Dysregulated expression and distribution of Kif5α in neurites of wobbler motor neurons
Kilian Kürten,Anne-Christin Gude,Aimo Samuel Christian Epplen,Jan Stein,Carsten Theiss,Veronika Matschke
Abstract Impaired axonal transport has been observed in patients with amyotrophic lateral sclerosis (ALS) and in animal models,suggesting that transport proteins likely play a critical role in the pathological mechanism of ALS.Dysregulation of Kinesin-family-member 5α (Kif5α),a neuron-specific isoform of heavy chain kinesin family,has been described in several neurological disorders,in humans and animal models,including ALS.In this study,we determined Kif5α expression by gene sequencing,quantitative reverse transcription-polymerase chain reaction,and western blot assay in the cervical spinal cord of wobbler mice and immunofluorescence staining in dissociated cultures of the ventral horn.Further,we observed the distribution of Kif5α and mitochondria along motor neuronal branches by confocal imaging.Our results showed that Kif5α expression was greatly dysregulated in wobbler mice,which resulted in altered distribution of Kif5α along motor neuronal branches with an abnormal mitochondrial distribution.Thus,our results indicate that dysregulation of Kif5 and therefore abnormal transport in motor neuronal branches in this ALS model could be causative for several pathological findings at the cellular level,like misallocation of cytoskeletal proteins or organelles like mitochondria.
Key Words:amyotrophic lateral sclerosis;cell culture;kinesin;mitochondria;neurodegeneration;spinal cord;transport
Introduction
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease that selectively affects the upper and lower motor neurons in the brain and spinal cord (Kiernan et al.,2011).It leads to ineluctable death within 2–5 years after the disease onset,usually due to respiratory failure (Bruijn et al.,2004).Approximately 5–10% of diagnosed ALS cases are familial with ALStypical mutations in several genes (Zou et al.,2017).ALS-typical mutations include a loss-of-function mutation in the kinesin family member 5A (KIF5A)gene (Brenner et al.,2018;Nicolas et al.,2018),leading to a disrupted Kif5α C-terminal sequence,potentially affecting the binding of specific cargo proteins (Naruse et al.,2021).
Kif5α is a neuron-specific isoform of a heavy chain kinesin (Aizawa et al.,1992;Niclas et al.,1994;DeBoer et al.,2008).This molecule carries out anterograde transport of proteins and organelles including a vast variety of vesicles and mitochondria from the perikaryon to the periphery (Hirokawa et al.,2010;Kamata et al.,2017).Studies on different model systems show the great importance of Kif5α for the long-distance axonal transport (Uchida et al.,2009;Wang et al.,2019) because mutations or manipulations on the expression of this protein lead to altered kinetics of transport,maldistribution of mitochondria,and subsequently degeneration of neurons (Campbell et al.,2014).Interestingly,impaired axonal transport was shown to be linked to mitochondrial dysfunction (Ferraiuolo et al.,2001;Moser et al.,2013).Kif5α loss-of-function mutations in the C-terminus of the protein have been recently detected in ALS patients (Brenner et al.,2018;Nicolas et al.,2018),indicating a link between mechanisms of altered cytoskeletal dynamics and the disease ALS (Taylor et al.,2016;Chia et al.,2018).Moreover,a decreased survival rate of motor neurons compared to less affected sensory neurons has been shown in Kif5α knockout mice.In particular,mitochondrial transport velocity and axonal growth decrease through the loss of Kif5a,affecting motor neurons more sverely (Karle et al.,2012).Inactivation of Kif5a,via a Cre-mediated postnatal loss,has been shown to lead to accumulation of neurofilaments (NF),NF-H (high molecular weight),NF-M (medium molecular weight),and NF-L(low molecular weight) in cell bodies of sensory neurons in mice (Xia et al.,2003).Interestingly,both ALS patients and wobbler mice (established animal model of ALS) show an altered distribution of phosphorylated NF in motor neurons,with accumulation at the perikaryon that does not differentiate between moderate and severe disease severity (Manetto et al.,1988;Pernas-Alonso et al.,2001).
In this study,we used the wobbler mouse,which is derived from a C57BL/Fa strain suffering from a spontaneous loss-of-function mutation in the vacuolar protein sorting-associated protein 54 (Vps54
) gene.TheVps54
gene encodes for a part of the Golgi-associated retrograde protein (GARP) complex that is involved in retrograde transport processes from the early and late endosomes to the trans-Golgi network (Schmitt-John et al.,2005).Only homozygous wobbler mice develop the wobbler phenotype,which is strikingly similar to that of ALS patients (Boillée et al.,2003;Moser et al.,2013;Ott et al.,2015).Three developmental phases of the disease can be observed in the wobbler mouse.While at presymptomatic phase (postnatal day 0–19 [p0–p19]) no clinical symptoms are obvious,wobbler mice start to show pathological symptoms 20 days after birth (evolutionary phase;p20-p39),beginning with muscle weakness at the front feet and typical head tremor,finally reaching back feet and tail.In addition,these mice are small in size compared to healthy littermates (Boillée et al.,2003;Ott et al.,2015).Finally,at the stable phase (from p40),the wobbler phenotype is fully developed.Remarkably,motor impairment and pathologies such as altered protein patterns that might lead to neuronal cell death occur in the cervical part of the spinal cord at certain phases of the disease without being altered in the lumbar region(Bastone et al.,2009;Moser et al.,2013).Since compromised axonal transport has been shown both,in ALS patients and in animal models like wobbler mice,these processes are crucial to understand the role of transport proteins in the pathomechanisms of ALS(Mitsumoto et al.,1990;Ferraiuolo et al.,2001;Pernas-Alonso et al.,2001;Kiernan et al.,2011;Moser et al.,2013).In the current study,we used the wobbler mouse to investigate the expression of Kif5a,which is involved in the transport of proteins and organelles,like NF and mitochondria,in the cervical spinal cord and more specifically the distribution of this motor protein in motor neurons of the animals at different phases of the disease.Our results may provide a valuable contribution to the understanding of the role of Kif5α in the development and evolution of ALS.
Methods
Animals
All animals were handled in accordance with the standards of the German federal state of North Rhine-Westphalia and the European Communities Council Directive 2010/63/EU on the protection of animals used for scientific purposes.According to current German and European legislation,the removal of organs or cells from vertebrates for scientific purposes is not considered an animal experiment if the animals have not been subject to surgical interventions or invasive treatments prior to sacrifice.Thus,the sacrifice of laboratory animals in this study is not subject to approval and does not have to be confirmed by any committee in Germany.
The mouse strain used was C57BL/Fa carrying the wobbler mutation.The mice were bred and genotyped as previously described (Ott et al.,2015).All mice were kept at a 12-hour day/night cycle in an open cage,in which food and water were availablead libitum
.Cervical spinal cord tissue (C1–C8)was collected under a microscope at three developmental phases:postnatal day (p) 0,p20,and p40.Four exclusively homozygous couples of wobbler and wild-type mice were sacrificed at each of the three time points for later experiments.Both genders were used.Quantitative reverse transcription-polymerase chain reaction
Total RNA (tRNA) was obtained from the cervical spinal cord tissue of healthy wild-type and wobbler mice at the time points mentioned above using the NucleoSpin miRNA Kit (Macherey-Nagel,Düren,Germany,Cat# 740971) in accordance with the original manufacturer protocol.cDNA was synthesized using Reverse Transcription System (Promega,Madison,WI,USA,Cat#A3500),as described in the manufacturer protocol using 1 µg tRNA and oligo(dT) primer.cDNA was stored at–20°C until use.Standard quantitative reverse transcription-polymerase chain reaction (qRT-PCR) was performed on a CFX96 Real-Time PCR Detection System (Bio-Rad,Hercules,CA,USA) using GoTag qPCR Master Mix (Cat# A6001,Promega),100 ng of cDNA per reaction,and 0.7 µM primer.The primer sequences forGapdh
were sense:5′-GGA GAA ACC TGC CAA GTA TGA-3′,and antisense:5′-TCC TCA GTG TAG CCC AAG A-3′.The primer sequences for Kif5α were sense:5′-GAA GGA GAA GGA GAA GAC CAA G-3′,and antisense:5′-CAG TCT CAG GCA CAT TCT CTC-3′.After each PCR run (see the manufacture protocol for“reaction protocols”).Melting curves regarding single PCR products were plotted.Expression levels of Kif5α and the housekeeping geneGapdh
were analyzed in triplicate.The 2method (Livak and Schmittgen,2001) was used to analyze obtained data.Western blot assay
Western blot assay was performed as previously described (Röderer et al.,2018).In brief,protein was isolated from cervical spinal cord tissue with RIPA buffer (150 mM NaCl,20 mM Tris-HCl,0.1% sodium dodecyl sulfate,1%Triton-X100,1% sodium deoxycholate,1 mM Na2EDTA).The concentrations of obtained protein were measured using the PierceBCA Protein Assay Kit (Thermo Fisher Scientific,Waltham,MA,USA,Cat# 23225).50 µg of total protein per lane was used.Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted onto nitrocellulose membranes.Following a blocking procedure at room temperature in 1×phosphate buffered saline (PBS) containing 1% RotiBlock (Roth,Karlsruhe,Deutschland,Cat# A151) for a minimum of 1 hour,primary rabbit polyclonal IgG anti-Kif5α antibody (1:500;Thermo Fischer Scientific,Cat# PA5-29820,RRID:AB_2547294) and secondary horseradish peroxidase-conjugated goat anti-rabbit IgG antibody (1:5000;Santa Cruz Biotechnology,Dallas,TX,USA,Cat# sc-2054,RRID:AB_631748) were used to detect Kif5α.To detect control protein actin,primary mouse polyclonal IgG anti-β-actin antibody(1:100;Sigma-Aldrich,St.Louis,MO,USA,Cat# A-2668,RRID:AB_258014)and secondary horseradish peroxidase-conjugated donkey anti-mouse IgG antibody (1:5000;Santa Cruz Biotechnology,Cat# sc-2314,RRID:AB_641170)were used.Protein bands were visualized with Western Blotting Luminol Reagent (Santa Cruz Biotechnology,Cat# sc-2048).The band intensity was quantified by arithmetic analysis using the ImageJ 1.53f51 software (U.S.National Institutes of Health,Bethesda,MD,USA).Data were normalized to actin.Normalized values of wobbler samples were compared with normalized wild-type samples at each phase of age and displayed as a percentage in a bar chart.
Molecular cloning and sequencing of mouse Kif5α
cDNA of homozygous wild-type and wobbler spinal cord was obtained as described above and used for sequencing.The sense primer sequences used are as follows:5′-AGA GCT GGT TTA GTG AAC CG-3′,5′-TGG ATG TGA TCG ATG AGG GG-3′,5′-GAG AAC GAT GCT GCG AAG G-3′,5′-AAG ACA AGG AGC CAG ACA CA-3′,5′-GTA TCA GCT ACA CCA ACA AC-3′ and 5′-CAC GCT CAT GTT TGG ACA G-3′.The homozygous wild-type and wobbler sequences were compared with the published sequence NM_001039000.4 with MView 1.63 (Brown et al.,1998;Additional Figure 1
).Dissociated cell culture of the ventral horn of the spinal cord
The dissociated spinal cord cell cultures were cultivated as previously described (Zwilling et al.,2020).In brief,the spinal cord was isolated at the age of p40.The meninges were carefully plucked off and the anterior horn was separated from the posterior horn.The anterior horn was chopped into small pieces.The tissue pieces were digested with papain (36 U/mL papain) isolation medium (self-prepared;0.5 mM GlutaMax,100 U/mL penicillin/streptomycin,2% B27 supplement in Hibernate A) for 10 minutes at 37°C.After trituration of the tissue,the isolated cells were separated by density gradient centrifugation on an OptiPrep(PROGEN Biotechnik GmbH,Heidelberg,Germany,Cat# 1114542) as previously described (Brewer and Toricelli,2007).The cells from fractions 2 and 3 were pelletized by centrifugation (244 ×g
for 6 minutes at 10°C) and plated in motor neuron feeding medium [selfprepared;30% C2C12 myocyte-conditioned medium (Zwilling et al.,2020),0.5 mM glutamine,100 U/mL penicillin/streptomycin,2% B27 supplement,125 mM cAMP,1 ng/mL brain-derived neurotrophic factor (BDNF),0.1 ng/mL glial cell line-derived neurotrophic factor (GDNF) in Neurobasal A] at a density of 70,000 cells per well onto poly-D-lysine (50 µg/mL)coated cover glasses.The cells were cultivatedin vitro
for 10 days at 37°C and 5% COwith medium change with motor neuron feeding medium after 2–3 days.Immunofluorescence staining and confocal laser scanning microscopy
To investigate the distribution of Kif5α along the motor neuronal neurites,dissociated motor neuronal cell cultures were fixed after 10 daysin vitro
with 4% paraformaldehyde (PFA) in PBS for 10 minutes at room temperature.For immunostaining,cultures were permeabilized with 0.3% Triton-X 100 in PBS for 10 minutes before blocking with 5% goat serum in PBS for 30 minutes at room temperature.The cells were further incubated with the primary antibodies (1:500,mouse Tuj1 antibody,R&D systems,Minneapolis,MN,USA,Cat# MAB1195,RRID:AB_ 357520;1:200,rabbit Kif5α antibody,Thermo Fischer Scientific,Cat# PA5-29820,RRID:AB_2547294) at 4°C overnight.The samples were then reacted with secondary antibodies for 2 hours at room temperature (1:400,anti-mouse-AlexaFluorPlus594,Thermo Fisher Scientific,Cat# A32744,RRID:AB_2 762826;1:300,anti-rabbit-AlexaFluor488,Thermo Fisher Scientific,Cat# A11008,RRID:AB_143165).Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI,1 µg/mL;Sigma-Aldrich,Cat# D9542).Finally,slides were rinsed in PBS and cover slipped in fluoromount (Cat#F4680,Sigma-Aldrich).To investigate the distribution of mitochondria in motor neuronal cells,CellTracker (10 µM,Thermo Fisher Scientific,Cat# C7025) and MitoTracker(100 nM,Thermo Fisher Scientific,Cat# M7512) were used.Samples were imaged using an inverted confocal Laser Scanning Microscope (LSM 800,Carl Zeiss Microscopy GmbH,Jena,Germany) equipped with the respective filter sets in combination with a 40× objective (Plan-Apochromat 40×/1.4 Oil,Carl Zeiss Microscopy GmbH).The settings of the recordings were not changed between the individual phenotypes.Secondary antibodies were tested for specificity and showen no unspecific binding.
Analysis of Kif5α immunofluorescence staining
Tuj1-positive axons of motor neuronal cells were measured using Zeiss ZEN blue software (Carl Zeiss Microscopy GmbH,Jena,Germany).Within each axon,Kif5α positive signals were evaluated.To compare the number of kinesins between wild-type and wobbler samples,Kif5α positive signals were normalized to the length of the axon.The counting was performed in a double assessment in a blind study.To investigate the distribution of kinesin molecules along the axon,the length of each axon was set to 100% and the distribution of Kif5α signal counts was compared between the genotypes.
Statistical analysis
Data were statistically analyzed with GraphPad Prism 6.1 software (GraphPad Software,San Diego,CA,USA).The Kolmogorov-Smirnov normality test was used to confirm normal distribution.Data are expressed as means ± SEM.Data were tested for significance using the Student’st
-test.Results withP
<0.05 were considered statistically significant.Results
Dysregulated Kif5α expression in cervical spinal cord of symptomatic wobbler mice
We utilized qRT-PCR and western blotting to detect possible dysregulation of Kif5α expression at mRNA and protein levels during three developmental phases of wild-type and wobbler mice.Gene expression ofKif5α
was dysregulated at p20 and p40 (Figure 1A
).At p0,there was no significant difference in gene expression level between wild-type and wobbler mice(Figure 1A
).A significant upregulation ofKif5α
mRNA was found to be present in the cervical part of the spinal cord of wobbler mice at p20,whereas a highly significant downregulation was observed at p40,compared with wildtype mice (Figure 1A
).These findings were confirmed by blotting performed at p40 at protein level.However,at p20,no dysregulation of Kif5α protein level was shown (Figure 1B
andC
).No mutations in Kif5α gene of wobbler mice
To rule out aKif5α
mutation as a cause for possible dysregulation in wobbler mice,we performed sequencing ofKif5α
in wild-type and wobbler mice.There was no difference in the sequence ofKif5α
between homozygous wild-type and wobbler sequences (Additional Figure 1
).It can therefore be excluded that a mutation is causative for the dysregulation observed at the mRNA and protein levels in the cervical spinal cord of wobbler mice.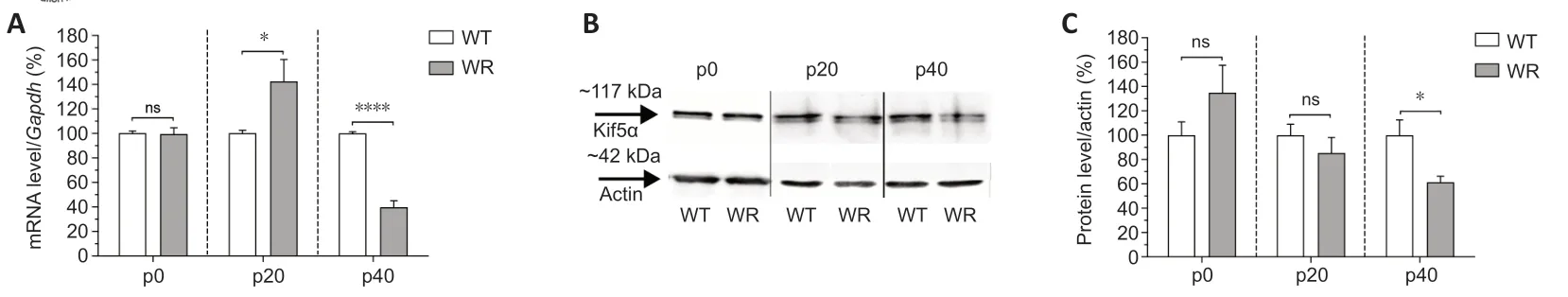
Figure 1 | Age-dependent differential expression of Kif5α in the cervical spinal cord of the wobbler mouse.
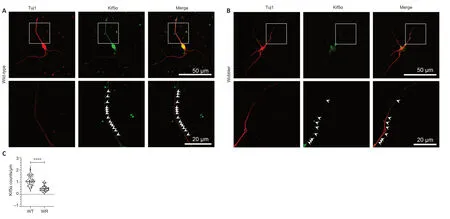
Figure 2 | Decreased Kif5α signals in neurites of motor neurons of wobbler mice at the stationary phase.
Altered Kif5α signals in the neurites of wobbler motor neurons
Due to their long axons,motor neurons are extremely dependent on axonal transport.As they are specifically affected by ALS,they are a very important target for further investigation.Since dysregulation of Kif5α expression in clinical wobbler mice was dysregulated at p40,we investigated the possible intracellular expression differences caused by this dysregulation using immunofluorescence staining and confocal laser microscopy.We found significantly lower kinesin spots in wobbler motor neuronal neurites compared with wild-type neurites (Figure 2
).The signal distribution of Kif5α in wild-type and wobbler neurites generally followed the same trend,with the neurites of wobbler mice uniformly showing fewer kinesin spots and accumulations (Figure 3
).It becomes obvious that kinesin signals could be detected as accumulations of molecules along the motor neuronal neurite of wild-type mice.The number of signals in the neurites of wobbler motor neurons was generally lower and accumulations occurred in a smaller quantity and did not reach the growth cone.Since existing literature suggests that Kif5α is important for mitochondrial transport and distribution,we performed experiments to qualitatively investigate the distribution of mitochondria in motor neurons of both genotypes at p40 (Additional Figure
2
).While mitochondria in wild-type motor neurons are distributed relatively homogeneously over all branches with accumulations along the neurites(Additional Figure 2A
),first hints could be gained on an altered distribution of mitochondria in Wobbler cells (Additional Figure 2B
).However,these results still need to be supported by further studies.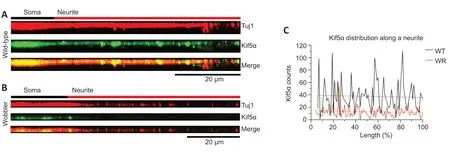
Figure 3 | Altered signals of kinesin spots in motor neuronal neurites of wobbler mice.
Discussion
Impaired axonal transport plays a crucial role in the pathogenesis of ALS and various ALS models (Mitsumoto et al.,1990;Taylor et al.,2016;Brenner et al.,2018;Chia et al.,2018;Nicolas et al.,2018).The neuron-specific kinesin subunit Kif5α is therefore of special interest for investigation of ALS,a degenerative disease of the motor neuron system.
It is known that impairment of Kif5α leads to different pathological conditions like,(I) transport disorders and NF accumulation (Xia et al.,2003),(II)decrease in mitochondrial transport quantity and velocity (Karle et al.,2012;Wang et al.,2019),and (III) persistence of mitochondria in the main stem of the axons combined with a general intracellular decrease of mitochondria(Campbell et al.,2014;Stein et al.,2021).Therefore,Kif5α seems to be not only part of a potentially impaired neuronal intracellular transport but also a reason for other intracellular impairments such as NF accumulation and an impaired mitochondrial distribution process.
Our results reveal that wobbler mice did not show any dysregulation of Kif5α expression at birth.It can be assumed that the effects of the wobbler mutation at birth had not yet caused extensive cellular impairments,thus not altering the expression levels of Kif5α.Many of the factors investigated in wobbler mice also showed no differences in expression at this stage of age(Pernas-Alonso et al.,2001;Moser et al.,2013;Saberi et al.,2016).These findings correlate with the fact that newborn wobbler mice did not show any symptoms (Boillée et al.,2003).
At p20,we showed that wobbler mice still did not show severe symptoms,however,at the molecular level,a significant upregulation of Kif5α mRNA was detected compared to wild-type mice.Nevertheless,the protein level was not altered.There could be several reasons for this phenomenon.On one hand,it could be a compensation reaction in which a reduced protein level was to be balanced by increased mRNA expression.On the other hand,dysregulated miRNAs might also play a role in the translation control of Kif5α,as have been shown for other molecules in the wobbler mouse (Klatt et al.,2019;Rohm et al.,2019).A recent study could show an upregulation of Kif5 via miRNA when distal axon growth is inhibited through chondroitin sulfate proteoglycans (Li et al.,2019).Similar upregulation might be induced by impaired pathways due to the wobbler mutation.
In the current study,at the symptomatic phase,a significant downregulation of Kif5α was shown at both mRNA and protein levels,although Kif5α does not reach the growth cone either.At this point,cellular impairment was observed in motor neurons (Boillée et al.,2003),suggesting a correlation between the continuous aggravation of Kif5α synthesis and motor neuron degeneration.
We showed reduced expression of Kif5α in the cervical spinal cord which was preceded by increased mRNA expression during the evolutionary phase (p20).Since Kif5α in cerebrospinal fluid has already been used as an experimental biomarker to monitor the severity of multiple sclerosis (Hares et al.,2021),similar trials could be conducted among family members of patients with familial ALS.In this way,it might be possible to detect pathological changes before symptoms appear and initiate treatment at an early phase.Even though the wobbler mutation affects the process of vesicle tethering ubiquitously and is causing cellular impairment not exclusively in motor neurons,they are especially affected (Moser et al.,2013).Since motor neurons are extremely large cells,reaching over extremely long distances,dysregulation of the neuron exclusive subunit Kif5α can affect them more severely,similar to the decrease of motor neurons inKif5α
knockout mice(Karle et al.,2012).Our results showed a reduced expression level of Kif5α in spinal cord of wobbler mice and abnormal Kif5α distribution that did not extend into the growth cone.Accordingly,our very first results showed a decreased quantity of mitochondria along the neurites of Wobbler motor neurons,which also did not reach the distal parts of the observed branches.We speculate that the deficiency of Kif5α might be responsible for a maldistribution and reduced quantity of mitochondria in motor neuronal branches.This is in line with the observations described above in theKif5α
knockout model suffering from the generally reduced number of mitochondria and their persistence at the stem axon (Campbell et al.,2014).Dysfunctional mitochondria are found in wobbler mice,which further deteriorate in the stable phase (Xu et al.,2001;Dave et al.,2003).It is possible that mitochondrial dysfunction is caused by maldistribution due to impaired Kif5α regulation,possibly exacerbated by the reduced rate of mitochondrial transport,which was not investigated in this study,but was described inKif5α
knockout models (Karle et al.,2012).Since mitochondria enable the axon to extend,branch,and regenerate (Smith et al.,2019),their decline in the neuronal periphery and growth cone was likely to further deteriorate neurodegeneration.In addition to mitochondria,other cellular components may also suffer from Kif5α deficiency,such as the cytoskeleton.NF are essential for axonal structural stability and their radial growth (Yuan et al.,2012) and provide as part of the cytoskeleton structural stability to the neuron and its organelles(Yuan et al.,2017).If NF are no longer transported along the axon due to a malfunction or an actual decrease of Kif5α in the neuron periphery,they have a higher risk of degeneration (Yuan et al.,2012).In particular,the stability of neurons with large axons,such as motor neurons,is at risk.Recent studies show that detection of the light subunit of NF-L in cerebrospinal fluid or plasma is indicative of axonal damage and neuronal cell death (Brureau et al.,2017) in ALS and Alzheimer’s disease (Loeffler et al.,2020).In this context,the increase in NF-L in cerebrospinal fluid reliably indicates ALS progression(Loeffler et al.,2020;Sun et al.,2020).
An accumulation of NF in motor neurons was described as a hallmark of ALS in humans (Lu et al.,2011) and was shown inKif5α
knockout models (Xia et al.,2003;Karle et al.,2012).Kif5α is of particular importance for the transport of these cytoskeletal proteins in neurites (Uchida et al.,2009).In consequence,the decrease of Kif5α quantity and its altered distribution along the neurites of wobbler motor neurons at the stationary phase shown in this study likely affect NF distribution.Our findings therefore might explain the accumulation of NF in cell bodies and axonal swellings ofKif5α
knockout models (Karle et al.,2012;Nicolas et al.,2018) and at the perikaryon of neurons in wobbler mice (Ott et al.,2017).We showed for the first time that Kif5α was not dysregulated due to a mutation in the correspondingKIF5α
gene in wobbler mice.However,its dysregulation may be a critical secondary effect of the wobbler mutation in theVps54
gene,although a link between Vps54 functionality and dysregulation of Kif5α remains elusive.This secondary effect may lead to an impaired distribution of organelles such as mitochondria in neurites of motor neurons (Additional Figure 2
) and might also be responsible for the known accumulation of NF in wobbler mice and ALS patients,leading to neurodegeneration.A recent study on a Charcot Marie Tooth model revealed that compensation for the loss of Kif5α dependent cargo inhibited axonal degeneration (Fukuda et al.,2021).If Kif5α impairments are a common denominator of motor neuron degeneration despite different origins,new therapeutic opportunities for ALS can be created by compensating the loss of function of Kif5α.Our data showed for the first time dysregulated expression and distribution of Kif5α in wobbler motor neurons.Furthermore,we could demonstrate that mitochondria,transported in particular by Kif5α,are differentially distributed in motor neurons of wobbler mice.Thus,our results indicate that dysregulation of Kif5α and therefore abnormal transport in motor neuronal branches in the wobbler mouse model of ALS may be causative for many pathological findings at the cellular level,e.g.a misallocation or abnormal distribution of cytoskeletal proteins or organelles such as mitochondria.Such a misdistribution of important cargos,especially mitochondria,can result in abnormal metabolic function and further degeneration.Accordingly,our results are in line with current publications that reveal Kif5 to cause certain forms of fALS (Brenner et al.,2018) or to be associated with ALS emphasizing the importance of Kif5 as a significant target for further research (Nicolas et al.,2018).
Acknowledgments:
The authors gratefully acknowledge Anke Lodwig,Claudia Grzelak,and Anja Harbecke for technical assistance,as well as Aja Lenz for secretarial work.We acknowledge support by the Open Access Publication Funds of the Ruhr-Universität Bochum.
Author contributions:
Study conception and design:VM;work supervision:VM,CT;experiment implementation:KK,ACG,ASCE,JS,VM;data analysis and validation:KK,ACG,VM;figure and table creation and manuscript drafting:KK,VM;providing critical revision and editing the manuscript:CT.All authors have read and approved the final version of this manuscript.
Conflicts of interest:
The authors declare that they have no conflicts of interest.
Availability of data and materials:
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement:
This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional files:
Multiple sequence alignment of WT,WR,and published Kif5α sequence NM_001039000.4.
Altered distribution of mitochondria in motor neuronal neurites of wobbler mice.
- 中国神经再生研究(英文版)的其它文章
- Neuroaxonal and cellular damage/protection by prostanoid receptor ligands,fatty acid derivatives and associated enzyme inhibitors
- Extracellular vesicles in Alzheimer’s disease:from pathology to therapeutic approaches
- Molecular approaches for spinal cord injury treatment
- Sex-biased autophagy as a potential mechanism mediating sex differences in ischemic stroke outcome
- Adipose tissue,systematic inflammation,and neurodegenerative diseases
- Interleukin-1:an important target for perinatal neuroprotection?

