Molecular approaches for spinal cord injury treatment
Fernanda Martins de Almeida ,Suelen Adriani Marques ,Anne Caroline Rodrigues dos Santos ,Caio Andrade PrinsFellipe Soares dos Santos CardosoLuiza dos Santos HeringerHenrique Rocha Mendonça,Ana Maria Blanco Martinez
Abstract Injuries to the spinal cord result in permanent disabilities that limit daily life activities.The main reasons for these poor outcomes are the limited regenerative capacity of central neurons and the inhibitory milieu that is established upon traumatic injuries.Despite decades of research,there is still no efficient treatment for spinal cord injury.Many strategies are tested in preclinical studies that focus on ameliorating the functional outcomes after spinal cord injury.Among these,molecular compounds are currently being used for neurological recovery,with promising results.These molecules target the axon collapsed growth cone,the inhibitory microenvironment,the survival of neurons and glial cells,and the re-establishment of lost connections.In this review we focused on molecules that are being used,either in preclinical or clinical studies,to treat spinal cord injuries,such as drugs,growth and neurotrophic factors,enzymes,and purines.The mechanisms of action of these molecules are discussed,considering traumatic spinal cord injury in rodents and humans.
Key Words:axonal regeneration;drugs;enzymes;growth factors;molecular therapy;neurotrophic factors;purines;spinal cord injury
Introduction
The central nervous system (CNS) responds differently to injuries when compared to the peripheral nervous system.In contrast to peripheral nerves,the central axons do not efficiently and adequately regenerate after lesion.
Spinal cord injury (SCI) occurs as a consequence of abrupt or sustained trauma to the spinal cord and represents a serious clinical condition.The extent of damage depends on the intensity of the trauma which directly interferes with the patient’s prognosis (Kuricova et al.,2014).SCI results in devastating social,physical,and financial burdens for patients and families.Recent studies have reported that the incidence of SCI worldwide has ranged between 10.4 and 83 cases per million per year (Singh et al.,2014;Hejrati and Fehlings,2021).
The primary mechanical trauma results in neuroglial cell death and axonal damage and,consequently,alteration in the network required for sensorimotor function.Following this initial insult,a secondary injury cascade is initiated,which is characterized by inflammatory cell infiltration,vascular effects such as hemorrhage,ischemia and edema,ionic imbalance,glutamate release,and excitotoxicity,free radical formation,and cytokine release,which generates further neuroglial cell death and aggravates neurological deficits and outcomes (Tator and Fehlings,1991;Bareyre and Schwab,2003;Rowland et al.,2008;Hejrati and Fehlings,2021).
The difficulty in regeneration is mainly attributed to the microenvironment of the injured spinal cord (Kamada et al.,2005;Yiu and He,2006).The proximal stumps of the injured nerve fibers are exposed to the inhibitory molecules of the reactive glial environment.The recruitment of inflammatory cells and astrocytes leads to the formation of a glial scar,usually accompanied by cavities filled with chondroitin sulfate proteoglycans (CSPGs).Other inhibitory molecules are Nogo,myelin-associated glycoprotein (MAG) and,oligodendrocyte myelin glycoprotein,components of the central myelin that also interfere in the regeneration of axons.Furthermore,after axonal injury,there is also a decrease in trophic factors supply,due to intrinsic neuronal changes such as atrophy and cell death (Kamada et al.,2005;Yiu and He,2006).
In an attempt to optimize functional restoration of the lesioned CNS,numerous neuroprotective and neuroregenerative therapeutic approaches are emerging,such as cell therapy,which has shown favorable results with the use of different cell types such as pre-differentiated embryonic stem cells (Marques et al.,2010),dental pulp cells (de Almeida et al.,2011) and mesenchymal cells (Čížková et al.,2006;de Almeida et al.,2015;Ramalho et al.,2018).On the other hand,there is a wide range of molecular compounds that are currently being used for neurological recovery,with promising results.
The goal of molecular therapeutic intervention consists of promoting axonal regeneration and sprouting,protection of neurons from cell death,and enhancement of nerve fiber conduction (Thuret et al.,2006).It is important to note that sprouting of afferent fibers in the thoracolumbar spinal cord can contribute to some conditions such as autonomic dysreflexia (Rabchevsky,2006).
Although most research aims at motor functional recovery as a major outcome,we acknowledge other comorbidities,such as neuropathic pain and neurogenic urinary tract dysfunction,as neglected SCI related conditions,gaining attention and being evaluated as treatment goals (Anderson,2004;Hunt et al.,2021;Morse et al.,2021;Wang et al.,2021).There are a variety of molecules that can be used to either attenuate the damage caused by the secondary injury or to stimulate regeneration and restore lost connections and functions that occur in the spinal cord after injury.In this review,we will discuss some molecular compounds that have been used after SCI and provide a summary of these strategies.
Search Strategy and Selection Criteria
We included data from pre-clinical experimental studies and human clinical trials registered in clinicaltrials.gov in this narrative review.All these studies cited in the current review have been performed in the last twenty years(2001–2021).They were searched on the PubMed database using the following keywords or terms:regeneration,SCI,pharmacological,drugs,fibroblast growth factor (FGF) and SCI,brain-derived neurotrophic factor(BDNF) and SCI,nerve growth factor (NGF) and SCI,epidermal growth factor(EGF) and SCI,ciliary neurotrophic factor (CNTF) and SCI,glial cell line-derived neurotrophic factor (GDNF) and SCI,platelet-derived growth factor (PDGF)and SCI,guanosine,adenosine,inosine,purines,chondroitinase,and sialidase.
Pharmacological Treatments for Spinal Cord Injury
A drug can be defined as any chemical substance,except for a nutrient or an essential dietary ingredient,which when administered to a living organism produces a biological effect.Thus,the pathophysiological processes elicited by SCI are theoretically possible to be targeted by pharmacological interventions.Despite decades of scientific studies,no drug tested so far has achieved clinically satisfactory results in phase III clinical trials making preclinical studies of pharmacological treatments to improve neurological function a priority in this research field.A selection of drugs that are currently employed for different pathophysiological aspects of SCI or that are being studied with promising results in both preclinical and clinical trials are listed below.
Dopamine,Atropine,Norepinephrine,Midodrine,Glibenclamide:Blood-Perfusion Controlling Drugs
The neurogenic shock that follows SCI enhances the hypoxic damage caused by traumatic-induced vascular rupture.To minimize this condition,vasopressin and cardiac stimulant drugs such as dopamine,norepinephrine,the muscarinic antagonist atropine,and the alpha1-adrenergic agonist midodrine are of utility in the acute clinical management of SCI patients(Markandaya et al.,2012).Additionally,prevention of further lesion of SC microvasculature by the employment of the sulfonylurea receptor 1 channel antagonist glibenclamide prevents progressive necrosis and improves neurological status after SCI in rats (Simard et al.,2007).Thus,a phase I/II clinical trial that analyzes the safety and efficacy of its oral administration over the first 3 days after the injury has been conducted,with results expected for 2022 (Minnema et al.,2019).
Methylprednisolone,Tirilazad,Minocycline:Anti-Inflammatory Compounds
The most traditional drug clinically employed to treat the secondary injury cascade after SCI is methylprednisolone (MP).Being used since NACIS trials’results in the 1980s,it is a synthetic glucocorticoid agonist,suitable to be employed after acute SCI at an early stage due to its anti-inflammatory and neuroprotective effects,acting mainly in the prevention of lipid peroxidation(Hall,2011).However,these clinical trials revealed modest functional recovery,associated with severe adverse effects concerning immunodepression,leading to recurrent infections,discouraging its use (Cristante et al.,2012).Due to the side effects revealed by the aforementioned clinical trials,drug designers were encouraged to develop medications with the protective effects of MP but without its adverse events.Among these,emerged tirilazad,a nonglucocorticoid steroid with anti-inflammatory and antioxidant properties.Although safe for human use,clinical trials revealed less effectiveness than MP (Bains and Hall,2012),pushing scientists towards the evaluation of other classes of anti-inflammatory drugs.Minocycline is a clinically available anti-inflammatory compound that inhibits neuroinflammation and neurodegeneration through retinoic acid signaling (Clemens et al.,2018).Preclinically,it has been shown to inhibit the secondary expansion of the lesioned site,reducing autonomic dysreflexia (Squair et al.,2018).Although no severe adverse effect was related to its use,a phase II clinical trial employing minocycline during the first week after SCI revealed only a nonsignificant tendency of the improved neurological score (Casha et al.,2012).
Pregabalin,Riluzole,4-Aminopyridine:Voltage-Gated Cation Channels Antagonists
Originally developed for epilepsy treatment,gabapentinoids and riluzole present interesting properties for spinal cord (SC) neuroprotection.Riluzoledriven blockade of voltage-gated sodium channels reduces both glutamate release and excitotoxicity after SCI in animal models (Cifra et al.,2013).Preliminary analyses revealed improved motor scores with no serious side effects when employed to patients during the first 2 weeks after SCI(Grossman et al.,2014).Also reducing glutamate-induced excitotoxicity,gabapentinoids,such as pregabalin,are α2-δ2 voltage-gated calcium channel subunit blockers that reduce microglial activation,through down-regulation of p38 mitogen-activated protein kinase (MAPK),impairing the secretion of the pro-inflammatory cytokines interleukin-1 (IL-1),interleukin-6 (IL-6),tumor necrosis factor-α,and prostaglandins (Ha et al.,2011).This antiinflammatory effect results in decreased apoptosis and improved locomotor function with reduced mechanical hypersensitivity and tactile allodynia,promoting neuroprotection and neural regeneration after SCI in rodents(Tedeschi et al.,2016).An observational cohort study analyzing data from the European Multi-centre Study about SCI revealed that gabapentinoids treatment within 1-month post-injury significantly improved motor recovery of SCI patients (Warner et al.,2017).On the other hand,inhibitors of the voltage-gated potassium channels,such as 4-aminopyridine,facilitate action potential conduction within demyelinated fibers after SCI in guinea pigs(McBride et al.,2007).Treatment of chronically diplegic patients after SCI with 4-aminopyridine resulted in improved locomotion,bladder,and anal sphincters control,somatosensory and erectile functions,but,unfortunately,also led to adverse events such as seizures (Grijalva et al.,2010).
Baclofen:Inhibitory Neurotransmission Stimulation
Beyond being useful to treat spasticity after SCI,the GABAB receptor agonist has shown both neuroregenerative and neuroprotective actions after SCI.Hilton and co-workers showed that oral baclofen treatment after SCI promotes axonal regeneration and/or sprouting,converting pre-synaptic sites into growth cones,through Munc13 down-regulation (Hilton et al.,2022).Moreover,acute baclofen treatment promoted white matter sparing,decreased microglial/macrophage content,modulated the cytokine milieu,and enhanced locomotor function and bladder control after SCI in mice (de Sousa et al.,2021).Its use within the first 4 weeks after SCI,besides proven safety,revealed sensory and motor improvements of SCI patients (Cragg et al.,2019).
Leukocyte Common Antigen Related Phosphatase Related Peptides,NogoA Neutralizing Antibody,Elezanumab:Inhibitors of Growth Cone Collapse
The SCI site remodeling is a challenge to axonal growth.The pro-inflammatory and neurotoxic epicenter is isolated from normal SC parenchyma by the glial scar,sparing the surrounding neural tissue.Conversely,scar-forming astrocytes secrete CSPG,which impairs axonal elongation (Yuan and He,2013).So,drugs that target these inhibitors are being tested for SC repair.Leukocyte common antigen related phosphatase (LAR) was identified as a CSPG receptor that,once activated,induces growth cone collapse.Its systemic blockade with LAR-targeting peptides allowed serotonergic regeneration and locomotor partial recovery in animal models (Fisher et al.,2011).Clinical trials employing LAR-targeting peptides were not conducted yet.
Additionally,degenerating myelin sheath after SCI exposes MAG,oligodendrocyte myelin glycoprotein,and Nogo-A,which also impair axonal remodeling and functional recovery (Lee et al.,2010).Thus,the blockade of the myelin inhibitors through intrathecal administration of the fragment crystallizable fraction of Nogo receptor 1 results in regeneration and/or sprouting of corticospinal fibers and behavioral improvements,both in mouse and non-humans’ primates (Li et al.,2004;Wang et al.,2020).Due to these results,a phase II clinical trial employing intrathecal NogoA neutralizing antibody administration has been conducted for SCI patients,revealing non-significant improvements in neurological symptoms (Kucher et al.,2018).Another inhibitory molecule of the remodeled SCI site,repulsive guidance molecule A (RGMa),is up-regulated in several cell types,where it signals through neogenin receptors,inhibiting axonal elongation (Mothe et al.,2017).Application of elezanumab,an anti-RGMa monoclonal antibody,promotes neuroprotection,neuroplasticity,and functional recovery following a thoracic hemicompression of SC in non-human primates (Jacobson et al.,2021).Inspired by these promising preclinical results,a phase II clinical trial is currently recruiting volunteers (NCT04295538).Finally,RGMa,myelin proteins,and CSPG activate RhoA in growth cones,in such a way that RhoA acts as an intraneuronal hub to different inhibitory molecules of the extracellular milieu,signaling growth cone collapse (Wu and Xu,2016).More recently,it was shown that both C3 transferase and Y27632 treatments inhibit RhoA and Rho-dependent kinase,respectively,promoting axonal regeneration and recovery of hindlimb function after SCI in rodents (Dergham et al.,2002).This approach was translated to clinical trials,where VX-210,a cell-permeable derivative of C3 transferase,was employed.Although it gave promising results in phase I and II clinical trials,it did not reach the pre-defined efficacy endpoint in phase III (Fehlings et al.,2021).
Phosphatase and Tensin Homolog Antagonist Peptide 2 and 4,Bisperoxovanadium,TTK21:Promoters of Axonal Growth Intrinsic Properties
Combinatorial treatments stimulating the intrinsic growth capacity of CNS axons were the only approaches that have achieved both full-length regeneration and partial functional recovery within the lesioned CNS so far(De Lima et al.,2017).Phosphatase and tensin homolog (PTEN) is an enzyme that mediates the dephosphorylation of phosphoinositide 3-kinase (PI3K)targets,such as protein kinase B.Protein kinase B signaling pathway leads to mammalian target of rapamycin and S6 kinase activation,promoting protein synthesis and axonal elongation.PTEN systemic inhibition,with antagonist peptides PAP2 and PAP4,promotes serotonergic and corticospinal tract regeneration beyond lesion site,and locomotor function recovery in SCI animal models (Ohtake et al.,2014).Similarly,systemic treatment with the non-specific PTEN inhibitor,bisperoxovanadium,promoted tissue sparing and functional forelimb recovery after cervical spinal cord lesion in mice (Walker et al.,2012).Although bisperoxovanadium theoretically has a potential negative influence on cancer prevention due to cell growth stimulation,its blockade of phosphatase activity impairs cell cycle progression required for cancer cell development (Scrivens et al.,2003).Thus,PTEN inhibition seems a promising target for future clinical trials for SC repair.Besides,protein synthesis-dependent axonal elongation requires genes to be transcripted within the neuronal nuclei.Transcription machinery access to the genes requires chromatin remodeling,which is accomplished by post-translational modifications of histones or DNA itself.Delivery of TTK21,an activator of cAMP response element-binding protein/P300 after SCI,increased acetylation of dorsal root ganglia neurons’ histones and promoted regeneration and sprouting of axons within the rodent’s SC,along with both sensory and motor improvements in behavioral assays (Hutson et al.,2019).The safety of histone acetylation promoters,such as valproic acid,is well known due to their use in the treatment of other pathologies in humans,such as epilepsy,encouraging clinical trials employing histone acetylation enhancers.
Therefore,besides the drugs available for SCI repair,several novel targets are being studied,aiming for the development of novel drugs that would eventually be able to modify other aspects of SCI pathophysiology.The combinatorial treatment with different drugs or with different regenerative therapies may have additive effects to overcome the inhibitory environment of the injured SC.Figure 1
exemplifies selected aforementioned drugs and their mechanisms of action.Enzymes
Chondroitinase
After SCI,the physical barriers formed by the glial scars and the chemical substances secreted by them are great hurdles that inhibit the growth of central nervous system axons.The glial scar is composed of several components,of which CSPG is the most abundant (Zhang et al.,2013b).CSPG is the most prevalent extracellular matrix component secreted by astrocytes(Zhang et al.,2013a).Its inhibitory effects are accomplished by acting on oligodendrocytes and neurons,where they impair remyelination and axonal growth,respectively.CSPG signals through different receptors,such as LAR,protein tyrosine phosphatase σ (PTPσ) and NgR 1,2,and 3 (Sapieha et al.,2005;Schwab and Strittmatter,2014;Lang et al.,2015;Dyck et al.,2018,2019).Early suppression of CSPG secretion from reactive astrocytes can reduce their inhibitory effect on nerve fiber regeneration (Zhang et al.,2013b).Therefore,focusing on early suppression of CSPG production by astrocytes may reduce their inhibitory effect on post-injury axonal regeneration (Profyris et al.,2004).
Chondroitinase ABC (ChABC) has been shown to promote regeneration of axons through the glial scar (Yick et al.,2003).ChABC is obtained from the bacteria Proteus vulgaris and acts by degrading the glycosaminoglycan side chains of CSPGs.Degradation of CSPG with the use of ChABC removes the inhibition of regeneration from the glial scar (Figure
2) (Raspa et al.,2019).It has been reported that ChABC treatment after SCI reintegrates postsynaptic activity below the lesion site and promotes functional recovery of locomotor and proprioceptive behaviors in rats (Bradbury et al.,2002;Mahajan,2018).Besides,ChABC infusion enhances recovery after experimental nigrostriatal lesions and in several animal models of SCI (Mountney et al.,2010).These promising results in animal models strongly drive the initiation of human tests(Mahajan,2018).In addition to acting individually,ChABC has been tested in combination with other pro-regenerative therapies as a potential treatment for SCI.Among them is the combination with enteric neural stem cells,bone marrow mesenchymal stem cells,hyperbaric oxygen therapy,treadmill rehabilitation,GDNF,and other enzymes,like sialidase (García-Alías et al.,2009;Zhang et al.,2013a;Shinozaki et al.,2016;Liu et al.,2018;Jevans et al.,2021).
Sialidases
These enzymes are glycosidases responsible for the removal of sialic acid(Sias) residues (desialylation) from glycan portions of either glycoproteins or glycolipids (Yuan et al.,2020).The Sias are a family of 9-carbon containing acidic monosaccharides found on both N-and O-linked glycans of either glycoproteins or glycolipids.Sias is involved in many biological processes,such as regulating cellular interactions in controlling activation,differentiation,transformation,and migration of cells (Mountney et al.,2013).
The hydrolytic removal of Sias (desialylation) from glycoproteins or glycolipids takes part in the regulation of various physiological and pathological pathways(Mountney et al.,2010).Desialylation of glycoconjugates influences cell signaling,adhesion,apoptosis,receptor activation,phagocytosis,cell migration,cell transformation,differentiation,migration,and neuritogenesis.Therefore,sialidases regulate many cellular processes in both physiological and pathological conditions by removing Sias from glycoconjugates (Yang et al.,2006).
The microenvironment of SCI is highly inhibitory for axonal regeneration.Endogenous inhibitors,including those on residual myelin,for example,MAG,contribute to regeneration failure.MAG binds to various neuronal receptors to inhibit axonal outgrowth,including Nogo receptors (NgR1 and NgR2),PirB,β1-integrin,and sialoglycans (Vyas et al.,2005;Mehta et al.,2007).Some neurons respond to MAG primarily via sialoglycans,whereas others use NgRs and other receptors.Gangliosides are the most abundant sialoglycans on nerve cells.MAG inhibition of axonal outgrowth in some neurons is reversed by treatment with sialidase,an enzyme that hydrolyzes sialic acids and eliminates MAG-sialoglycan binding (Figure 2
) (Vyas et al.,2002).Sialidase treatment enhances recovery after spinal cord contusion in the rat (Mountney et al.,2010).Therefore,sialidase emerges as a potential biological drug for the recovery of axons,validating sialoglycans as therapeutic targets for the treatment of SCI.Another important therapy,in addition to sialidase,is the use of polysialic acid(PSA).PSA is a natural,biodegradable,and negatively charged polysaccharide mainly attached to the neural cell adhesion molecule (Saini et al.,2016;Zhang et al.,2018).Several studies report its importance concerning its therapeutic possibility.Among the advantages discussed above,it is known that PSA decreases tumor necrosis factor-α and IL-6 release by inhibiting ionized calcium-binding adapter molecule 1,microglia/macrophage activation,and reduces apoptosis-associated caspase-3 protein expression.In addition,PSA inhibits axonal demyelination and glial fibrillary acidic protein expression,increases neurofilament 200 expression,and improves the functional outcome (Mehanna et al.,2010;Zhang et al.,2018).Therefore,PSA also stimulates regeneration in the central nervous system after SCI (Saini et al.,2016).
In conclusion,among axonal regeneration inhibitor-targeted experimental therapies,two bacterial enzymes have emerged as potential drugs to treat SCI.ChABC cleaves inhibitory CSPGs and sialidase cleaves sialoglycan receptors for MAG (Yick et al.,2003;Mountney et al.,2013). Therefore,including the combination of such therapies can favor a better axonal regeneration,reflecting in potential functional recovery after SCI (Bradbury et al.,2002;Mountney et al.,2013).
Growth and Neurotrophic Factors
Growth factors and neurotrophic factors are secreted biomolecules that are present in the nervous system during development and throughout adult life;they promote neuronal cells development,differentiation and survival,neurite outgrowth,synaptic plasticity,and neurotransmission,influencing the topography of axonal projections during development and regeneration.Growth factors can be produced by many different tissues;most neurotrophic factors belong to one of the three families:(A) neurotrophins (NGF,BDNF,NT3,and NT4),(B) glial cell-line derived neurotrophic factor family ligands(GDNF,Neurturin,Artemin,Persephin),and (C) neuropoietic cytokines (CNTF,leukemia inhibitory factor (LIF),IL6,and others).In general,growth factors and neurotrophic factors exert their trophic effects by signaling through tyrosine kinases receptors,which activates distinct cell signaling pathways as PI3K/AKT/mTOR,PKC,RAS/MAPK,and Jak-STAT,as seen in Figure 3,although the cellular responses elicited by them often overlap.
Fibroblast growth factor
When discovered,FGF was an unknown protein extracted from the cow’s pituitary gland and was named NIH-LH-B8 (Armelin,1973).It seemed to have a highly specific activity,turning resting-state 3T3 fibroblast lineage into a mitotic state (Armelin,1973),and later its name was changed to“fibroblast growth factor”(Gospodarowicz,1974).Nowadays,FGF is better described as a protein family composed of 23 trophic factors which exert a wide variety of effects such as proliferation,differentiation,migration,chemotaxis,neurogenesis,and axonal growth.Among these proteins,the basic fibroblast growth factor (bFGF or FGF2) emerges as a potential treatment in the medical regenerative field (Itoh and Ornitz,2008).
FGF2 promotes mitogenic activity,stem cell-state steadiness,and cell survival (Ding et al.,2010;Mossahebi-Mohammadi et al.,2020).These are useful properties for foreseeing a novel treatment for SCI.Using a thoracic spinal cord transection model in C57BL/6J mice,Huang and colleagues observed,12 weeks after injury,increased density of axons and betterspared tissue after intravenous injection of human umbilical cord-derived stem cells overexpressing bFGF (bFGF-MSCs) as compared to vehicle administered control mice and to umbilical cord-derived stem cells without bFGF overexpression animals (Huang et al.,2021).After 60 days in Sprague-Dawley rats of a contusive model of SCI,similar results were noticed with allotransplantation of AAV-transfected neural stem cells carrying the bFGF gene inside the lesion’s epicenter.It was also observed more spared neurons,axonal growth,and discrete astrogliosis in the bFGF treated group compared to its controls (Zhu et al.,2021).Studies evidence that to exert its regenerative functions,bFGF binds to its transmembrane receptor deflagrating PI3K/Akt/mTOR,RAS/MAPK,and PLCy (Figure 3
) pathways (Zhou et al.,2018;Cai et al.,2021).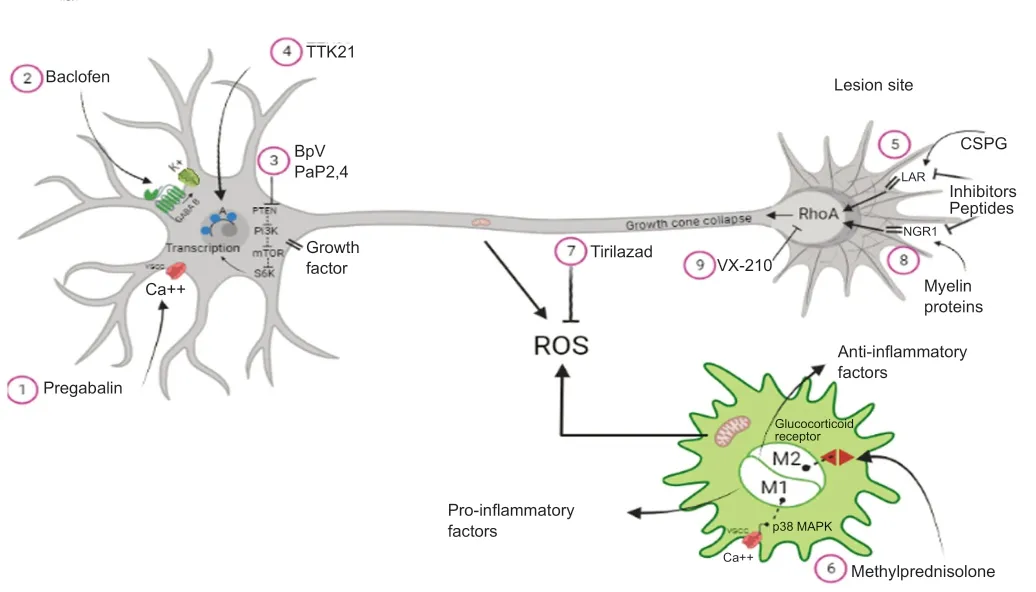
Figure 1 | Mechanisms of action of drugs after spinal cord injury.

Figure 2 | Role of enzymes after spinal cord injury.
bFGF similar drugs were synthesized and membranes with drug-delivering scaffolds are being developed as possible treatments to spinal cord injuries(Zhou et al.,2018;Edamura et al.,2020;Imagama et al.,2020).Despite these promising results,bFGF research is still in the pre-clinical stage.
Platelet-derived growth factor
PDGF was initially identified as having a growth-promoting activity in platelets,being later verified its production by many varieties of cell types,such as glial cells and neural progenitors.There are four different PDGF genes (PDGF A-D)that encode the five dimeric proteins (PDGF-AA,-BB,-AB,-CC,and -DD).They bind with different affinities to one of the three tyrosine kinase receptors(PDGFR-αα,PDGFR-αβ,and PDGFR-ββ) and signal by various intracellular pathways associated with cell division and growth,including the PI3K/AKT/mTOR pathway,RAS/MAPK,and STAT families,besides PLC-γ activation as seen inFigure 3
(Andrae et al.,2008).
Figure 3 | Schematic representation of the components of growth and neurotrophic factors signaling pathway.
In an experimental study,PDGF protects against blood-spinal cord barrier disruption after SCI by remodeling the neurovascular units,upregulating tight and adherens junctions,and promoting autophagic flux activation(Ye et al.,2021).The blood-spinal cord barrier plays a vital role in SCI recovery,thus its preservation can reduce other degenerative events such as loss of microvasculature,infiltration of blood-derived macrophages,neuroinflammation,oxidative stress,glial scar formation,and cell death.
Inflammatory response and M1 or M2 subtype polarization of macrophages/microglia are essential to glial scar formation.M2 macrophages can secrete PDGFB acting on PDGFRβ to promote PDGFRβpericytes migration,in bothin
vivo
andin vitro
conditions,to form the fibrotic core of the glial scar around the spinal lesion (Li et al.,2021a).PDGF-AA can influence the oligodendrocyte precursor cells to migrate and differentiate into oligodendroglial cells,increasing myelination and improving functional recovery (Yao et al.,2017).Additionally,PDGF can promote motor neurons survival,reduce scar tissue,improve the remyelination and promote SCI recovery when administered locally or subcutaneously (Guo et al.,2019;Ye et al.,2021),or when secreted by different genetically modified cells (Plemel et al.,2011;Yao et al.,2017).These results suggest that PDGF administration may be a promising treatment for acute SCI.
Epidermal growth factor
Previously known as urogastrone,EGF presents regenerative properties,is already used as a treatment for diabetic skin ulcers (Berlanga et al.,2013).In preclinical studies,it is being evaluated as a potential therapy for SCI,but the literature is still inconclusive,facing contradictory results about its therapeutic efficiency.Some studies indicate that EGF promotes neurogenesis,diminishes oxidative stress and apoptosis after SCI in mice (Ozturk et al.,2018;Xue et al.,2020).One research demonstrated that EGFFGF treatment is less effective than FGFEGFGDNF treatment to enhance axonal growth across the lesion site (Anderson et al.,2018).Despite these beneficial results,some studies show that EGF may hamper central nervous system regeneration by facilitating blood-brain barrier disruption and enhancing astrogliosis after injury (Wu et al.,2010).One study observed a reduction of NF-κB and MAPK levels within astrocytes nuclei and,because of that,secondary complications were diminished after SCI by blocking EGF receptors with PD168393 experimental drug (Li et al.,2021b).At the moment,no published clinical trials use EGF as a treatment for SCI.
Glial cell line-derived neurotrophic factor
GDNF was first described in glial cells but is also expressed in neurons,astrocytes,oligodendrocytes,Schwann cells,and skeletal muscle fibers.GDNF binds to tyrosine kinase receptors,forming a GDNF-GDNF family receptor alpha-1 (GFRα1) binary complex,then assembly with co-receptor tyrosine kinase rearranged during transfection (RET),forming the GDNFGFRα1-RET ternary complex domains that signal through the activation of RAS/MAPK,PI3K/Akt/mTOR pathway and Jun N-terminal kinases-mediatedtranscription (Chen et al.,2009).The GDNF-GFRα1 complex can also signal through a neural cell adhesion molecule (Figure 3
),with a lower affinity,in an independent manner (Zhang et al.,2009).GDNF expression is higher during CNS development,being reduced in healthy adults.However,SCI promotes its immediate up-regulation,mainly in microglia and macrophages,which sustain its enhanced expression during four weeks (Satake et al.,2000).GDNF contributes to astrogliosis modification by glial fibrillary acidic protein and CSPGs down-regulation,resulting in secondary damage reduction and robust axonal regeneration in adult rats (Deng et al.,2011;Anderson et al.,2018).Also,it stimulates the myelination (Zhang et al.,2009) and exerts chemoattractive effects on axonal regrowth (Anderson et al.,2018),being involved in neuronal survival and formation and maturation of the neuromuscular synapse during development and disease (Deng et al.,2011;Anderson et al.,2018).
Since GDNF does not cross the blood-spinal cord barrier,it demands local administration or conjugating GDNF with other molecules,such as viral proteins,antibodies,or genetically modified cells that secrete GDNF,overcoming this limitation.Combining GDNF administration and SC transplantation has been proposed as a possible strategy to promote axonal regeneration and myelin formation after SCI (Deng et al.,2011),and the combination of GDNF with other neurotrophic factors enhances its therapeutic capability (Anderson et al.,2018).
Nerve growth factor
After its isolation and identification in 1953 by Rita Levi Montalcini,Viktor Hamburger,and Stanley Cohen,NGF revealed its protagonist role among trophic factors in regeneration after SCI (Sharma,2007).The presence or absence of NGF in the CNS microenvironment,or even its precursor form,proNGF,is responsible for intricate molecular mechanisms,which will lead to neuronal survival or apoptosis via TrkA or low-affinity NGF receptor (LNGFR/p75NTR),respectively.Succinctly,NGF will either induce transcription factor activity such as NF-κB,CREB,ELK1,and regulator factor bcl2,which are involved in cell survival or will induce c-Jun/c-Fos AP-1 apoptotic activity.To induce cell survival or cell death,NGF and proNGF propagate signal amplification downstream RAS/MAPK or PI3K/Akt/mTOR pathways (Figure
3
) which have also been implicated in other trophic factor mechanisms and axonal regeneration (Lee et al.,2001;Freeman et al.,2004).Experimental studies indicate that NGF released from neural progenitor cells transplanted inside the cerebral cortex of an organotypic model promoted axonal growth similar to a corticospinal tract (Kamei et al.,2007).Neuron survival,axonal growth,decreased apoptosis,spared parenchyma,reduced formation of cavities,reduced astrogliosis,and better motor function recovery were seen in murine models after SCI when treated with different NGF delivering methods (Song et al.,2021;Xia et al.,2021).Preclinical studies have shown encouraging results.Therefore,NGF is one of a few trophic factors being already studied in a clinical trial.In one study,46 patients with motor and sensory functions were hampered by lumbar intervertebral disk herniation (Chen-yang et al.,2021).In this research,the group of patients treated with intramuscular methylcobalamin-NGF injection presented,weeks after the decompression surgery,better neurological outcomes compared with the group that underwent decompression surgery followed by just methylcobalamin intramuscular injection.
Brain-derived neurotrophic factor
BDNF is another extensively studied neurotrophin.Like NGF,BDNF has neuronal survival and axonal elongation properties,also participating in neural plasticity,memory formation,and cognitive processing.BDNF binds to TrkB and LNGFR/p75NTR (Figure 3
),promoting a signaling pathway similar to NGF (Huang and Reichardt,2001;Yamada and Nabeshima,2003;Kowiański et al.,2018).Strüder and Rojas Vegas (2008) observed that resting paraplegic athletes with thoracic SCI have high venous blood BDNF concentration and,after exercising,this concentration rises even more (Rojas Vega et al.,2008).They hypothesize that these enhanced BDNF levels would promote the preservation of the spinal cord or compensatory plasticity,leading to functional recovery.Although there are diverse preclinical studies in the literature evidencing BDNF benefits,there is no clinical trial using it.Ciliary neurotrophic factor
CNTF release is enhanced after SCI by invading Schwann cells,local astrocytes,and muscle fibers and maintains survival and differentiation of various neuronal and nonneuronal cell types.CNTF is a glycoprotein that belongs to the IL-6 family,and its heterotrimeric receptor is composed of the CNTF receptor alpha (CNTFRα),glycoprotein-130 (gp130),and the leukemia inhibitory factor receptor (LIFRβ,also known as CD118).The CNTF/CNTFRα complex subsequently binds to gp130 and LIFRβ,and this heterodimerization activates the JAK/STATs pathway,RAS/MAPK,and PLCγ (Figure 3
),which promote diverse gene transcription regulation (Chen et al.,2009;Pasquin et al.,2015).Alternative activation of IL6Rα/gp130/LIFRβ tripartite receptor by high CNTF concentration might contribute to the extreme weight loss observed in humans and animals upon CNTF systemic administration (Sleeman et al.,2000).On the other hand,local administration of CNTF does not show side effects and can promote motor neuron survival,protect neurons in the red nucleus,promote axonal regeneration,increase sensory and motor neuron survival,enhance tissue sparing,modulate astrocytic and microglial activity in the vicinity of the injury,increase the survival and differentiation of adult oligodendrocyte precursor cells,enhance remyelination,and improve functional outcomes (Ye et al.,2004;Cao et al.,2010).Besides,CNTF influences neuroinflammation by macrophage chemoattraction by reactive astrocytes.CNTF can regulate the reactive astrocyte polarization from A1(neurotoxic) to the A2 (neuroprotective) phenotype and promote A2-type reactive astrogliosis by activating the STAT3 signaling pathway.Also,STAT3 might be essential for glial scar formation and astrocytic neuroprotective profile after SCI (Zhang et al.,2021).
Purines
In the field of CNS injuries treatments,molecular therapies have shown promising results in regeneration,neuroprotection,and other effects.Nowadays,it is widely known that purines nucleosides,such as adenosine,adenosine’s metabolic subproducts inosine and guanosine,exert more biological effects than only those related to the nucleic acid constitution and cell energy metabolism (Ribeiro et al.,2016).Here we review the contribution of purines on SCI treatment (Figure 4
).
Figure 4 | Schematic representation of purine’s receptors and functions.
Adenosine
Over the years,since the discovery of the functions of adenosine,some researchers observed that adenosine could regulate the formation of cAMP(Ribeiro et al.,2016).This regulation is mediated through its interaction with four G protein-coupled receptors:A2aR e A2bR composed of a Gs unit,stimulating adenylyl cyclase and enhancing the level of cAMP;and A1R and A3R,composed of a Gi unit,inhibiting adenylyl cyclase and decreasing the level of cAMP.The four adenosine receptors (ARs) share a common structure,which crosses the plasma membrane seven times and is linked by three intracellular and three extracellular loops (Ribeiro et al.,2016;Borea et al.,2018).The concentration of adenosine,both intracellularly and extracellularly,is regulated by nucleoside transporters,which are located in the cellular membrane (Borea et al.,2018).Regarding the expression of ARs in the spinal cord,A1 and A2A receptors are most prevalent within the spinal cord.The A1R is highly expressed and was shown to inhibit neurotransmitter release.In stressful excitotoxic conditions after an SCI,this inhibition can promote neuroprotection,and reduction of the secondary damage.The A2AR is mainly expressed in astrocytes,oligodendrocytes,and microglia,and they mediate many functions,including attenuation of glial reactivity,blood-brain barrier permeability,uptake of GABA by astrocytes,and immunomodulation of microglia (Ribeiro et al.,2016;Borea et al.,2018).
Although adenosine has a potential pharmacological activity,it has a fast metabolization and clearance from the bloodstream,due to its short half-life(<1 second),reducing its beneficial therapeutic effects (Gaudin et al.,2014;Borea et al.,2018).Thus,to bypass adenosine’s short half-life,Gaudin et al.(2014) used the squalenylacetic acid linked to the amino group of adenosine(SQAd) that spontaneously forms nanoparticles,promoting a controlled liberation of this nucleoside,in a contusive SCI model.SQAd animals had no visible traumatic area on the spinal cord 72 hours post-injury,resulting in an improvement of hindlimb function,extending to almost complete recovery,28 days after injury.They also showed that the SQAd treatment attenuated the degenerative impact on small-and medium-size nerve fibers.These results are probably due to the improvement in the microcirculation,leading to secondary parenchyma neuroprotection (Gaudin et al.,2014).A recent study,using 5 days administration of synthesized adenosine analog COA-Cl,in the acute phase of a contusive SCI model,showed a decrease in the volume of the cavity,1 month after the injury,and also an improvement in motor function in the Basso,Beattie and Bresnahan score and inclined plane test,due to the effect of the COA-Cl on suppressing the neuronal apoptosis around the injured site (Sakamoto et al.,2021).In conclusion,using the SQAd and the COA-Cl enhanced the potential of adenosine after SCI and improved the recovery of function,mainly due to the neuroprotective effects of adenosine in the spinal cord.No clinical trials are being conducted with adenosines.
Inosine
The deamination of adenosine by the action of adenosine deaminase results in inosine that can act as an agonist,binding directly to A1,A2A,and A3,and can trigger the well-known effects of these receptors (Haskó et al.,2004;Welihinda et al.,2016;Vincenzi et al.,2020).Like adenosine,inosine concentration,both intra and extracellularly,is regulated by nucleoside transporters (Doyle et al.,2018).Unlike adenosine,inosine is more stable and has a half-life of approximately 15 hours (Welihinda et al.,2016).Besides the binding to ARs,inosine can diffuse into neurons and activate the mammalian sterile 20-like kinase 3b,a protein kinase that is part of a signal transduction pathway that regulates axonal growth (Kim et al.,2013).
After spinal cord lesion,injured and uninjured axons can form compensatory circuits,extending collateral branches (Kim et al.,2013).In a model of spinal dorsal column transection,the use of inosine stimulated the sprouting of axons from the corticospinal tract to the contralateral side.Furthermore,these axons established synapses with long propriospinal interneurons,partially reestablishing cortical control at the lumbar level,improving motor function recovery (Kim et al.,2013).After a compressive SCI,the oral administration of inosine showed a major sparing of the white matter and survival of ventral horn motorneurons,and recovery of motor function(Kuricova et al.,2014).A model using complete transection or compressive SCI induced neurogenic detrusor overactivity.Two ways of inosine administration were performed:(1) immediately (in both compression and transection groups);(2) 8 weeks (only in compression group) after injury.Inosine administered both 6 weeks (immediate administration) and 14 weeks (delayed administration) after injury showed significant attenuation of detrusor overactivity and presented a reduction in both frequency and amplitude of spontaneous non-voiding contractions (Chung et al.,2015).Inosine also showed attenuation of apoptosis,a secondary degenerative event,which reduced the damaged spinal cord area,after a compressive SCI (Liu et al.,2006),and immunomodulatory effects,reducing the volume fraction of tissue occupied by activated macrophage/microglia around the lesion epicenter (Conta and Stelzner,2008).Regarding the time of administration,our group investigated the administration of inosine 2 and 24 hours in mice with compressive SCI.Inosine improved the motor and sensitive functions,and the animals presented a great number of regenerated myelinated fibers when compared to the control group,in both time points of administration(Cardoso et al.,personal communication).In conclusion,inosine presents many beneficial effects such as regenerative potential,neuroprotection,and immunomodulation,due to the activation of ARs and mammalian sterile 20-like kinase 3b.Thereby,its use improves the function reestablishment.No clinical trials are reported.
Guanosine
In contrast to adenosine and inosine,guanosine acts by binding to its GCPR,in the extracellular space,activating MAPK pathways,such as MEK-ERK1/2,PI3K/AKT,and p38 MAPK.It also activates soluble guanylyl cyclase,enhancing the levels of cGMP.Thus,there are two ways in which guanosine can be formed to act extracellularly:(1) intracellularly by the breakdown of GMP,and then released into the extracellular milieu,through membrane nucleoside transporter;(2) extracellularly by the breakdown of guanine nucleotides GTP/GDP/GMP,via 5-ectonucleotidase (Rathbone et al.,2008;Ribeiro et al.,2016).Traumatic SCI induces a prominent local inflammatory reaction and persistent demyelination in the white matter around the lesion site (Jiang et al.,2003).Using a compressive SCI model and intraperitoneal (i.p.)administration,guanosine demonstrated effects over myelination,stimulating oligodendrocytes progenitors to differentiate into mature cells.This was confirmed using a specific marker of mature oligodendrocyte,the monoclonal antibody Rip,and observing a great number of Rip-positive cells.They also immunostained sections for MBP and showed the presence of MBPpositive cells in the injured area,corroborating the observed improvement in myelination which resulted in better locomotor function (Jiang et al.,2003).Guanosine also has a neuroprotective effect after SCI.Using a compressive SCI model and i.p.administration,guanosine preserved the function of long tracts,enhanced the recovery of bladder function within 7 days,exerted an immunomodulatory effect,attenuating the activation of microglia/macrophage,and significantly suppressed apoptosis (Jiang et al.,2007).The i.p.administration can rapidly increase the levels of guanosine and its metabolites (guanine,xanthine,and uric acid) in the brain and SC,despite purine metabolism in peripheral tissue.Immediately after the i.p.injection,the proportion of guanosine:guanine is 2:1.Thirty minutes after the i.p.injection,the proportion is 1:3 (Jiang et al.,2008).In conclusion,guanosine improves myelination,by the activation of PI3K/Akt,a pathway responsible for myelination,enhances axonal regeneration,and exerts neuroprotection,improving functional recovery,after SCI.Up to now,no clinical trials have been reported employing guanosine after SCI.
Conclusion
Central axons fail to regenerate appropriately after a traumatic lesion.Therefore,the damaged connections are not reestablished after SCI,leaving permanent dysfunctions.Among several therapeutic interventions,molecular therapy has been showing promising results in terms of functional recovery.Pharmaceuticals,growth and neurotrophic factors,enzymes,and purines are being tested as a treatment after SCI.The mechanism of action of these molecules targets the axon collapsed growth cone,the inhibitory microenvironment,and the survival of neurons and glial cells,particularly the oligodendrocytes that form the central myelin sheath.Despite several decades of experimental studies,most of these molecules are still at the preclinical stage;therefore,there is a growing need for more judicious and well-designed experiments.Furthermore,there are still few studies focusing on the combination of molecules or association of molecular therapy with different strategies,such as cell therapy,exercise,among others.Experimental studies focusing on combinations of molecules and/or pro-regenerative strategies are needed and,hopefully,will provide better outcomes in terms of functional recovery after SCI.
Author contributions:
AMBM and FMA contributed to the conception and design of the manuscript.AMBM,HRM,and FMA contributed to the revision of the manuscript.All authors drafted the manuscript and approved the final version of the manuscript.
Conflicts of interest:
The authors declare no conflicts of interest.
Open access statement:
This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Neuroaxonal and cellular damage/protection by prostanoid receptor ligands,fatty acid derivatives and associated enzyme inhibitors
- Extracellular vesicles in Alzheimer’s disease:from pathology to therapeutic approaches
- Sex-biased autophagy as a potential mechanism mediating sex differences in ischemic stroke outcome
- Adipose tissue,systematic inflammation,and neurodegenerative diseases
- Interleukin-1:an important target for perinatal neuroprotection?
- Neurotrophic fragments as therapeutic alternatives to ameliorate brain aging

