thus even a minor improvement in signs and symptoms of this disease would be most welcome by the patients and their caregivers.Unfortunately,a milieu of endogenous agents and inter-connected pathways conspire to induce AD,and thus a singular curative therapeutic agent or device is unlikely to be successful in combating AD.However,in addition to the accumulation of amyloids and Tau and related misfolded proteins causing brain cellular damage in AD,multifactorial chronic inflammation appears to be one major root cause of AD,and it is now believed that PGs may play a role in the onset of AD pathology and/or exacerbating the condition (Giovannini et al.,2003;Phillis et al.,2006;Figure 3
).Corroborating evidence for this includes the fact that the neurofibrillary tangles and β-amyloid plaques associated with AD are pro-inflammatory,causing migration of leukocytes and microglia to the injury site.These cells then release numerous cytokines and chemokines that up-regulate and activate PLA2 that generates more PGs.Consequently,PGElevels are elevated leading to further amplification of the inflammatory cascade in the patient’s brain.Corollaries to this situation are that in experimental animal models of AD,a chronic intake of anti-inflammatory inhibitors of COX-1/2 (Figure 4
) diminishes the risk,and retards the onset and progression of the disease (McGeer et al.,1990;Giovannini et al.,2003;Farooqui et al.,2007;Kotilinek et al.,2008).It is worth noting,however,that compensatory endogenous anti-inflammatory lipid metabolites derived from DHA are simultaneously produced to provide a level of homeostasis and keep the inflammation in check.If the balance between the arachidonic acid-derived PGs/leukotrienes,and DHA-derived lipoxins,D-and E-resolvins and neuroprotectin D1 (Bazan,2007;Figure 2
) shifts towards the former,chronic inflammation sets in and the grey matter (neuronal cell bodies and their dendritic tree) begins to atrophy.Since the ensuing death of the associated axons occurs next,the overall pathology of AD continues,with further deposition of misfolded proteins and scar tissue produced by invading astrocytes/fibroblasts stimulated by locally released pro-fibrotic cytokines (e.g.,transforming growth factor-β and connective tissue growth factor).Observations of prolonged inflammation-based biomarkers and signs of neural damage (including deposits of complement proteins;activated microglia and lymphocytes;elevated PLA;neuronal lysis) have been reported for postmortem AD patient brains (McGeer et al.,1987,1989;Rogers et al.,1988;Stephenson et al.,1996).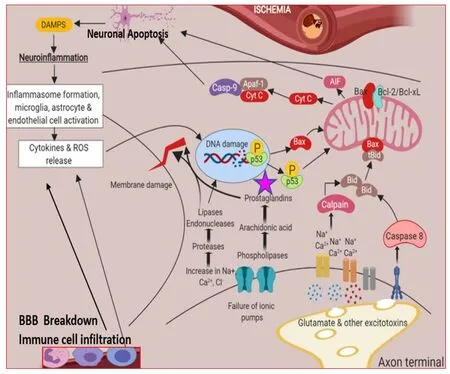
Figure 3 | The stress factors and their roles in the inflammatory processes encompassing the detrimental effect of PGs in the death of neurons.
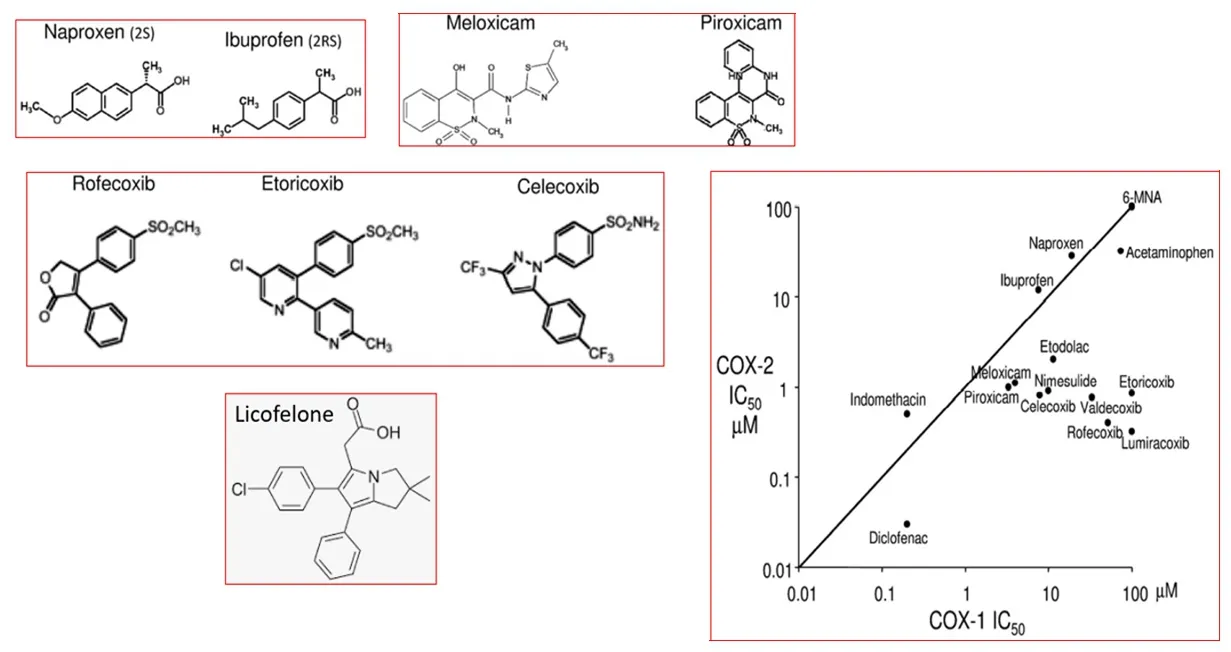
Figure 4 | The chemical structures of various COX-1/ COX-2 inhibitors and their relative enzymatic potencies and selectivities.
Additional evidence of PGs involvement in AD etiology concerns enhanced cerebral COX-1/2 expression (Yasojima et al.,1999;Hoozemans et al.,2001),detection of abnormally high levels of PGEin the cerebrospinal fluid of AD patients (Montine et al.,1999),and EPreceptor-induced elevation of amyloid-β precursor mRNA and protein (Pooler et al.,2004;Hoshino et al.,2007;Herbst-Robinson et al.,2015).An interesting observation relates to an apparent selective down-regulation of EPreceptors by PGE,which continues to activate EPreceptors with a resultant increase of the β-amyloid concentrations (Hoshino et al.,2009).Linkage of EPreceptor activation to AD pathology has been confirmed in a mouse model of AD where knocking out the EPreceptor resulted in reduced oxidative cerebral damage and β-amyloid levels (Liang et al.,2005),and no apparent loss of mouse spatial memory (Savonenko et al.,2009).Additionally,microglia that lack EPreceptors are less prone to toxic effects of β-amyloid and appear to ingest and degrade these toxins (Shie et al.,2005).Furthermore,EPreceptor activation by TNF-α-mediated elevation of PGEin rat astrocytes elevates cellular nitrites and nitrates that can kill neurons (Hsiao et al.,2007).While EPreceptors appear to play a central role in AD (Wei et al.,2010;Prio et al.,2012),despite a recent report that illustrates protective effects of an EP/EPreceptor agonist (misoprostol;Tian et al.,2016),elevated PGDconcentration and increased DP-receptor expression on microglia and astrocytes within senile plaques of AD patient brains,and in animal models of the disease,suggest an involvement of DPreceptors in AD as well (Mohri et al.,2007).Consistent with these reports is the intriguing possibility that other PGs derived from PGD,for example by dehydrogenation leading to the formation of detrimental J2-PGs (derived from PGD),also play a role in causing or amplifying the disease process by dampening the ubiquitin-proteasome/mitochondrial pathway (Figueiredo-Pereira et al.,2015).
Based on the intense search for potential beneficial remedies towards AD,numerous therapeutic approaches have been proposed and some are in late-stage development and entered clinical trials (Cummings et al.,2017).Whilst recognizing the role that PGs (in particular PGEand PGD) play in the inflammatory cascade-connected to AD pathogenesis (see above),only first-generation COX-inhibitors (indomethacin;ibuprofen),and recently,novel COX/5-LOX inhibitors (e.g.,Licofelone;Kumar et al.,2020;Razavi et al.,2021),seem to have been tested in the AD patients,and no other PGreceptor-related targets appear under investigation (Cummings et al.,2017).The latter enzyme inhibitors appear not to show any significant benefits in the AD-patients.However,there is some new hope on the horizon.With the recent advent and characterization of small molecule antagonists of DPand EPreceptors,it now becomes important to investigate whether such drugs could impart beneficial effects in animal models of AD and to AD patients.Many preclinical studies would need to be performed first to select the optimum compound(s) for testing in the clinic.DPreceptor antagonists(e.g.,BW-A868C,S-5751,ONO-AE3-237,and Laropiprant;Figure 5
) and EPreceptor antagonists (e.g.,PF-04418948,TG4-155,TG6-10-1,TG6-129,and AH-6809;Ganesh,2014;Figure 6
) are recommended to be screened for their efficacy against oxidative stress,amyloid-β-induced toxicity,etc using cortical/hippocampal neurons and astrocytes obtained from mice with experimentallyinduced AD.Efficacious DPand EPreceptor antagonists could then be tested alone or in combination.Additional combination therapy could exploit suitable COX-1 and COX-2 inhibitors since they improved spatial learning and memory in triple transgenic mice by lowering amyloid accumulation and tau phosphorylation (Cakala et al.,2007;Choi et al.,2013).Should the afore-mentioned non-clinical strategy be successful,the stage would be set for evaluating and translating these findings in the clinical setting with AD patients.However,as COX-2 is induced after local inflammation,it may be better to stratify the patient population so that AD pathology and thus treatment could be staged to account for different development and severity of the disease.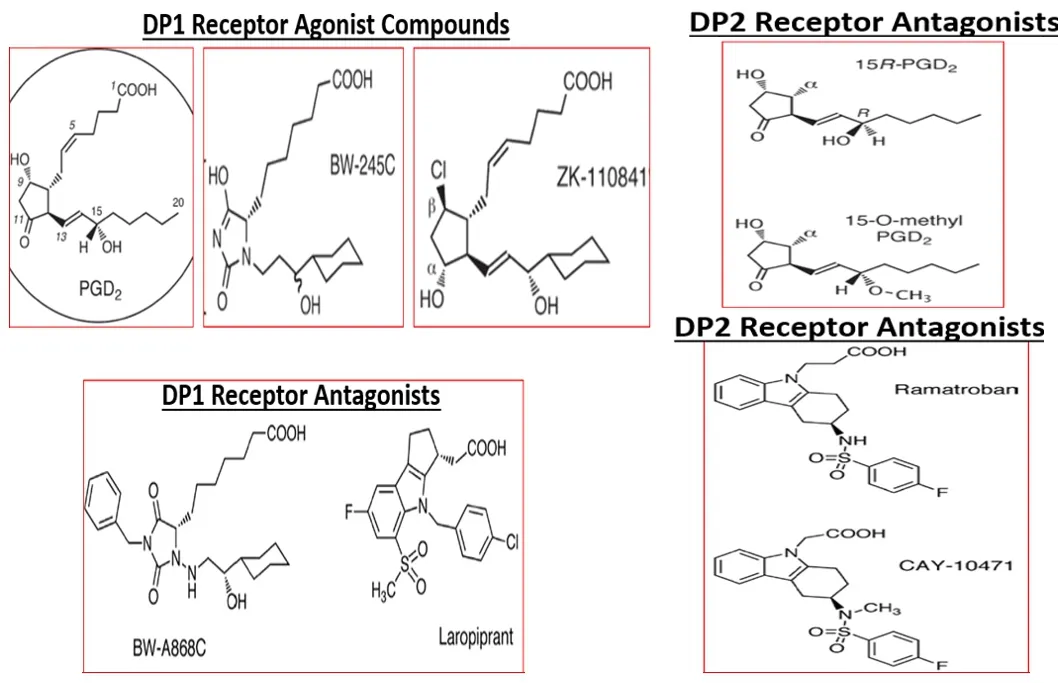
Figure 5 | Chemical structures of DP1 and DP2 receptor agonists and antagonists.
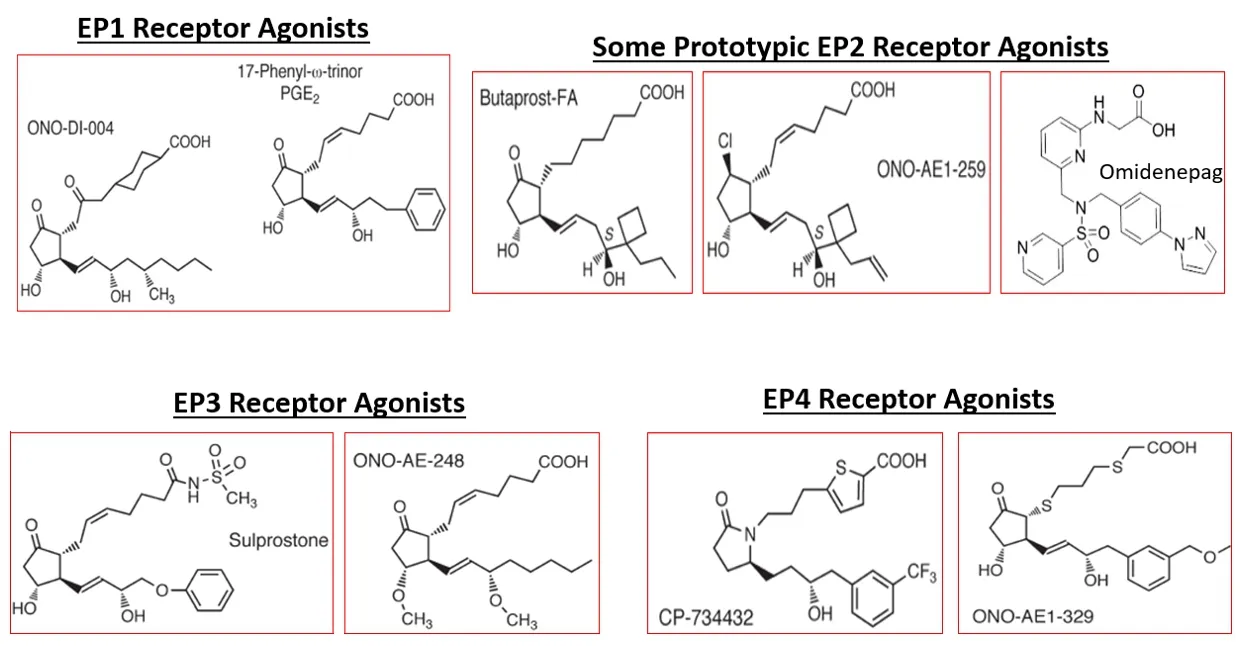
Figure 6 | Chemical structures of EP1,EP2,EP3,and EP4 receptor agonists.
There is a paucity of information about the role of IP receptors in CNS functions/dysfunctions.However,the following few examples indicate the beneficial effects of IP-receptor agonists.Thus,adenoviral induction of PGI synthase and hence stimulation of PGI2 generation in neuronal glia lowered inflammatory mediator expression stimulated by bacterial lipopolysaccharide(Tsai et al.,2005).Similarly,IP-receptor agonists protected hippocampal neurons challenged with various chemical insultsin vitro
(high O,xanthine +xanthine oxidase,or serum deprivation;Satoh et al.,1999).In conclusion,the complexity and multiplicity of factors involved in the disease causation and progression in AD,with some linkage to COX-1/2 involvement,renders future therapeutic approaches to be also combinatorial rather than a single target approach.Huntington’s Disease
As a degenerative disease that impacts neurons within the striatum/globus pallidus and motor cortex,Huntington’s disease (HD) afflict >30,000 people in the US.The debilitation associated with the HD including movement disorders and dementia appears to have some linkage to PGs and inflammation since the intake of COX inhibitors in animal models of HD helped reduce some of these symptoms (Kumar et al.,2007,2011;Kalonia et al.,2010,2011).In HD patient brains,there is also evidence of neuroinflammation including a high number of activated microglia (Sapp et al.,2001;Qian et al.,2010).A recent report,however,described a neuroprotective role of misoprostol (EP/EPreceptor agonist) in reducing memory reduction in the R6/1 mouse model of HD through the release of brain-derived neurotrophic factor (Anglada-Huguet et al.,2016).However,the role of other PGs in the etiology of HD remains unclear and is complicated by the lack of translation of animal disease model data to the human condition.Thus,further research is needed to delineate the pathophysiology of HD and to unearth the potential pathways and molecular culprits involved,including the possible role of endogenous PGs.
Parkinson’s Disease
As many as 7–10 million people suffer from Parkinson’s disease (PD)worldwide.Damage to the dopaminergic neurons in the nigrostriatal tract is responsible for the associated movement disorder (Roberts et al.,1981).While the precise etiology of PD is unclear,the potential involvement of inflammatory PGs seemed indicated since elevated COX-2 expression (Knottet al.,2000;Teismann et al.,2003) and raised PGEconcentration in CSF and substantia nigra of PD patients (Mattammal et al.,1995) was observed.Furthermore,COX-1 and COX-2 inhibitors protected substantia nigra neurons in rodent models of PD (Teismann et al.,2001;Vijitruth et al.,2006).Unfortunately,the latter findings have not fully and consistently translated to PD patients,and thus there’s controversy surrounding the therapeutic utility of COX-inhibitors in the treatment of PD (Samii et al.,2009;Driver et al.,2011).While an EPreceptor agonist,butaprost (Figure 7
),protected isolated dopaminergic neurons in culture (Carrasco et al.,2008),whether such efficacy can be realized in PD patients remains to be determined.The recent availability of a novel COX/5-LOX inhibitor,licofelone (Kumar et al.,2020;Razavi et al.,2021) may help in this regard.A recent study involving 6-hydroxydopamine induced neurotoxicity in a neuroblastoma cell-line (SH-SY5Y) via oxidative stress demonstrated protective effects of FP-receptor agonists,fluprostenol,and PGF(Figure 8
;Sano et al.,2021).These compounds decreased the intracellular reactive O-species levels and promoted activation of the endogenous anti-oxidant gene expression systems,activities that were blocked by the FP-receptor antagonist,AL-8810 (Figure 8
).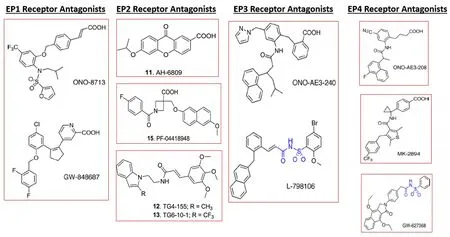
Figure 7 | Chemical structures of EP1,EP2,EP3,and EP4 receptor antagonists.

Figure 8 | Chemical structures of FP-receptor agonists and antagonists.
Amyotrophic Lateral Sclerosis
Death of neurons and their axons located in the spinal cord,brainstem motor nuclei,and motor cortex are hallmark characteristics of amyotrophic lateral sclerosis (ALS).This disease afflicts >20,000 people in the US at any one time,with as many as 6000 newly diagnosed patients each year.While the sporadic form accounts for >90% of the total ALS cases with unknown etiology,the inherited form is associated with mutations of the superoxide dismutase-1(Julien,2001).Elevated levels of COX-2 (Almer et al.,2001) and PLAactivity(Shibata et al.,2010) were reported in postmortem spinal samples ALS patients,in both sporadic and familial forms of ALS.Consequently,increased levels of PGEwere observed in the CSF of ALS patients (Almer et al.,2002),and EPreceptors participate in accelerating the progression of the disease in an animal model of ALS (Liang et al.,2008).Therefore,antagonism of the EPreceptor may prove beneficial in slowing the progression of ALS.However,an earlier report had described the beneficial effects of PGE(Bilak et al.,2004),but the receptor subtype(s) involved was not delineated.With the relatively recent advent and availability of selective EP-receptor antagonists (Woodward et al.,2011;Ganesh,2014;Figure 6
),the detrimental or beneficial role(s) of PGs in combating ALS could be ascertained shortly,at least in animal models of ALS.Multiple Sclerosis
The global prevalence of multiple sclerosis (MS) is estimated to be around 2.5 million people of which nearly 400,000 cases exist in the US.MS is an autoimmune disease characterized by the loss of myelin around the axons of neurons that form the nervous system (Pachner et al.,2011).Since nerves control every organ,MS adversely impacts all bodily functions.Unfortunately,however,it has been very difficult to study MSin vitro
andin vivo
to delineate the precise etiology of MS.Some progress has been made using a rodent model of MS involving experimental autoimmune encephalomyelitis in which PGs are major culprits (Kalyvas and David,2004;Kalyvas et al.,2009;Ayoub et al.,2011).The latter is supported by observations of concomitant elevation of COX-1,COX-2,and PLA2 activity,and levels of PGEand PGFin the spinal cord and cerebellum of experimental autoimmune encephalomyelitis-affected rodents (Bolton et al.,1984;Pollak et al.,2003;Ayoub et al.,2011).Furthermore,inhibitors of COX-1/2 and PLA2 reduced the symptoms and behavioral effects associated with experimental autoimmune encephalomyelitis (Reder et al.,1994;Marusic et al.,2008).One additional promising therapeutic intervention pertains to the use of an FP-receptor antagonist (AL-8810;Griffin et al.,1999;Sharif et al.,2000;Sharif and Klimko,2019;Figure 8
) in reducing myelin loss and movement disorder in a mouse model of MS using copper-based toxin (cuprizone;Iwasa et al.,2014).Such data offer some hope for future symptoms-relief in MS-related motor dysfunctions.Since a more potent FP-receptor antagonist than AL-8810 has recently been discovered,BAY-6672 (Figure 8
;Beck et al.,2020) may be worth evaluating in animal models of MS and perhaps tested for clinical efficacy and utility.Stroke,Traumatic Brain Injury,and Epilepsy
Serious debilitating disorders of the brain such as stroke (the single largest cause of adult disability in the developed world accounting for 800,000/year cases in the US),epilepsy,and traumatic/mechanical injury to the head are usually caused by vascular abnormalities or damage to cerebral blood vessels and thus the accompanying ischemia/hypoxia.The resulting reduction in blood flow deprives the neurons of precious energy and oxygen and causes profound oxidative stress (Figure 9
).Permanent middle cerebral artery occlusion of rodents has been used as a reliable and predictive model of stroke.Using such a mouse model of stroke,prior intravenous treatment with an FP-receptor (FP-R) antagonist (AL-8810) markedly decreased the cortical infarct volume and provided a faster commensurate motor recovery in the mice (Kim et al.,2012).Such structural and functional outcomes were confirmed in other mice whose FP-Rs had been genetically deleted (Kim et al.,2012),thereby confirming the involvement of endogenous PGFin mediating the stroke symptomatology and the ischemic events.In addition,in oxygenglucose-deprived mouse hippocampal slices and cultured hippocampal neurons,the FP-R-antagonist also afforded protection by curbing the generation of highly reactive oxygen species and thus reducing oxidative damage (Kim et al.,2012).These studies,therefore,provided strong evidence that AL-8810 and perhaps other FP-R-antagonists,such as BAY-6672 (Beck et al.,2020) may be useful therapeutic agents in the treatment of stroke and other ischemic diseases (Figure 8
).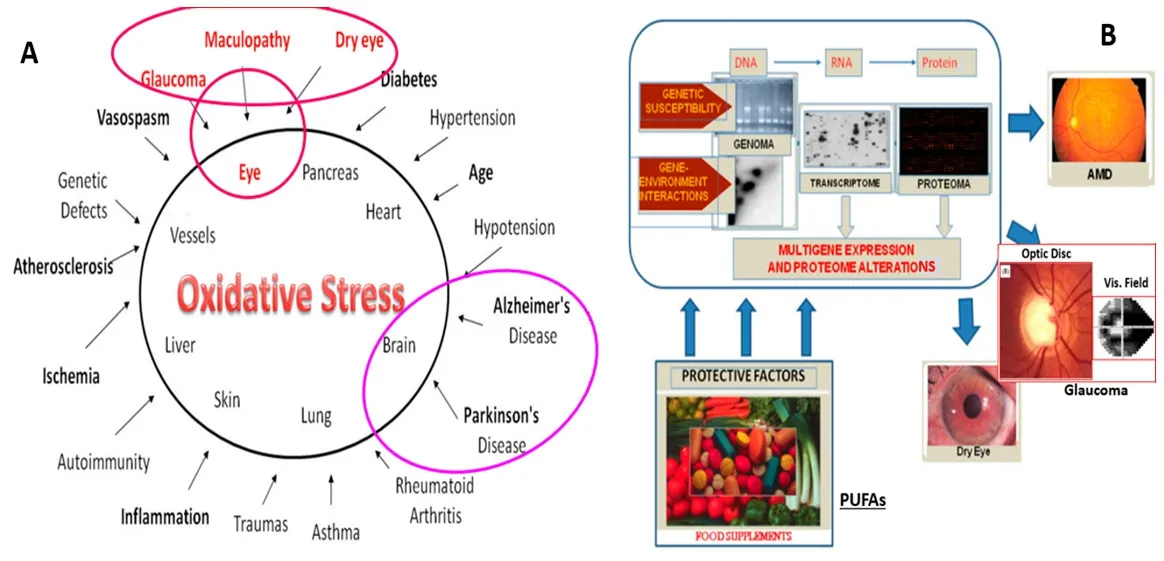
Figure 9 | Impact of oxidative stress related to disease causation,and the role of epigenetics and PUFAs in health and disease.
The potential use of EPreceptor antagonists may also be beneficial in the management of stroke since SC51089 and SC51322 prevented the damage to rat hippocampal slices under O-glucose-deprivation conditions (Zhou et al.,2008).However,a report by McCulloch et al.(2002) using isolated hippocampal neurons and slices placed under NMDA-induced cytotoxic and hypoxic conditions indicated that activation of EP2 receptors may be neuroprotective in cerebral ischemic challenges.Likewise,15-deoxy-(16-m-tolyl)-17,18,19,20-tetranoriso-carbacyclin methyl ester,a selective CNStype IP receptor agonist,abrogated brain damage in an animal model of stroke (Takamatsu et al.,2002).Since a milieu of prostanoids appears to be implicated in the pathophysiology of stroke,however,a cocktail of COX-and lipoxygenase inhibitors may be beneficial in tackling signs and symptoms of stroke and related cerebral aneurysms (Singh et al.,2014).Much more research is needed using modern techniques and novel PG agonists and antagonists to tease out the exact involvement of PG receptors in causing or preventing damage in the hippocampal and cerebral cortical tissues during and after stroke or stroke-like trauma of the brain.
In an allied field to stroke,traumatic brain injury (TBI) is common and is often linked to brain damage and death in the elderly.An initial contusion followed by hematoma,subarachnoid hemorrhage,and diffuse injury,and death of the axons of brain neurons is hallmark features of TBI.Neuronal inflammation combined with oxidative stress and Ca-overloading of cerebral neurons represents the second phase of TBI and the accompanying loss of various bodily functions including speech and neuromuscular activity.Several researchers have reported elevated generation and release of proinflammatory PGs,including PGF,during and after TBI as a result of increased availability of arachidonic acid and enhanced COX-2 activity (Glushakov et al.,2013a,b).Interestingly,increased COX-2 levels have been observed in the ischemic neonatal and adult human brain,and in an experimental mouse model of TBI,Glushakov et al.(2013a,b) also showed that intraperitoneal injection of an FP-R antagonist (AL-8810) decreased hippocampal swelling and improved the neurological deficit scores 1-and 2-days post-TBI insult.Therefore,it appears that FP-R antagonists could be utilized to reduce the damage and help preserve the functional integrity of the cerebral cortex and hippocampus after and during the TBI events.Recently,an EP-receptor agonist (AH6809) lowered the production of inflammatory mediators in the hippocampus after cerebral concussion in rats (Li et al.,2018),indicating that endogenously released PGEmay have protective effects in TBI,a finding supported byin vitro
studies in hippocampal slices (Yousif et al.,2018).The potential utility of other PG agonists to chronic brain injury (e.g.,caused by aluminum) is exemplified by the neuroprotective effects of an IP-receptor agonist,beraprost (Pan et al.,2015).Disturbance of brain electrophysiology via loss of ionic homeostasis leads to a phenomenon known as cortical spreading depolarization (or depression)(CSD) (Kramer et al.,2016;Cozzolino et al.,2018).CSD often leads to vascular defects such as vasospasm and vasoconstriction resulting in reduced cerebral blood flow and volume (oligemia) and is implicated in several neurological disorders including migraines,epilepsy,intracranial hemorrhage,and TBI.These conditions result in significant disability and morbidity around the world.The involvement of endogenous PGs during CSD/oligemia development was demonstrated in rats by Gariepy et al.(2017).The initial phase of oligemia/CSD involved activation of COX-1 and production of TXA,while the second phase was mediated by PGFderived from stimulation of COX-2 activity.Consistent with this was the finding that AL-8810 prevented only the second phase of oligemia and consequently increased cerebral blood flow/volume (Gariepy et al.,2017).Taken together,such studies provide support for the potential clinical utility of TXA2-R and FP-R antagonists (Gariepy et al.,2017;Sharif and Klimko,2019;Beck et al.,2020) and also EPreceptor antagonists (e.g.,L- 161982;Varga et al.,2016) to treat CSD and overcome the pathologically reduced brain blood flow/volume.
Epileptic seizures have numerous instigators,and several medicinal products are available to treat this disorder (Chung et al.,2011).However,there’s always a need for new drugs to treat recalcitrant patients or those where current therapies are insufficient or are contraindicated.Relative to the potential roles of endogenous and exogenous PGs in either starting or curtailing seizure activity,kainic acid-induced seizure models using rodents have been utilized.In such studies,Kim et al.(2008) showed that COX-1 and COX-2 inhibitors,or FP-R antagonist AL-8810 injected intracisternally before kainic acid-induced seizures potentiated the seizure activity.As a corollary,ic-injection of PGF,but not PGDor PGE,significantly reduced kainic acidinduced seizures (Kim et al.,2008),thereby suggesting the involvement of FP-Rs in the beneficial effects of endogenously released PGFin maintaining cerebral/hippocampal electrical activity homeostasis.However,additional studies are needed to corroborate these observations and to elaborate on the complex roles of PGs and inflammatory mediators in modulating seizure activity (Shimada et al.,2014;Santos et al.,2017).Also,since numerous FPreceptor-selective agonists are now available (e.g.,latanoprost,travoprost,and tafluprost) the role of FP-receptors in controlling seizure activity is worthy of pursuit.With continuing research,the role of EP2 receptors mediating epileptic conditions has been recently verified where high-affinity brainpermeable EP2-receptor-selective antagonists (TG4-155,TG6-10-1,TG8-260) ameliorated seizure-induced damage in various rodent models of status epilepticus (Nagib et al.,2020;Rojas et al.,2021).Likewise,since a new generation of COX/5-LOX inhibitors like licofelone (Payandemehr et al.,2015;Razavi et al.,2021) exhibited anti-convulsant characteristics in mice,this class of enzyme inhibitors for future treatment of epilepsy may be warranted,especially as adjunctive therapies.
Prostaglandins in Mediating Pain
PGs have been associated with mediating inflammation and pain for several decades leading to the discovery of aspirin as a universally acceptable COX-1-inhibitor (Vane,1971).However,due to different types of peripheral and deep pain categories and various noxious stimuli that can trigger pain,COXinhibitors alone are not particularly effective analgesics due to their relatively short duration of action and stomach-irritation causing effects although their antipyretic and pain-reducing properties are still much appreciated.To delineate the various roles of DP-,EP-,FP-,IP-and TXA-receptors in stimulating nociceptor responses due to heat,mechanical and formalininduced pain,mice lacking these receptors due to genetic deletions were utilized.Popp et al.(2009) demonstrated that COX-1 and EP-receptor knockout mice exhibited reduced heat-evoked pain,while COX-2 and EP-receptor knockout mice had reduced licking response to formalin injections.Interestingly,heat-induced pain sensitivity was enhanced in FP-,EP-and EP-1+3 receptor-deficient mice,whilst DPand EP-knockout mice displayed greater responses in the formalin test (Popp et al.,2009).IP-and TXA-deficient mice exhibited normal behavior in all the afore-mentioned paininducing challenges.These data strongly suggested that pain processing signaling due to various stimuli is differentially propagated and handled at the level of the spinal cord and the brain,thereby reinforcing the notion that a single PG receptor subtype-directed drug is unlikely to provide a high magnitude and long duration of analgesic efficacy.Even though the usefulness of such gene-knockout strategies is limited by potential compensation by the remaining PG receptors in these animals,other studies have shown that PGEvia EP-receptors and prostacyclin via IP-receptors sensitize TRPV1 capsaicin-channels (Moriyama et al.,2005),DP(Wright et al.,1999) and EP-receptors mediate spinal inflammatory hyperalgesia(Reinhold et al.,2005),and EP-receptors and kappa-opioid receptors cooperate in transducing tactile pain (Minami et al.,2003).Moreover,Kunori et al.(2009) reported that mechanical allodynia induced by intrathecal administration of PGFand ATP was propagated via capsaicin-insensitive primary afferent pathway within the spinal cord,and supportive evidence was gathered using FP-R knock-out mice in which neither αβ-methylene ATP nor PGFelicited allodynia.However,since an FP-R antagonist,AL-8810,curtailed the allodynic pain induced by intrathecal αβ-methylene ATP in wild-type mice,it was concluded that activation of the PXreceptors to cause the pain-response was ultimately being mediated via FP-Rs that are co-located with PXreceptors in the spinal cord (Kunori et al.,2009).Gatta et al.(2012) then reported that direct spinal administration of PGFinto healthy mice potently excited nociceptive neurons and this activity was also abolished by AL-8810.Collectively,there is the active participation of various PG-receptors in mediating different forms of algesic signals and thus future combination medicinal products can be envisaged being formulated in different permutations encompassing COX-1/2 inhibitors+FP-receptor antagonists (AL-8810;BAY-6672;Figure 8),or COX-1/2 inhibitors (Figure 4)+EP-and EP-(Figure 6) and FP-receptor antagonists (Figure 8),depending on the need of the patient.Such formulations would provide alternative therapies for combating pain without resorting to the use of opioid drugs and dealing with all the undesirable side-effects of the narcotics.
Schizophrenia and Refractory Depression
Only a few studies have been conducted to determine the potential role(s) of PGs in schizophrenia and refractory depression and have been summarized(Yui et al.,2015).Since elevated levels of PGEand cytokines have been reported in subsets of patients with both these psychiatric disorders,COX-2 inhibitors were tested for potential benefits.Unfortunately,mixed results were observed thereby warranting further investigations of the causal relationship between prostanoids and such diseases.
Pathological/Beneficial Role of Prostaglandins in Ocular Functions and Disorders
Based on early research in the ocular field,endogenous and exogenous PGs demonstrated inflammatory effects causing edema and pain,and thus earned a bad reputation (Vane 1971;Bhattercherjee and Coles,1977).However,in the late 1980s-early 2000s,it was discovered that certain esterified PGs have beneficial effects in the eye in certain cases when dosed topical ocularly (t.o.).One particularly useful effect of FP-receptor agonists pertained to eliminating aberrantly accumulated aqueous humor (AQH) fluid in the anterior chamber(ANC) of the eye to reduce elevated intraocular pressure (IOP),this being a major risk factor for the development of glaucoma,the second leading cause of blindness (Stjernschantz and Bito,1989;Sharif et al.,1999;Hellberg et al.,2002;Klimko and Sharif,2019).However,the widespread distribution of various PG receptor genes and receptors in the eye determined by RT-PCR(Schlotzer-Schrehardt et al.,2002;Sharif et al.,2002),in situ
hybridization and immunoblotting (Ocklind et al.,1997),immunohistochemistry (Zhao et al.,1995;Biswas et al.,2004;Nesher et al.,2015),receptor binding studies conducted on homogenized ocular tissues/cells (Csukas et al.,1993),and by qualitative and quantitative autoradiography (Matsuo and Cynader,1992;Sharif et al.,1999,2004;Davis and Sharif,1999;Figures 10
and11
) and using functional assays (Figures 12
and13
) suggested a variety of physiological and/or pathophysiological actions of PGs in the eye.Some aspects of such will now be discussed below.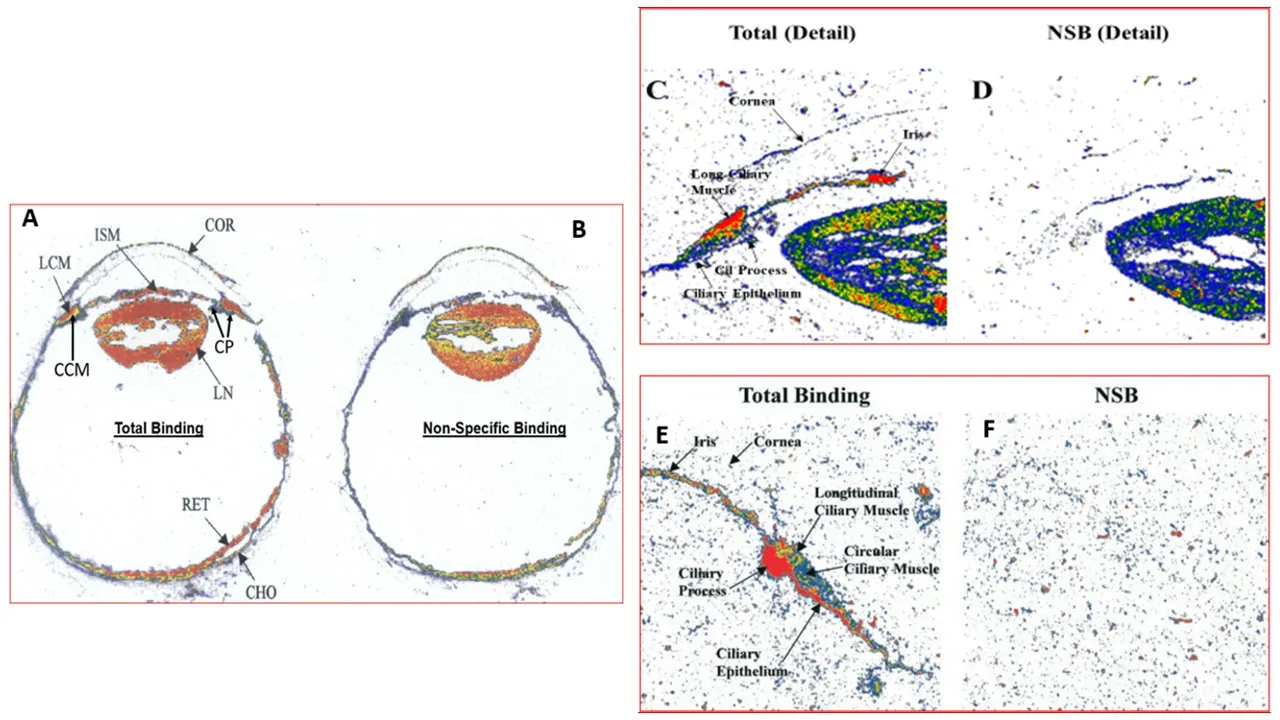
Figure 10 | Autoradiographic localization and visualization of various PG receptors in human ocular tissues using various radioligands.
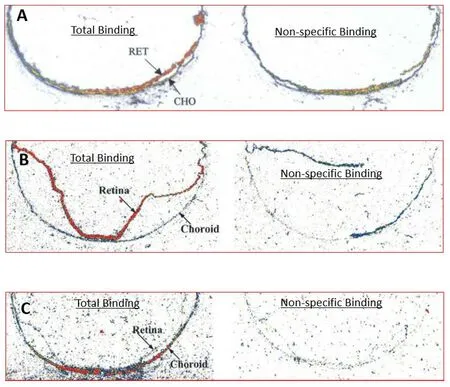
Figure 11 | Autoradiographic localization of PG receptors in the retina and/or choroid in posterior segment sections of postmortem human eyes.
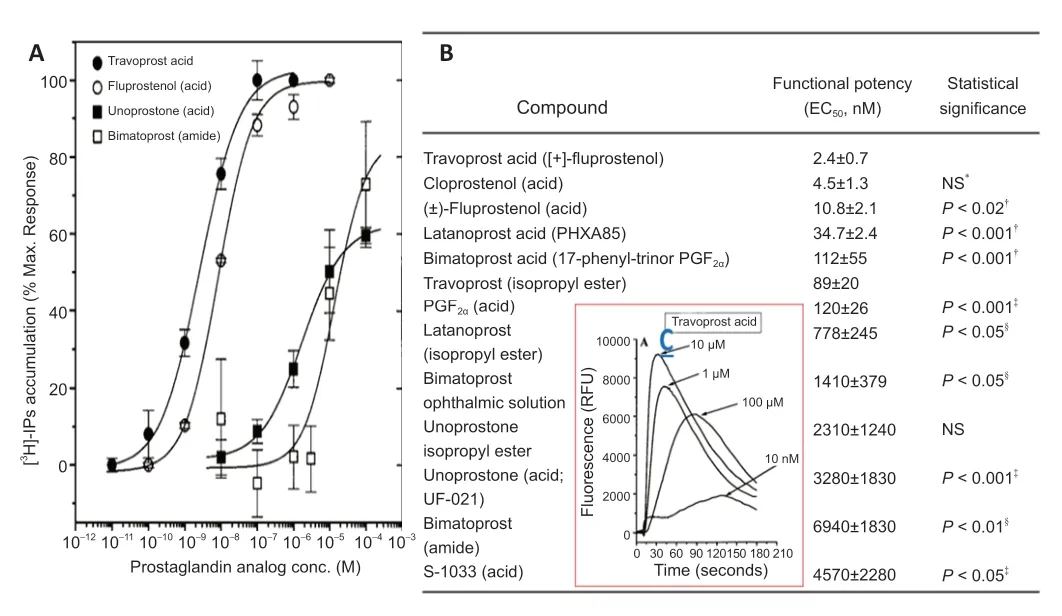
Figure 12 | Functional responses to various FP-receptor-agonist PG free acids and an amide and their relative potencies in isolated human trabecular meshwork (h-TM)cells.
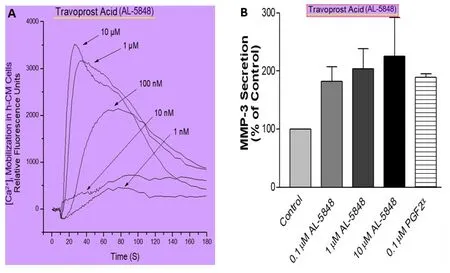
Figure 13 | The ability of travoprost free acid to activate functional FP-receptors on human ciliary muscle (h-CM) cells.
Ocular Allergies
It is well known that the major mast cell mediator culprit responsible for the intense itching and ocular surface (conjunctiva and cornea) redness is histamine,and H-antagonists with or without mast cell stabilizing properties treat such conditions (see for review Sharif,2020).However,as mast cells also release additional chemicals including PGDand PGEdepending on the instigating allergen,it was thought that these PGs may be also involved in causing the allergic conjunctivitis (AC).PGD,by activating DP-receptors,triggers chemotaxis of eosinophils and ocular surface inflammation in AC(Fujishima et al.,2005).However,Ueta et al.(2009,2011) have shown that activation of EP3-receptor located on conjunctival epithelial cells may be able to blunt the progression of experimental AC.In another study,Guenoun et al.(2005) demonstrated cytoprotective and antioxidative effects of latanoprost and travoprost against the toxicity induced by benzalkonium chloride in isolated human conjunctival epithelial cells.
Dry Eye Disease
Since the ocular surface is exposed to the environment,it is bombarded with pathogens,allergens,and sunlight.The electromagnetic waves associated with sunlight damage corneal,lens trabecular meshwork,and retinal cells through the generation of reactive oxygen species and thus through oxidative stress.The dryness of the ocular surface is characterized by the feeling of itchiness,foreign-body sensation,and mild to moderate pain (Pflugfelder and de Paiva,2017;Clayton,2018).The etiology of dry eye disease (DED) and the related Sjogren’s syndrome is still poorly understood but can be caused by a decrease in circulating female hormones,pollution,reduced blinking,and diseases of the meibomian and lacrimal glands (Clayton,2018).The latter results in reduced production and secretion of natural tears and associated lipids and proteins such as mucins.The only current treatments for DED encompass t.o.dosing with artificial tears,tear secretagogues like diquafosol (a purine receptor agonist),cyclosporin,and an integrin antagonist (Lollett and Galor,2018).Even though DED is considered an inflammation-based disorder,there is no apparent compelling data that supports the involvement of PGs.However,evidence has accumulated that supports the potential beneficial role of dietary PUFAs (Figure 2) in reducing some symptoms of DED via limiting oxidative stress (Zhu et al.,2014).
Ocular Inflammation/Pain
Release of endogenous PGs,mainly PGEand perhaps PGF,during eye trauma,inflammation of the anterior and posterior chambers of the eye(uveitis) (Battacherjee and Coles,1977),and during surgical procedures (e.g.,cataract removal;corneal transplants;LASIK procedures) is well documented(Jacobs,2017).Consequently,topical ocular COX-inhibitors and steroids are regularly utilized to curb the production of these PGs and thus reduce ocular inflammation,edema,and pain (Chen et al.,1997;Jacobs,2017).As COXinhibitors such as indomethacin,diclofenac,and flurbiprofen are effective therapeutics,there has not been much research performed in determining the potential utility of PG-receptor antagonists in combating ocular pain and inflammation.However,since many EP-receptor-sub-type selective antagonists are now available,these should be evaluated for possible ocular nociceptive efficacy (Biswas et al.,2007;Ganesh,2014;Rojas et al.,2021).
Optic Neuritis
Spontaneous inflammation,edema,and pain associated with optic nerve components can be initiated by many factors including endogenously released PGs such as PGE.This normally resolves by itself over 10–14-days but in severe cases,corticosteroids and COX-inhibitors are prescribed for oral and t.o.administration to treat this condition to reduce the potential severity and duration of the ailment.Additional research is needed to determine whether dietary supplementation of PUFAs and various PG receptor antagonists can impart benefits to patients with optic neuritis.
Myopia
The most prevalent and burdensome refractive eye disorder is myopia (nearsightedness).This ocular defect affects billions of people worldwide and starts in children of school-age (Resnikoff et al.,2019).Family history and environmental factors such as reduced time spent outdoors and close-eye work such as reading increases the risk and severity of myopia.Essentially the eye globe is enlarged and elongated such that the light reaching the back of the eye is focused in front of the retina,thereby making the distant images appear blurry while the nearby objects are well focused. Unfortunately,myopic patients are prone to headaches/eye strain and can be victims of retinal detachment,cataracts and closed-angle glaucoma,and potentially pigmentary glaucoma (Resnikoff et al.,2019).The underlying pathology causing the increased axial length of the eyeball is unknown but the signs and symptoms of myopia can be overcome by increasing exposure to sunlight,corrective spectacles,and contact lenses,and via topical eyedrop therapies that contain the muscarinic receptor antagonist atropine which has some mydriatic and other side-effects.
In search for better and safer anti-myopia drugs,two reports described the beneficial effects of FP-receptor agonist analogs latanoprost,dosed topical ocularly (El-Nimri and Wildsoet,2018),and PGFdosed peribulbarly (Yang et al.,2018).Since this improvement in axial length and other features of formdeprivation myopia in the guinea pig model induced by FP receptor agonists was blocked by prior treatment with AL-8810 (Griffin et al.,1999;Sharif and Klimko,2019),Yang et al.(2018) concluded that the beneficial effect was mediated by FP-receptors.An earlier study using form deprivation in chicks reported that no such therapeutic effects of free acids of PGF,PGE,and latanoprost dosed topically or subconjunctivally (Jin and Stjernschantz,2000).However,when PGFwas injected intravitreally,a significant attenuation of myopia development was observed.These collective data indicate that FPreceptor activation in the retina/choroid/sclera is responsible for proper maintenance of the axial length of the eyeball to provide good quality eyesight.Furthermore,these results suggest that in addition to the currently accepted treatment regimen of t.o.muscarinic antagonist (e.g.,atropine) to combat myopia (Tsai et al.,2021),use of FP-receptor PG agonists alone or in combination with atropine should also be considered.The additional benefits of using FP-agonists for treating and slowing the progression of pathological myopia would be the concomitant lowering of intraocular pressure that would reduce the potential for retinal detachment (see ahead).
Wet Age-Related Macular Degeneration and Diabetic Retinopathy
These ocular diseases primarily impact the back of the eye and in particular the retina and the associated blood vessels.While there may be a small component of the edema and neovascularization caused by locally-released PGs,the major culprit here is vascular endothelial growth factor (VEGF).Accordingly,intravitreally delivered anti-VEGF biologics are the current standard of care for these diseases.However,aberrant neovascularization appears to involve various PGs.For instance,PGFis increased in microvascular and retinal Muller glial cells under ischemic/hypoxic conditions as encountered in oxygen-induced retinopathy of prematurity (Barnett et al.,2010;Hu et al.,2017).Additionally,Savage et al.(2011) showed that latanoprost,a selective FP-R agonist,caused a significant increase in VEGF from isolated Muller cells and increased proliferation of human retinal microvascular endothelial cells,and these actions were blocked by AL-8810,an FP-R antagonist (Griffin et al.,1999;Sharif and Klimko,2019;Figure 8
).These reports suggested that FP-R antagonists may have potential clinical utility as anti-angiogenic drugs but such findings need to be confirmed and extended.Of course,combination therapy or multi-pharmacophoric drugs exhibiting simultaneous protective effects via inhibiting multiple PG receptors are also valid approaches to combating ocular diseases such as pathological neovascularization and uveitis.This may be even more important for patients who become recalcitrants to the standard of care anti-VEGF treatments.Some recent studies support the role of PUFAs as protective agents against choroidal neovascularization (CNV) in models of wet AMD (Gong et al.,2016).These authors demonstrated that inhibition of cytochrome 450 2C (CYP2C)activity by montelukast in a mouse model O-induced retinopathy and laserinduced CNV added to the cytoprotective effects of ω-3 long-chain PUFAs on retinal neovascularization and CNV by 30% and 20%,respectively.Additionally,montelukast reduced retinal neovascularization and CNV by 36% and 39% and reduced the plasma levels of CYP2C8 products in CYP2C8-overexpressing mice fed an ω-3 long-chain PUFA rich diet (Gong et al.,2016).Similarly,diets rich in omega-3 PUFAs reduced retinal lesions (Tuo et al.,2009) and protected the retina from age-associated degeneration in aged c57bl/6j mice (Prokopiou et al.,2019;Huang et al.,2020).
Globally,diabetic retinopathy (DR) affects millions of patients who can lose their sight rapidly if DR is not diagnosed quickly and treatment initiated.Retinal microvascular defects induced by diabetes in DR appear to greatly impact the pericytes on the microcapillaries.Early studies on human vitreous revealed significantly lower levels of prostacyclin,PGE,and PGFin DR and proliferative-DR patients than in the control eyes (Naveh et al.,1990;Douros et al.,2001).Additionally,these findings have some clinical relevance since PGFwas significantly decreased in the plasma of human subjects with nonproliferative diabetic retinopathy NPDR group compared to controls (Peng et al.,2018).Whether these deficiencies are biologically relevant and important was investigated by other researchers.Indeed,pericyte apoptosis induced by high glucose in diabetic mice could be reducedin vitro
by the inclusion of PGFthrough inhibition of the phosphoinositide-3-kinase/beta-catenin pathway (Cheng et al.,2021).This beneficial effect was FP-receptor-mediated since AL-8810 blocked the aforementioned effects.In vitro
,PGFaccelerated adhesion and migration of bovine retinal pericytes by increasing Rho kinase activity,and administration of a PGFanalogue reduced the damage on retinal capillaries in a diabetic mouse model (Peng et al.,2018).Collectively,these findings indicate that endogenous prostanoids play an important role in maintaining the health of the retinal microcapillaries,in particular the pericytes and that FP-receptor activation mediates these protective effects.Importantly,omega-3 PUFAs preserved retinal function in type-2 diabetic mice (Sapieha et al.,2019).Ocular Hypertension and Glaucoma
The IOP within the ANC of the eye is tightly regulated since it is responsible for maintaining the shape of the eye-ball and for providing a nourishing environment for the tissues lining the ANC.Since the ANC is avascular,the AQH fluid in the ANC provides nutrients and oxygen to the lens,corneal endothelial,and other important cells lining the trabecular meshwork (TM)and Schlemm’s canal (SC) drainage system (Figure 14A
).Additionally,due to its normal clarity,AQH provides an unhindered penetration of light to the retina for optimal sight.Non-pigmented ciliary epithelial cells produce the AQH at the same rate as it exits the ANC through the TM/SC system under normal circumstances.One major risk factor associated with primary open-angle glaucoma (POAG),the second leading cause of blindness worldwide (Weinreb et al.,2016a,b),is elevated IOP or ocular hypertension (OHT).The latter is caused by the accumulation of excess AQH in the ANC due to clogging of the TM/SC AQH drainage system.As a result of the aging process and oxidative stress induced by the diminished autophagy,many of the TM cells die,the IOP keeps rising,and deleteriously affects the retinal ganglion cells (RGCs) and their axons at the back of the eye.Since this damage remains undetected over many decades,the patient continues to lose vision due to reduced connections of the RGC axons in the optic nerve to the brain.Further eyesight deterioration is prevented when the patient receives topical medications to reduce the IOP.Consequently,much research has been conducted and suitable drugs discovered and introduced into the clinical management of elevated IOP(Weinreb et al.,2016a,b;Klimko and Sharif,2019).Specifically,topically applied FP-R PG agonist analogs (e.g.,latanoprost,travoprost,bimatoprost,and tafluprost) effectively reduce IOP by promoting drainage of the AQH through the newly created and/or expanded spaces between ciliary muscle bundles and the sclera (uveoscleral pathway) and also through the TM/SC pathways (Figure 14A
).The mechanism of action of these FP-R agonists involved the release of endogenous matrix metalloproteinases that digest the excess extracellular matrix (ECM) ECM that blocks the TM/SC drainage system.While FP-R agonist PG analog drugs are now first-line treatment options for treating OHT (Weinreb et al.,2016a,b),other classes of PGs have also shown efficacy in animal models of OHT/POAG (e.g.,DP,EP,EP,FP/EP,and TP receptor agonists),and even in OHT/POAG patients (DP-R agonist [AL-6598;Hellberg et al.,2002;Sharif et al.,2004],EP-R agonists [DE-177,omidenepag isopropyl (Kirihara et al.,2018a,b)],and FP/EPreceptor agonist (ONO-9054 or sepetaprost;Suto et al.,2015).A conjugated PG analog (latanoprostene bunod;Cavet and DeCory,2018;Weinreb et al.,2016b) was recently approved for the clinical utility to treat the latter ocular disease.This drug lowers IOP by releasing nitric oxide in the ANC to relax TM/SC cells and by co-releasing latanoprost that stimulates egress of AQH through the uveoscleral pathway and conventional TM pathway(Weinreb et al.,2016b;Cavet and DeCory,2018).
As noted above,being avascular,the cells and tissues in the ANC of the eye are dependent on the AQH to supply them with glucose,O,antimicrobial proteins,amino acids required for protein synthesis,anti-oxidants like ascorbic acid and glutathione,and a whole range of vital minerals and trace elements for efficient intracellular,membrane-bound and mitochondrial enzyme activities.When the AQH drainage is restricted the ambient AQH is stagnant and the nourishment to the ANC tissues is severely disrupted.Toxic metabolites (e.g.,urea,lactate,and reactive Ospecies),lipids (e.g.,sphingomyelins and ceramides),large proteins (fibronectin,collagen,tissue growth factors),and cell debris continue to accumulate thereby stressing the surrounding tissues within the ANC.It has been documented that indeed in glaucoma patients the composition of the AQH changes to create such an unhealthy environment within the ANC that reduces the ability of TM cells,for instance,to release matrix metalloproteinases and to phagocytose the offending materials in the AQH (Ashworth-Briggs et al.,2015;Cabrerizo et al.,2017;Adav et al.,2019;Hubens et al.,2020).Thus,FP-R agonist analogs not only help lower and control IOP over 24-hours by removing excess AQH from the ANC of the eye,but their particular mechanism of action also helps replenish the fluid in the ANC with fresh AQH and thus they promote better health of the cells of the lens,corneal endothelial cells,TM,and SC.This feature is important since healthy and well-nourished TM/SC cells are far better at phagocytizing debris (autophagy;Porter et al.,2015) and any foreign materials in the ANC than sick ones and thus prevention of future occlusion and damage of the TM/SC is achieved.
As mentioned above,since TM/SC/ciliary muscle cells are negatively and deleteriously affected by aberrant ECM deposits within the angular space and within the latter tissues,that rigidifies the latter cells and that eventually increases the resistance to AQH flow through and around these tissues,it is deemed important to protect TM/SC cells from the ensuing oxidative stress(Camras et al.,2012;Stamer and Acott,2012).Recent studies by Kalouche et al.(2016) revealed that EP2-receptor activation by butaprost of human TM cells in culture protected them from endoplasmic reticulum stress and associated apoptotic cell death.A subpopulation of myofibroblastic cells in TM impart an important contractile property that is important for cell volume changes (Dismuke et al.,2009,2010),the release of local matrix metalloproteinases (Yang et al.,2016),and for positively impacting closely associated SC cells for enhancing conventional outflow of AQH to reduce IOP.In this regard,Kalouche et al.(2016) demonstrated that latanoprost free acid significantly reduced collagen deposition and contracted TM cells,both processes being antagonized by AL-8810,an FP-receptor antagonist (Sharif and Klimko,2019).In contrast,the EP-R agonist,butaprost,relaxed TM cells and reduced collagen deposition induced by transforming growth factor-β2,a damaging cytokine.These beneficial effects of latanoprost free acid on human TM cells appeared to involve calcipressin,a regulator of calcineurin that requires an elevation of intracellular and extracellular Ca,an action that was also strongly blocked by AL-8810 (Fautsch et al.,2011). In additional studies,PGFstrongly induced Nur77 expression,an orphan nuclear receptor involved in cell growth,in the cultured human ciliary muscle and human TM cells (Liang et al.,2004).The PGF-induced up-regulation of Nur77 in these cells was effectively inhibited by AL-8810 (Liang et al.,2004) indicating that FP-R analog agonists not only promote AQH efflux by local release of ECMdigesting enzymes but they can also promote good health of TM/ciliary muscle and most likely SC cells by stimulating the local release of beneficial growth factors.
Another factor implicated in the etiology of GON/ POAG is hypoxia and resultant oxidative stress (Lei et al.,2013;Zode et al.,2015).These conditions lead to a decline of ATP energy source in the TM and SC cells in the ANC of the eye,and also of RGCs in the retina (Thomas et al.,2000) that deleteriously reduces the normal functionality of these cell types (Weinreb et al.,2014).During ocular ischemia/hypoxia,numerous classes of cellular toxins (e.g.,reactive Omolecules;endothelin [ET],ATP,excess glutamate;cytokines and chemokines) are released into the cellular microenvironment in the ANC and within the retina/choroid (Figures 14A
andB)
.Whilst FP-R PG agonists enhance the provision of fresh AQH fluid to the ANC by inducing drainage of AQH (see above),and thus also removing locally released toxic chemicals,they also reduce the direct oxidative damage to TM/SC cells,a process that could be blocked by FP-receptor antagonists (Yu et al.,2008).Regarding the potentially damaging effects of ET at the TM-level,Thieme et al.(2006) demonstrated that PGF2α and fluprostenol blocked the ET-induced increase in [Ca]and the contraction of isolated human TM cells,with similar effects being noted for latanoprost derived from latanoprostene bunod (Cavet et al.,2015).Since these effects were reversed by prior treatment of cells with FP-R antagonist AL-8810,the protective effects of the FP-receptor activation were demonstrated.Therefore,FP-R agonist treatment of OHT in PAOG has apparent triple therapeutic effects:(1) directly enhancing AQH egress from ANC to reduce IOP;(2) by increasing flow of AQH through the ANC,the perfusing AQH removes metabolic and other toxic chemicals and free radicals;(3) reducing oxidative damage of TM/SC cells due to provision of antioxidants and other nutrients.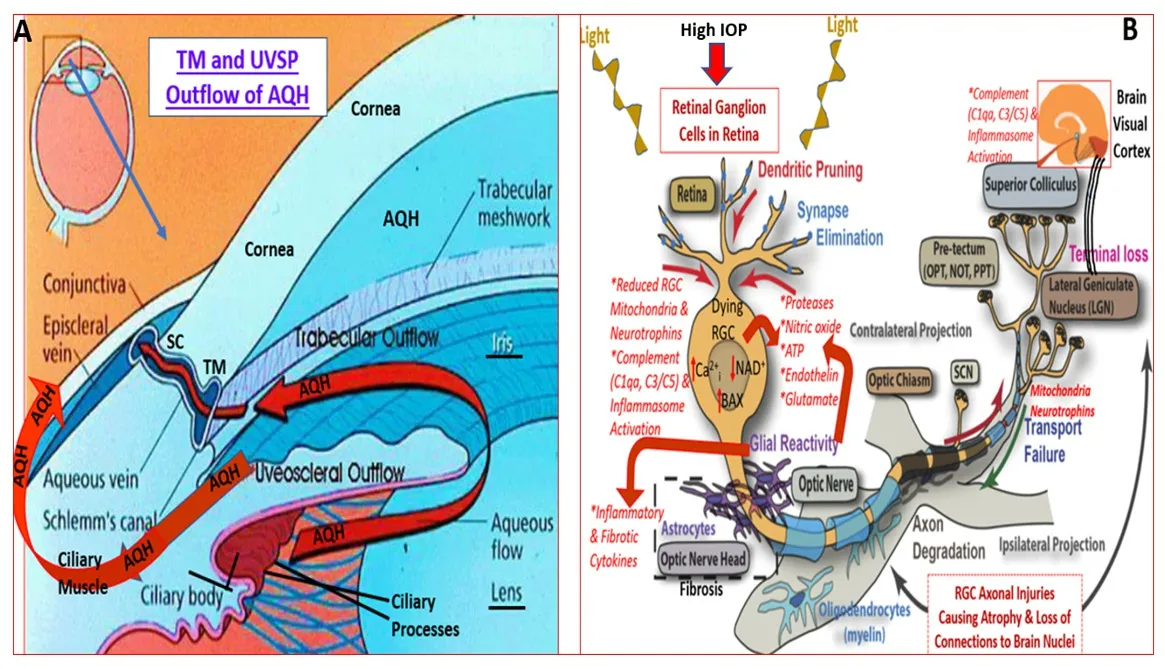
Figure 14 | Aqueous humor dynamics in the human eye and the multiplicity of factors involved in the death of retinal ganglion cells and their axons due to elevated IOP.
Lastly,several reports have described malfunctions in lipid metabolism as being associated with different forms of glaucoma.Specifically,AQH concentrations of autotaxin,the enzyme that converts different types of membrane-bound phospholipids to lysophosphatidic acid (LPA;Figure 15A
),and the levels of LPA and lysophosphatidyl choline were substantially elevated in patients with normotensive glaucoma,POAG,secondary openangle glaucoma,and exfoliation glaucoma (Nagano et al.,2019;Figure 16A–C
).Inhibitors of autotaxin dosed intravitreally (e.g.,S32826;Iyer et al.,2012)or t.o.(Nagano et al.,2019) in animals lowered IOP (Figures 15B
,and16D
,E
).The differences in the magnitude of the ocular hypotensive responses induced by the two autotaxin inhibitors are related to their potencies and due to their different routes of administration.Nevertheless,reducing the intraocular concentrations of the LPA,and most probably selective antagonists of LPA receptors (Figure 15A
),results in beneficial outcomes in terms of enhancing AQH drainage and replenishing the ANC’s AQH with freshly produced fluid from the ciliary body thereby keeping the tissues surrounding the ANC well nourished.Whether autotaxin inhibitors and/or LPA antagonists have similar direct or indirect protective effects in the retina/choroid remains to be determined.Retinal Cells and Neuronal Protection by PGs
During chronic OHT the elevated IOP in the ANC of the eye is constantly transmitted throughout the eyeball and seriously causes mechanical strain and distention at the back of the eye inducing local inflammation at the optic nerve head.Over time,this damages the RGC axons and eventually kills the RGCs via multiple factors and pathways (Figure 14B
),resulting in visual impairment (Weinreb et al.,2014).Over many years,the patient loses peripheral vision and can become blind if left untreated.To preserve eyesight,OHT/glaucoma patients receive IOP-lowering topical eyedrops.Even though this slows down the speed of visual impairment,the patients continue to progress towards serious vision loss.Glaucoma patients whose IOPs are normal similarly progress towards debilitating eyesight and visual field defects.Therefore,drugs that can directly or indirectly preserve RGCs and their axons are eagerly being sought.
Figure 15 | The generation of and signaling via the lysophosphatidic acid (LPA)receptor system.
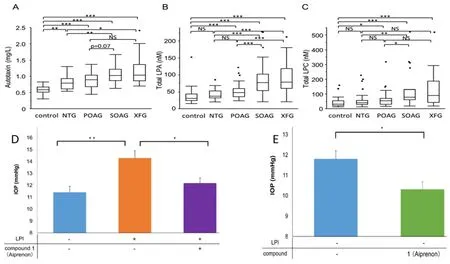
Figure 16 | The levels of autotaxin and lysophospholipids in AQH of glaucoma patients and the ability of an autotaxin inhibitor to lower IOP in mice.
Direct neuroprotective effects of PG agonists have been demonstrated using isolated rat primary RGCs subjected to a variety of insults presumed to occurin vivo
at the back of the eye in chronic OHT,POAG,and other forms of glaucoma (He et al.,2018).Thus,in a comprehensive comparative series of studies,Yamagishi et al.(2011) found that 100 nM free acids of tafluprost,latanoprost,and bimatoprost significantly (P
<0.001) protected rat RGC cells from glutamate-induced and hypoxia-induced apoptotic cell death.Interestingly,PGF,unoprostone and travoprost free acids were marginally protective.The RGC-rescuing effects of latanoprost free acid in the above studies confirmed earlier reports by Drago et al.(2001) using both retinal cellbased assays involving glutamate-toxicity and hypoxia-reoxygenation and anin vivo
rat model of ischemia-reperfusion.Likewise,latanoprost increased the survival of retinal neuro-glial cells by inhibiting caspase-3 (Nakanishi et al.,2006).Furthermore,latanoprost acid derived from a hydrogen sulfide-releasing agent conjugated to latanoprost free acid protected rat photoreceptors in a cell line exposed to oxidative stress and protected RGCs in the rat OHT model of POAG (Osborne et al.,2010).Interestingly,tafluprost was also shown to increase photoreceptor survival in the cell-line exposed to glutamate-induced toxicity and serum-deprivation insults,and it exhibited neuroprotective properties in an optic nerve crush model of POAG/OHT (Kanamori et al.,2009).Similarly,latanoprost enhanced the growth of“neurites”in the same photoreceptor cell line (Zheng et al.,2011).Moreover,an EP-receptor agonist,ONO-AE1-259-01 injected intravitreally abolished the neurotoxic effects of NMDA on RGCs (Mori et al.,2009).All these latter beneficial actions of EP-and FP-receptor agonistsin vitro
andin vivo
are mediated by the receptors located in the retina and choroid (Figure 11
).In various animal models of OHT/POAG disease involving either NMDAinduced neurotoxicity,optic nerve crush/axotomy,or ischemia-reperfusion procedures,intravitreally delivered latanoprost (Kudo et al.,2006;Hernandez et al.,2008;Emre et al.,2009) and unoprostone (Melamed 2002;Mukuno et al.,2004) demonstrated RGC protective activity in rats and rabbits.As mentioned above,tafluprost also enhanced RGC survival in the optic nerve crush model (Kanamori et al.,2009).In a similar vein,various FP-R agonist PGs (including tafluprost,travoprost,and latanoprost) have been shown to enhance ocular blood-flow at or close to the optic nerve head in rabbits/rats (Inan et al.,2004;Ohashi et al.,2008;Akaishi et al.,2010;Kurashima et al.,2010) and in human subjects (Cardascia et al.,2003;Koz et al.,2007;Alagoz et al.,2008;Giannico et al.,2016).This is regarded as a beneficial effect of these PGs since low blood perfusion pressure at the optic nerve head,along with other ocular vascular abnormalities,is associated with POAG development (Flammer et al.,2013;Pasquale,2016).One culprit responsible for such local ischemia/hypoxia is ET,a well-known vasoconstrictor peptide that is elevated in glaucomatous conditions in animal models of OHT and OHT/POAG patients (Choritz et al.,2012).ET mediates these detrimental effects by enhancing intracellular Ca([Ca]) and thus contracting blood vessels,and in case of individual cells like RGCs,its toxic effect results from Ca-overloading (Prasanna et al.,2011;Stankowska et al.,2017).As such,tafluprost protected retinal injury after intravitreal injection of ET-1 (Nagata et al.,2014).In another study,Kurashima et al.(2010) showed that tafluprost,latanoprost,and travoprost concentration-dependently relaxed ET-1-induced ciliary arteryin vitro
indicating that indeed these FP-R agonists may directly enhance ocular blood-flowin vivo
by counter-acting any locally produced ET or other vasoconstrictor substances.Additional therapeutic approaches that have shown promise to stabilize and rescue apoptotic or injured RGCs and their axons involve the up-regulation and release of endogenous neurotrophic factors by different pharmacological classes of agents.These have included alpha-2-adrenergic agonists such as brimonidine (Gao et al.,2002) and certain beta-adrenergic antagonists (e.g.,betaxolol;Wood et al.,2001),wherein vitro
andin vivo
efficacy was observed.Likewise,exogenously delivered ciliary neurotrophic factor,brain-derived neurotrophic factor,or gene therapy delivery brain-derived neurotrophic factor effectively provided RGC neuroprotectionin
vitro
andin vivo
(Martin et al.,2004;Chadder et al.,2012).Thus,the ability of EPreceptor agonists(butaprost and PGE) to promote brain-derived neurotrophic factor secretion from immortalized human glial cells (Hutchinson et al.,2009) suggests that this class of PGs may be clinically useful for not only lowering and controlling IOP but also directly protecting RGCs and their axons if suitably formulated EPagonists could be delivered to the retina.Some promising approaches involve sustained release vehicles such as a polycaprolactone drug delivery implant where omidenepag isopropyl (DE-117;a non-PG EP-receptor agonist has been utilized as a test agent (Kim et al.,2016) and biodegradable microparticles as in the case of dorzolamide (Pitha et al.,2018).Such innovative technologies will be important in future endeavors to combat retinal diseases including GON.Conclusions
It should be apparent from the above treatise that PGs and their receptors are intimately involved in many homeostatic and thus beneficial actions in the CNS and the eye.In contrast,endogenous PGs when released chronically produce detrimental effects due to their pro-inflammatory properties.This pluripotency of PGs makes it hard to always ascribe destructive or neuroprotective features of these ubiquitous endogenous mediators.Therefore,it is important to bear in mind that not only is the relative concentration of the PG molecule(s) present at the site of action important,but the interplay between the different PG receptors activated by the PGs is also very relevant.The relatively low specificity of endogenous PGs complicates matters further and necessitates the use of synthetic PG analogs,which have conclusively demonstrated receptor selectivity;a particular case is for FP-receptors (Table 2
).Furthermore,the homologous and heterologous dimerization of PG-receptors with receptors such as those for angiotensin-II may also be another important factor that would determine the net outcome of the cellular/tissue activity being studied.Partial agonism (e.g.,unoprostone free acid)vs
.full agonism (e.g.,travoprost free acid) of the PGs,and allosteric modulation of the PG-receptors,are additional aspects to be considered in the overall scheme of what and how endogenous and exogenous PGs may impart their actions in the CNS and the eye.It is fortuitous that at least PGreceptor,antagonists generally behave as pure receptor blockers and their activityin vitro
andin vivo
is more easily interpreted.Much progress has been made in discovering,characterizing,and developing novel agonists and antagonists for numerous PG receptors and their sub-types (Figures 5–8
),and it behooves us to exploit the rich pharmacology of the PG systems to explore their utility to combat CNS and ophthalmic disorders.In disorders where prostanoids have damaging effects,the receptor antagonists have a major role to play to abrogate those activities.However,although it seems intuitive that suppression of the production of deleterious PGs,being upstream to the receptors,would be preferred,COX-1 and COX-2 inhibitors generally inhibit the synthesis of all PGs (Figures 1
and2
).Therefore,in some ways,these enzyme inhibitors lack the degree of specificity to address the selectivity offered by receptor-selective antagonist drugs.Nevertheless,as described above,COX-1 and COX-2 blockers have demonstrated some efficacy in several neurological and ocular disorders and represent useful drugs.Similarly,while PUFAs and their therapeutically useful derivatives offer benefits in certain diseases discussed above,the major challenge is the ability to upregulate the de novo synthesis of these molecules and/or to deliver in a sustained manner sufficient amounts of the agents to exert their protective effects.Thus,much more needs to be learnt and applied to rescue damaged and dying cells in the CNS and ocular tissues to tackle many disorders associated with PGs,PUFAs,and COX-enzymes.Author contributions:
The author was responsible for all literature searches,data collection,writing and submission of the manuscript,and approved the final version of the manuscript.
Conflicts of interest:
The author has no conflict of interest to declare.The main purpose of the review article is to gather relevant research-based information and to timely disseminate it to help students and early career life scientists involved in neuroscience and ophthalmology research.Through this endeavor,the author hopes to inspire further research into neurological and ophthalmic diseases and to help discover suitable treatment modalities for them.
Editor note: NAS is an Editorial Board member of Neural Regeneration Research.He was blinded from reviewing or making decisions on the manuscript.The article was subject to the journal’s standard procedures,with peer review handled independently of this Editorial Board member and their research groups.
Open access statement:
This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Extracellular vesicles in Alzheimer’s disease:from pathology to therapeutic approaches
- Molecular approaches for spinal cord injury treatment
- Sex-biased autophagy as a potential mechanism mediating sex differences in ischemic stroke outcome
- Adipose tissue,systematic inflammation,and neurodegenerative diseases
- Interleukin-1:an important target for perinatal neuroprotection?
- Neurotrophic fragments as therapeutic alternatives to ameliorate brain aging

