Pointing fingers at blood contact:mechanisms of subventricular zone neural stem cell differentiation
Subash C.Malik,Yu-Hsuan Chu,Christian Schachtrup
The limited ability of the central nervous system(CNS) to regenerate in adult mammals after injury or disease is a significant problem.Intriguingly,neural stem/progenitor cells (NSPCs) offer great promise for regenerating the CNS.Endogenous or transplanted NSPCs contribute to repair processes,but their differentiation and function are abnormal in CNS injury and disease.The main reasons for these abnormalities are changes in the extracellular environment in the injured CNS that affect signaling pathways and transcriptional regulation in NSPCs.In CNS disease with vascular permeability or blood-brain barrier disruption,blood-derived fibrinogen enters the parenchyma and drastically changes the extracellular environment of brain cells,including NSPCs.Fibrinogen is present in the brain in a wide range of CNS pathologies,such as multiple sclerosis,Alzheimer’s disease,stroke,and traumatic brain injury.Here,within this perspective,we focus on how the blood-derived coagulation factor fibrinogen alters the subventricular zone (SVZ)stem cell niche environment to activate the bone morphogenetic protein (BMP) receptor (BMPR)signaling pathway in NSPCs.The activated BMPR signaling increases p75 neurotrophin receptor(p75) and inhibitor of DNA binding 3 (Id3)abundance in NSPCs,and thus,regulates NSPC migration and differentiation in a mouse model of cortical ischemic stroke (photothrombotic ischemia) and cortical brain trauma (stab wound injury) (Pous et al.,2020;Deshpande et al.,2021).NSPCs located in the adult mammalian SVZ are an endogenous source for cell replacement and brain repair.The fine-tuned cellular and molecular niche environment controls the cardinal features of the SVZ NSPCs:an unlimited capacity for self-renewal,indefinite ability to proliferate,and multipotency for the different neuroectodermal lineages of the CNS.Pathological states induce dynamic changes in this niche and trigger a regenerative response,but the regulatory mechanisms that control NSPC differentiation in CNS disease are largely unknown.In contrast to the human brain SVZ,where production of new neurons is highly reduced by 2 years of age and little to no neurogenesis is observed after childhood,the adult rodent brain SVZ continuously produces new neurons throughout life and reacts to CNS injury and disease.Therefore,the adult rodent SVZ is ideally suited for studying cellular signaling cascades and transcriptional programs in adult NSPCs and for identifying potential pharmacological and regenerative cell-based therapies for neuronal regeneration.Improved control of the endogenous or transplanted NSPC fate and functions will provide optimized therapeutic effects by replacement of lost neurons and severed axons and creation of a permissive microenvironment to promote CNS tissue repair.
The SVZ stem cell niche:a vulnerable site for pathological states?
Determining the identity and principal source of specific fluid-borne signals within the adult SVZ stem cell niche could provide a tremendous boost for unlocking plasticity for brain repair.SVZ NSPCs have a unique cytoarchitecture and specialized niche vasculature that allow them to contact components from the cerebrospinal fluid (CSF) and circulating blood(Tavazoie et al.,2008;Silva-Vargas et al.,2016).In addition to the NSPCs,the adult SVZ stem cell niche includes the ependymal cell layer lining the ventricle,a specialized extracellular matrix (e.g.,fractones),and an extensive planar vascular plexus(Obernier and Alvarez-Buylla,2019).Quiescent NSPCs (B1 cells) extend a single primary cilium into the lateral ventricle contacting the CSF on their apical side and a long process to the endothelial cells of the vascular plexus on their basal side.Activated type B1 stem cells continuously generate mobile doublecortin+neuroblasts (young,immature neurons) via proliferating Mash1+type C transit-amplifying cells in the SVZ.Neuroblasts are able to leave the SVZ and migrate long distances through the rostral migratory stream to the olfactory bulb to become γ-aminobutyric acid-releasing inhibitory interneurons,including granule cells and periglomerular cells,important for odor detection and discrimination behaviors.In addition,type B1 stem cells are capable of,besides the large fraction of interneurons,continuously generate a small proportion of glia in the healthy brain (Obernier and Alvarez-Buylla,2019).We were intrigued by the unique situation of adult SVZ NSPCs,and so,we revisited the SVZ as a sensor for environmental changes under pathological states.We found that,immediately after a distant cortical injury,large amounts of the blood-derived coagulation factor fibrinogen were deposited inside the entire SVZ stem cell niche,and the fibrinogen induced the BMP signaling pathway in NSPCs (Pous et al.,2020).Notably,among NSPCs in the SVZ,BMP-2 expression was greatly increased after cortical injury (Deshpande et al.,2021),and so,early fibrinogen leakage into the SVZ stem cell niche environment might be the primary signal that triggers expression and activation of components along the BMP signaling pathway.Thus,early and robust changes of the SVZ environment with deposition of a provisional fibrin matrix implicate the stem cell niche as a vulnerable indicator of pathological states.
BMPR signaling in the SVZ stem cell niche:a key for NSPC control?
BMPR signaling maintains adult neural stem cell niches and neurogenesis at basal level and inhibits neurogenesis at an elevated level.Understanding the underlying mechanisms might enable a targeted manipulation of NSPC fate and functionality.Increased BMPR signaling in the SVZ controls NSPC differentiation and migration (Pous et al.,2020;Deshpande et al.,2021).By enhancing BMPR type I association in lipid rafts through its αC domain-β1-integrin binding,fibrinogen enhances BMPR signaling in a ligand-independent manner and directs lineage specification of SVZ NSPCs into newborn astrocytes(Pous et al.,2020).Furthermore,increased levels of BMPR-signaling induces SVZ-derived NSPCs and their progeny to migrate towards the lesion area(Deshpande et al.,2021).BMPR signaling regulates the levels of p75in NSPCs and genetic depletion of p75in mice reduced SVZ NSPC migration towards the lesion area after cortical injury.p75,a member of the tumor necrosis factor receptor superfamily,participates in multiple intracellular signaling pathways to regulate a wide range of biological functions that promote or inhibit the overall process of tissue repair (Malik et al.,2021).Increased p75expression results in cytoskeletal organization necessary for NSPC migration and equips the SVZ NSPCs to sense the brain-derived neurotrophic factor radiant in the lesion area and to redirect their migration path towards the cortical lesion.Our data suggest that p75controls Rho GTPase activating and inhibiting proteins in adult SVZ NSPCs.Rho family proteins are active in response to extracellular signals,including soluble cytokines,growth factors,and neurotrophins.Full-length p75in SVZ NSPCs may be a sensor for increased cortical lesion brain-derived neurotrophic factor gradients to modulate Rho family activity and facilitate the SVZ NSPC cytoskeletal rearrangement necessary for migration.Yet,the underlying mechanisms of how p75regulates Rho GTPase activating and inhibiting proteins and the cytoskeletal rearrangements in NSPCs are unknown and will be an attractive research topic.We suggest that BMPinduced p75abundance regulates the migration of SVZ-derived NSPCs and their contributions to regeneration at injury sites (Deshpande et al.,2021).Overall,our data revealed that early and robust changes of the stem cell niche environment with deposition of the provisional fibrin matrix alters the magnitude of BMPR signaling and orchestrates an SVZ-originating astrogenic response contributing to the regeneration process in CNS disease.Linking environmental changes with NSPC function:
The Id transcriptional regulator:Reprogramming NSPC differentiation and functionality by modulating transcription is a promising way to promote CNS regeneration.However,the transcriptional network that regulates those actions in SVZ NSPCs under pathological conditions is only poorly understood.Pioneering studies identified the basic helixloop-helix (bHLH) transcription factor (TF) Olig2 as a repressor of neurogenesis in CNS disease.Antagonizing Olig2 functionin vivo
resulted in a significant number of immature neurons(Buffo et al.,2005).Ectopic expression of Olig2 in adult ependymal cells,which are NSPCs in the spinal cord,led to efficient production of oligodendrocytes enabling spinal cord repair(Llorens-Bobadilla et al.,2020).These findings support the concept that targeting the bHLH TF protein family might be an attractive avenue for harnessing endogenous NSPCs for repair.Inhibitors of DNA binding proteins function as dominant-negative regulator of bHLH TFs and are thus critical players that control stem cell function (Chu et al.,2021).Our results showed that early and robust changes to the stem cell niche environment trigger Id expression in NSPCs and orchestrate the SVZ-originating astrogenic response.Specifically,we found that BMPR signaling-induced Id3 expression in the stem cell niche promotes NSPC differentiation into astrocytes by restraining the transcriptional activity of the bHLH TF E47 (Bohrer et al.,2015;Pous et al.,2020).In addition to astrocyte-specific genes (e.g.,glial fibrillary acidic protein and GLAST (also known asSlc1a3
)),the Id3-E47 axis regulates several solute carrier (SLC) family members (e.g.,Slc1a2
,Slc25a18
,Slc38a3
,Slc39a14
,Slc7a11
),suggesting a potential role of Id proteins in regulating NSPC homeostasis and metabolism upon environmental changes.The functional diversity of each Id member under different diseases remains to be investigated,but will probably be derived from their conformational flexibility and preference to interact with divergent binding partners.Yet,the power of Id proteins to orchestrate altered environmental cues into changes of a repertoire of gene expression and cellular activity implies a potential of cell-and/or disease-dependent modification of specific Id members as a promising therapeutic intervention (Chu et al.,2021).Interestingly,blood-derived fibrinogen activates BMP signaling in oligodendrocyte progenitor cells inhibiting oligodendrocyte differentiation in multiple sclerosis (Petersen et al.,2017).While Id3 upregulation in NSPCs induces astrogliogenesis(Bohrer et al.,2015;Pous et al.,2020),Id2 upregulation in oligodendrocyte progenitor cells might inhibit oligodendrocyte differentiation (Chu et al.,2021).Overall,our findings show that early and robust changes of the stem cell niche environment with deposition of the provisional fibrin matrix alters the magnitude of BMPR signaling orchestrating a transcriptional network that mediates the SVZoriginating regenerative response in CNS disease.
Perspective:
In our studies,we showed that the adult mammalian SVZ stem cell niche is a primary site of vulnerability to CNS injury and disease and that fibrinogen is rapidly deposited in the SVZ stem cell niche environment and alters SVZ NSPC behavior (Figure 1
).Fibrinogen’s pleiotropic roles (e.g.,activating CNS inflammation,inducing scar formation,and modulating neural stem cell behavior) place it at the nexus of coagulation,immunity,and regeneration processes at the brain-vascular interface.Fibrinogen-regulated NSPC-derived astrogenesis from the SVZ niche might have unique,beneficial effects in the lesion area,such as contribution to ‘glial scar formation’and ‘cell replacement’ as well as ‘neuroprotection’and ‘immunomodulation’,which overall promote the repair process.On the other hand,massive fibrinogen deposition in the stem cell niche might be a sensitive indicator for detrimental effects and potential initiation of CNS disease.Microglia are early responders to the altered environment,and the SVZ niche is susceptible to T-cell infiltration in aged mice and humans(Dulken et al.,2019).Early changes of the SVZ niche environment might lead to changes in SVZ niche cells,e.g.,microglia and ependymal cells that affect the CSF composition and long-range communication,such as reducing endothelial cell barrier properties and inflammation (Figure 1
).In addition,misguided BMP signaling triggered by excess fibrinogen deposition in the SVZ leads to Id and p75upregulation (Bohrer et al.,2015;Deshpande et al.,2021).Misregulation of Id family members and p75are connected to the selfrenewal and tumor-initiating capacity of stem cells and tumor invasion,respectively (Johnston et al.,2007;Lee et al.,2016).SVZ NSPCs are suspected to be the origin of glioblastoma (Lee et al.,2018),and misregulated Id or p75expression due to an altered SVZ environment might contribute to glioblastoma formation (Figure 1
).However,the cell population of origin for glioblastoma and the contribution of an altered extracellular environment besides genetic alterations are still under debate and will be the content of future research directions.Overall,these observations suggest several interesting research questions:How do early environmental changes in the SVZ affect local SVZ cell-cell communications,such as reciprocal interaction between SVZ NSPCs and microglia? Does the altered SVZ cell-cell communication contribute to peripheral immune cell infiltration and disease initiation? Can we apply our knowledge about identified molecular pathways involved in the differentiation of rodent adult brain SVZ NSPCs to control fate and functions of transplantable NSPCs tailored to promote CNS repair in humans (Figure 1
)?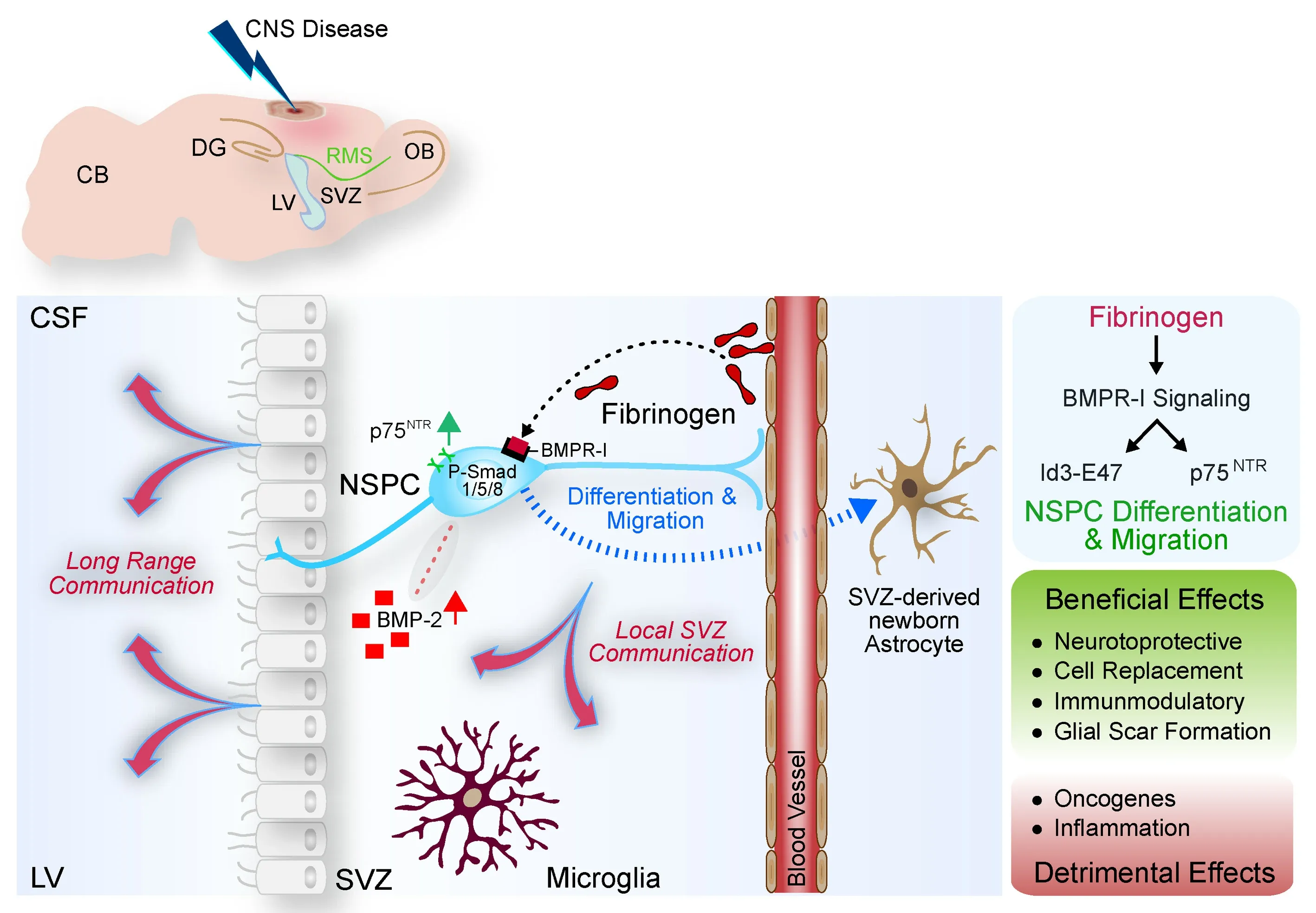
Figure 1 | The SVZ is a vulnerable site in CNS disease.
Future studies will shed light on the mechanisms of how a vulnerable SVZ stem cell niche instructs NSPC behavior and local and long-range communication and how these mechanisms might be harnessed to promote CNS repair.
We are grateful to Andreas Schober for graphics,to Gary Howard for editing and for support from the German Research Foundation Grants SCHA 1442/8-1,and 1442/9-1 to CS.
Subash C.Malik,Yu-Hsuan Chu,Christian Schachtrup
Institute of Anatomy and Cell Biology,Faculty of Medicine,University of Freiburg,Freiburg,Germany (Malik SC,Chu YH,Schachtrup C)Faculty of Biology,University of Freiburg,Freiburg,Germany (Malik SC,Chu YH)
Center for Basics in NeuroModulation(NeuroModulBasics),Faculty of Medicine,University of Freiburg,Freiburg,Germany(Schachtrup C)
Christian Schachtrup,PhD,christian.schachtrup@anat.uni-freiburg.de.https://orcid.org/0000-0001-9851-6299(Christian Schachtrup)
Date of submission:
October 25,2021Date of decision:
December 23,2021Date of acceptance:
January 14,2022Date of web publication:
May 31,2022https://doi.org/10.4103/1673-5374.338998
Malik SC,Chu YH,Schachtrup C (2023) Pointing fingers at blood contact:mechanisms of subventricular zone neural stem cell differentiation.Neural Regen Res 18(1):137-138.
Availability of data and materials:
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement:
This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:
Kyle D.Fink,University of California Davis Health System,USA.
Additional file:
Open peer review report 1.
- 中国神经再生研究(英文版)的其它文章
- Neuroaxonal and cellular damage/protection by prostanoid receptor ligands,fatty acid derivatives and associated enzyme inhibitors
- Extracellular vesicles in Alzheimer’s disease:from pathology to therapeutic approaches
- Molecular approaches for spinal cord injury treatment
- Sex-biased autophagy as a potential mechanism mediating sex differences in ischemic stroke outcome
- Adipose tissue,systematic inflammation,and neurodegenerative diseases
- Interleukin-1:an important target for perinatal neuroprotection?

