Extracellular vesicles in Alzheimer’s disease:from pathology to therapeutic approaches
Marta Garcia-Contreras,Avnesh S.Thakor
Abstract Alzheimer’s disease is a progressive and fatal neurodegenerative disorder that starts many years before the onset of cognitive symptoms.Identifying novel biomarkers for Alzheimer’s disease has the potential for patient risk stratification,early diagnosis,and disease monitoring in response to therapy.A novel class of biomarkers is extracellular vesicles given their sensitivity and specificity to specific diseases.In addition,extracellular vesicles can be used as novel biological therapeutics given their ability to efficiently and functionally deliver therapeutic cargo.This is critical given the huge unmet need for novel treatment strategies for Alzheimer’s disease.This review summarizes and discusses the most recent findings in this field.
Key Words:Alzheimer’s disease;brain;diagnostic;extracellular vesicles;isolation methods;microglia;neurodegenerative diseases;neuroinflammation;neurons;therapy
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disorder characterized by loss of memory and cognitive abilities and is the most common form of dementia.AD is a heterogeneous disorder with multiple phenotypes,which prevents an accurate early diagnosis and targeted interventions.The early diagnosis of AD is a major challenge due to the lack of reliable biomarkers and,unfortunately,AD also lacks effective treatment options.
Extracellular vesicles (EVs) are a heterogeneous group of membrane-bound structures including exosomes,microvesicles,and apoptotic bodies (van Niel et al.,2018).EVs contain proteins,lipids,and a wide variety of genetic materials,such as DNA,mRNA,and non-coding RNAs and are found in most biological fluids (Valadi et al.,2007).Moreover,EV cargo is protected from proteases and nucleases in the extracellular space.Current assessment and evaluation of AD use neuroimaging parameters together with biomarkers in the cerebrospinal fluid (CSF),which has to be obtained by an invasive procedure.EVs represent a promising alternative given they can be isolated from different sources using minimally or non-invasive techniques (Scarth et al.,2021).Given that brain-derived exosomes contain cargo from their original cells,and they can be isolated from peripheral blood and/or other biological fluids,they can therefore be used as biomarkers for the diagnosis,screening,prognosis,and monitoring of AD (Lugli et al.,2015;Chen et al.,2016;Arioz et al.,2021;Scarth et al.,2021).On the therapeutic side,there are no new drugs for AD which highlights the urgency for new therapeutic approaches.In recent years,EVs have also emerged as a novel therapeutic tool for neurodegenerative diseases given their ability to carry a therapeutic cargo to target cells.Hence,EVs may also represent an exciting new therapeutic intervention for AD,not only for treatment in established disease states but also for the prevention of neuronal damage and neuroinflammation in early disease states.
Search Strategy and Selection Criteria
In this narrative review,we used PubMed and Google Scholar to search articles published from 1998 to 2021 with the following keywords:extracellular vesicles,neurodegeneration,Alzheimer’s,therapeutics,pathology,and biomarkers.
Extracellular Vesicles
EVs are a heterogeneous population of membrane-bound structures released by most cells into the extracellular space and can be found in most body fluids such as serum,plasma,urine,saliva,and cerebrospinal fluid (van Niel et al.,2018).EVs can be classified based on their size,origin markers,content,or source (Table 1).
Extracellular Vesicles Isolation Methods
EVs can be isolated from cell culture media or biological fluids including blood,urine,cerebrospinal fluid,tears,and saliva. Due to the complexity of biological fluids,current isolation methods isolate either exclusively exosomes or a mixture of EVs and other components.An overview of these methods can be found inTable 2
.
Table 1 | Classification of extracellular vesicles
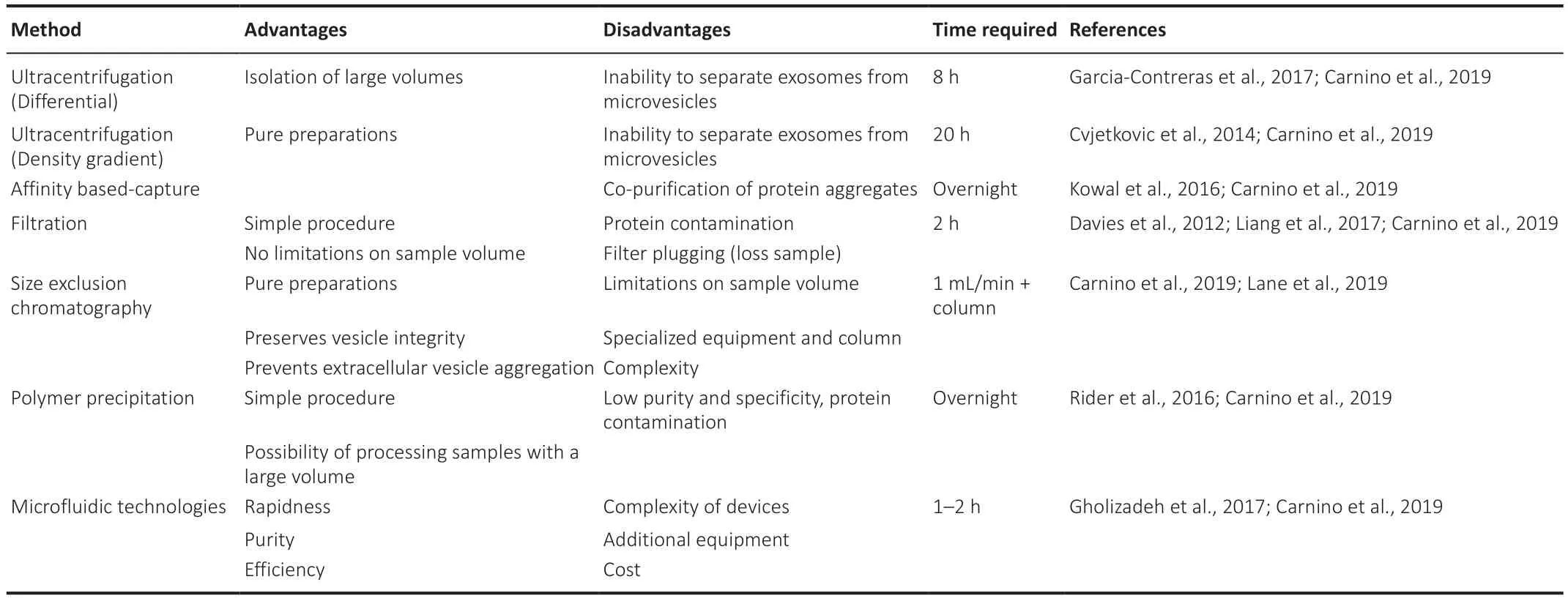
Table 2 | Extracellular vesicle isolation methods
Ultracentrifugation
Ultracentrifugation is the most commonly used method to isolate EVs.There are two types of ultracentrifugation methodologies:differential or density gradient.Differential centrifugation consists of sequential centrifugation steps:a 300 ×g
spin for 10 minutes followed by a 10,000 ×g
spin for 30 minutes to eliminate intact cells,dead cells,and cell debris.After depletion of cells and large apoptotic bodies by low-speed centrifugation,the EVs are pelleted in the final step at 100,000 ×g
for 70 minutes (Garcia-Contreras et al.,2017).Density gradient centrifugation is a combination of ultracentrifugation with a sucrose gradient to separate the EVs based on their density (Cvjetkovic et al.,2014).Affinity-based capture
Affinity-based isolation enables the selective capture of specific EVs subpopulations using antibodies to specific membrane markers such as CD63,CD81,or CD9 (Kowal et al.,2016).For this reason,selecting a proper membrane marker is one of the most significant steps in these immunoassays.A bead-based affinity approach is a method that uses magnetic beads or latex beads for capturing and isolating EVs.Another affinity based-capture method is a lipid nanoprobe system,which labels the EVs with a labeling probe for magnetic enrichment using a capture probe (Bano et al.,2021).
Filtration
Filtration is used in combination with ultracentrifugation to isolate EVs using porous membranes that trap molecules or particles of a specific size,allowing smaller molecules and particles to flow through the membranous filter (Liang et al.,2017).Samples are then processed using a centrifugal filter,followed by recovery with a reverse spin.Although this method is simple and allows the processing of many samples,filter plugging might result in loss of sample,in addition,to sample contamination by proteins.
Size Exclusion Chromatography
Size Exclusion Chromatography separates EVs based on the size using chromatography or microfiltration.Column chromatography allows for sequential elution of EV size fractions from a single column (Lane et al.,2019).Once the column is set,the sample is loaded into the column and eluted samples are collected from the column.While this method allows relatively fast collection of EVs by size,there are sample volume limitations and complex equipment requirements.
Polymer precipitation
Polymers such as polyethylene glycol are used for the precipitation of EVs.There are several commercial polymer-based isolation methods such as ExoQuick,Invitrogen Total Exosome isolation reagent,or miRCURYExosome Isolation Kit.These methods allow EV isolation from large samples with a high yield.However,contamination of EV pellets with non-exosomal materials remains a problem for polymer-precipitation methods and the polymer may interfere with the downstream analysis.
Microfluidic technologies
Microfluidic-based technologies can isolate EVs from biological fluid in an easily reproducible,convenient manner (Gholizadeh et al.,2017).Microfluidic devices allow the rapid and low-cost separation and detection of targets,and the devices can be simply made which lowers the production cost thereby making them commercially viable.
Extracellular Vesicles Characterization Methods
Electron microscopy
Morphological characterization is carried out using electron microscopy.Transmission electron microscopy is a standard technique used to characterize EV preparations.EVs analyzed by transmission electron microscopy often show a cup-shaped appearance,which is an artifact of the preparation procedure.Transmission electron microscopy can be combined with immunogold staining using gold conjugated antibodies to detect the presence of specific markers.Scanning electron microscopy is another approach to analyze EV morphology and structure,while atomic force microscopy is a type of scanning microscopy that allows imaging of the topology of EV surfaces with nanometer resolution.
Western blot assay and enzyme-linked immunosorbent protein assay
Western blot assay is a widely used method to characterize and detect EV proteins.Enzyme-linked immunosorbent protein assays (ELISA) are also used to quantify the number of exosomes based on the level of the exosomeassociated proteins including CD9,CD63,and CD81.ELISA is more sensitive and provides more accurate protein quantification compared to western blots.
Flow Cytometry
Flow cytometry or imaging flow cytometry of EVs is performed by using beads coupled to antibodies that detect EV surface markers (Campos-Silva et al.,2019).The use of beads is due to the small size of EVs and the difficulty to detect EVs with most conventional flow cytometers.The challenge with flow cytometry of EVs relates to their small particle size and low refractive index,which makes them difficult to separate from background signals.
Nanoparticle Tracking Analysis
Nanoparticle tracking analysis uses light diffraction patterns to measure the size and the concentration of EVs.Direct size and concentration quantification can also be performed using the tunable resistive pulse sensing principle.
Direct Counting of Single Extracellular Vesicles
There are new techniques that allow the study of single EVs,thereby enabling the study of specific EV subpopulations.Super-resolution microscopy allows separating focal spots to visualize and directly count the number of single EVs as well as quantify their content (Lennon et al.,2019).Other than fluorescence-based EV visualization,there is an interferometric reflectance imaging method for single EVs.EVs can also be captured in an antibody array on a chip followed by the acquisition of interferometric images by a chip reader where the number and size of EVs can be acquired (Daaboul et al.,2016).
Extracellular Vesicles in the Pathogenesis of Alzheimer’s Disease
EVs are membrane-bound structures that transport cargo between cells in the body (Mathieu et al.,2019),including the nervous system where they have been shown to be involved in several neurodegenerative diseases such as AD,Parkinson’s,and amyotrophic lateral sclerosis.AD is characterized by the intracerebral accumulation of amyloid-β (Aβ) leading to Aβ plaque formation(Villemagne et al.,2018) and EVs isolated from the CSF have been shown to contain high levels of Aβ which is toxic to neurons culturedin vitro
(Sardar Sinha et al.,2018) and in animal modelsin vivo
(Elsherbini et al.,2020).One explanation for this is that EVs stimulate Aβ aggregation (Dinkins et al.,2014)and Aβ aggregates are less efficiently cleared and degraded by astrocytes and microglia (Dinkins et al.,2016),thereby contributing to their dysregulation in AD.Tau aggregates are another major hallmark of AD (Villemagne et al.,2018) and EVs from the CSF of AD patients have also been shown to contain Tau and these can be transmitted to neurons where they induce Tau aggregation (Saman et al.,2012;Fiandaca et al.,2015;Wang et al.,2017).In neurodegenerative conditions,microglia have been shown to release higher levels of EVs in comparison to inactivated microglia (Clayton et al.,2021)and in AD these EVs contain Tau which can be propagated to neuronsin vivo
contributing to the progression of tauopathy (Asai et al.,2015;Clayton et al.,2021).Furthermore,the dysregulation of EV cargo in iPSCs neurons has been associated with the AD familial A246E mutant form of presenilin 1,which could participate in propagating tau pathology.EVs derived from the iPSCs carrying the A246E presenilin 1 mutation contain high levels of the amyloid precursor protein and are capable of inducing tau deposits in the mouse brain afterin vivo
injection (Podvin et al.,2021) (Table 3).Extracellular Vesicles as Biomarkers of Alzheimer’s Disease
Proteins cargo
Several proteins have been found to be altered in EVs derived from AD patient samples (Muraoka et al.,2020).Specifically,neuron-derived EVs in AD patients have been shown to have increased levels of alpha-globin,beta-globin,and delta-globin compared to healthy controls by liquid chromatography-tandem mass spectrometry proteomics analysis (Arioz et al.,2021).Furthermore,lysosomal proteins are also altered in neural-derived plasma EVs in preclinical AD samples.These autolysosomal proteins could distinguish preclinical AD patients from controls (Goetzl et al.,2015).Synaptic proteins such as NPTX2,AMPA4,NLGN1,and NRXN2α have also been reported to be decreased in neuron derived EVs from plasma of patients with AD.These proteins decreased significantly from the time of normal cognition in preclinical AD to the time of the development of AD dementia (Goetzl et al.,2018).Moreover,astrocyte-derived EVs have been shown to contain dysregulated protein cargo in AD samples such as β-site amyloid precursor protein-cleaving enzyme 1 and soluble amyloid precursor protein β (Goetzl et al.,2016).Microglia-derived EVs also have been shown to have altered protein cargo in AD mouse models and these appear to correlate with disease progression (Muraoka et al.,2021).
Lipid cargo
In addition to proteins,lipids have also been found to be dysregulated in EVs from AD samples (Su et al.,2021),which can be attributed to the lipid imbalance associated with AD.Human frontal cortex brain-derived EVs from AD patients are enriched in glycerophosphoethanolamine and polyunsaturated fatty acyl containing lipids (Su et al.,2021).Amyloid-induced astrocyte-derived EVs have been shown to be enriched in ceramide species,which might also contribute to AD (Wang et al.,2012).
miRNAs and mRNAs cargo
miRNAs in circulating EVs have been shown to serve as biomarkers for agerelated cognitive decline.Several miRNAs are dysregulated in AD samples from plasma,serum,CSF,and brain tissue (Lugli et al.,2015;Cheng et al.,2020).Specific miRNAs such as miR-132 and miR-212 are dysregulated in neural EV samples from AD patients compared to controls (Cha et al.,2019),while miR-9-5p and miR-598 have also been found in EVs obtained from the CSF of AD patients (Saman et al.,2012) (Table 4
).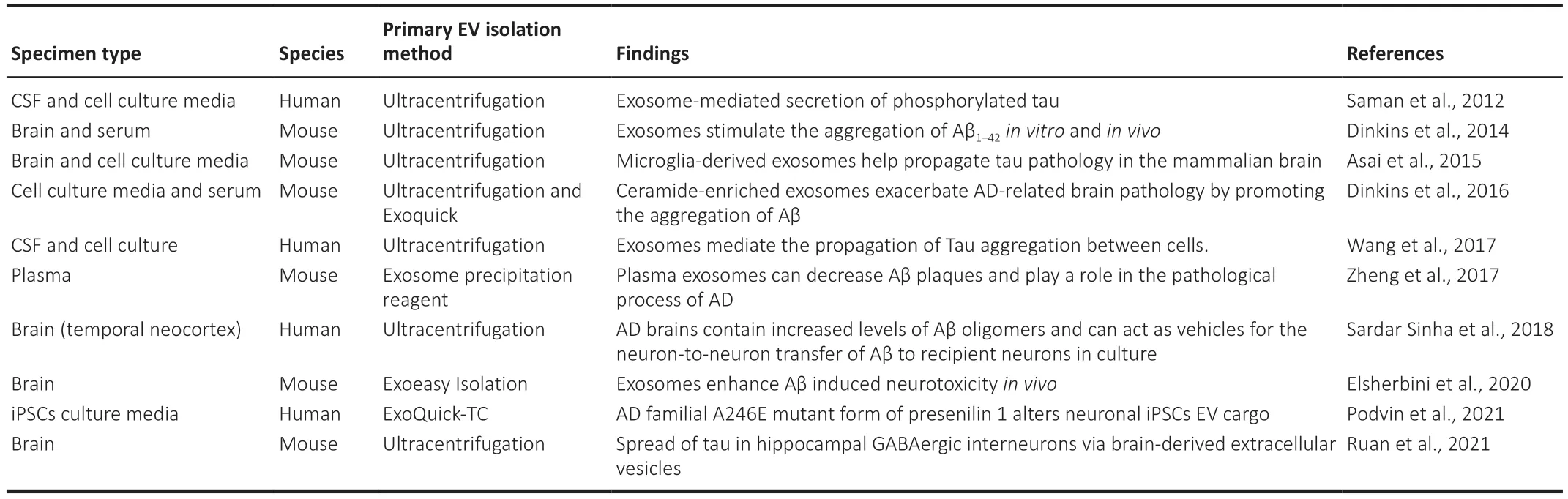
Table 3 | Studies showing EVs involved in Alzheimer’s pathology

Table 4 | Studies of findings on extracellular vesicles biomarkers in Alzheimer’s disease
Extracellular Vesicles as a Therapeutic Tool in Alzheimer’s Disease
EVs can also function as a novel therapeutic tool for neurodegenerative diseases given they can efficiently target the brain and penetrate through the blood-brain barrier (BBB) (Alvarez-Erviti et al.,2011).Specifically,stem cell-derived EVs have been shown to have neuroprotective and immunomodulatory properties in neurodegenerative diseases (Niu et al.,2020;Garcia-Contreras and Thakor,2021;Kim et al.,2021).In AD,the mechanism of Aβ degradation by glial cells is altered,including the production of proteases (such as neprilysin),which can hydrolyze Aβ at different cleavage sites.Previous studies have shown that administration of mesenchymal stem cell (MSCs) derived EVs overexpressing neprilysin reduced plaque deposition in AD mice models (Katsuda et al.,2013).Furthermore,MSC-derived EVs have shown to be neuroprotective in animal models of AD by protecting neurons against Aβ-induced oxidative stress and synaptic damage (de Godoy et al.,2018;Ma et al.,2020).This neuroprotection can be mediated by the delivery of MSC-EV specific cargo such as miR-29c-3p to neurons,which then inhibits BACE1 expression while activating the Wnt/β-catenin pathway (Sha et al.,2021).Neural stem cell-derived EVs have also been shown to have neuroprotective effects and can restore fear extinction memory consolidation and reduce anxiety-related behaviors (Apodaca et al.,2021).Finally,given that a lack of physical exercise contributes to several cerebral diseases,including AD,increasing EVs in the brain in response to physical exercise has been proposed as a potential therapeutic strategy (Zhang et al.,2021).New emerging engineering strategies are being developed to explore the specific targeting of EVs (Jang et al.,2021).EVs can be modified by manipulating their parent cells,with changes then subsequently incorporated into the secreted EVs for specific delivery/targeting (Dooley et al.,2021) (Table 5).
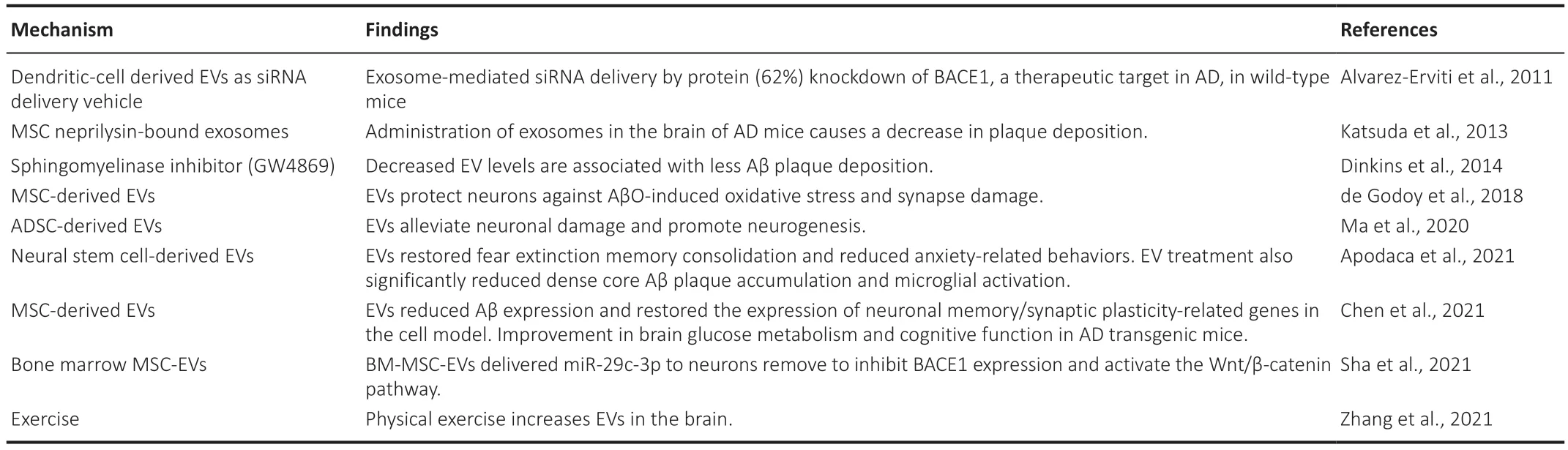
Table 5 | Studies showing therapeutic applications of extracellular vesicles in Alzheimer’s disease
Conclusion
AD is an increasingly common form of dementia that worsens over time.While Aβ and tau are recognized as key factors in AD,many important details remain unknown.EVs could provide new insights into the underlying drivers of AD,with dysregulated EV cargo possibly representing an underappreciated driver of AD pathology.In addition,some of these EV cargos could also be reflective on the stage of AD and thus be used as biomarkers.Existing efforts in the field are trying to investigate how to analyze cell-type-specific EV content.Current biomarkers for AD are obtained from the CSF,which is obtained in an invasive manner and sometimes is not able to differentiate AD from other types of dementia.In contrast,EVs could be obtained from blood in a minimally invasive manner with studies suggesting they could even reflect the disease stage of AD (Goetzl et al.,2015;Lugli et al.,2015).Brain-derived EVs are thought to be present in the circulation given that neuroinflammatory responses and pro-inflammatory cytokines promote the breakdown of the BBB.However,given their low concentrations,the development of highly sensitive methods for EV detection and isolation is needed.
Existing AD treatments have a low efficacy partially due to the difficulties in crossing the BBB.However,EV-based therapies could represent a more efficient,specific,and functional delivery system.EVs have the ability to cross the BBB by interacting with the endothelial cells (the first line of defense in the brain).There is an urgent unmet need to develop new treatments to delay or prevent AD.Promising results using different types of EVs have been obtained,and these include reducing Aβ plaque deposition,oxidative stress,synaptic damage,and/or microglial activation (Katsuda et al.,2013;de Godoy et al.,2018;Apodaca et al.,2021).New engineering strategies to over-express brain cell-specific markers into EVs could develop an even more efficient and targeted drug delivery system for neurodegenerative diseases such as AD.
Author contributions:
MGC was responsible for the literature review and writing of the paper.AST was responsible for the writing and editing of the paper.MGC and AST approved the final version of the manuscript.
Conflicts of interest:
There are no conflicts of interest.
Open access statement:
This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Neuroaxonal and cellular damage/protection by prostanoid receptor ligands,fatty acid derivatives and associated enzyme inhibitors
- Molecular approaches for spinal cord injury treatment
- Sex-biased autophagy as a potential mechanism mediating sex differences in ischemic stroke outcome
- Adipose tissue,systematic inflammation,and neurodegenerative diseases
- Interleukin-1:an important target for perinatal neuroprotection?
- Neurotrophic fragments as therapeutic alternatives to ameliorate brain aging

