Characterization of retinal ganglion cell damage at single axon bundle level in mice by visible-light optical coherence tomography fibergraphy
Xiaorong Liu,Hao F.Zhang
Retinal ganglion cells (RGCs) receive synaptic inputs through their dendritic trees in the inner plexiform layer (IPL) and convey the visual information via their axons which form the optic nerve to the brain (Sanes and Masland,2015).In glaucoma,RGCs and their axons degenerate and die,leading to irreversible vision loss and eventually blindness if left untreated (Quigley,2016).The self-destructive programs in RGCs induced by glaucomatous insults are often spatially compartmentalized (Syc-Mazurek and Libby,2019),which results in changes in the IPL,the ganglion cell layer,and the retinal nerve fiber layer (RNFL) before cell death in humans and rodents (Wollstein et al.,2012;Chen et al.,2015;Grannonico et al.,2021).Characterizing RGC morphological changes is thus potentially pertinent for timely intervention to preserve RGCs and vision,but much remains unknown to establish a sensitive and specific marker of RGC damage for better diagnosis and management of glaucoma.
Among the non-invasive imaging techniques,near-infrared optical coherence tomography(OCT) can objectively measure the RNFL and the ganglion cell-inner plexiform layer(GCIPL) for assessing glaucoma progression(Schuman et al.,2020).However,two main challenges remain to be tackled before establishing reliable demarcation of RGC damage with high sensitivity and specificity.First,the commonly used parameters such as the RNFL or GCIPL thinning are not sensitive enough to reflect RGC damage,especially at the early stage of glaucoma.For example,detectable RNFL thinning by clinical OCT often reflects an already significant and irreversible RGC damage in glaucomatous eyes (Chen et al.,2015;Yi et al.,2016).In addition,the ganglion cell layer contains non-RGC cells,the displaced amacrine cells (Sanes and Masland,2015),which are largely unaffected by glaucoma insult (Quigley,2016).The IPL consists of synaptic connections among bipolar cells,amacrine cells,and RGCs,and the glaucoma insults induced sublaminar changes of the IPL could reflect changes in the morphology and function of inner retinal neurons (Chen et al.,2015;Ghassabi et al.,2022).In other words,the GCIPL thickness is not a specific indicator for RGC damage.Second,the axial resolution of existing clinical OCT varies from 5 to 10 µm,which is not sufficiently high to measure minute retinal layer thinning.The lack of high optical contrasts also makes it difficult to visualize the damage of RGC axon bundles directly (Shu et al.,2017).
Because every RGC extends one axon in the RNFL,we seek to directly quantify changes of individual RGC axon bundles for RGC damage rather than the bulk thickness of RNFL or GCIPL.As discussed above,the current near-infrared OCT systems,operating at center wavelengths from 830 nm to 1550 nm,cannot satisfy the needs for spatial resolution and imaging contrast.We have applied visible-light OCT (vis-OCT),operating from 510 nm to 610 nm,with the axial resolution reaching 1.3 µm in the retina (Shu et al.,2017;Miller et al.,2020).Within the visible-light spectral range,optical scattering contrast is much higher than the near-infrared spectral range in biological tissue (Miller et al.,2020).As a result,vis-OCT offers new anatomical and functional imaging capabilities to improve RGC damage evaluation (Shu et al.,2017).We recently developed vis-OCT fibergraphy (vis-OCTF)to visualize and quantify RGC axon bundles in mice (Miller et al.,2020;Beckmann et al.,2021;Grannonico et al.,2021;Figure 1
).First,we quantitatively validated the RGC axon bundle networks imaged byin vivo
vis-OCTF usingex vivo
confocal microscopy.We matched fiber-to-fiber between vis-OCTF and confocal microscopy results of the same retinas and confirmed vis-OCTF’s sensitivity to visualize RGC fiber bundles with a minimum diameter of 2.5 µm (Miller et al.,2020).We applied vis-OCTF to examine the developmental changes in wild-type mice(Beckmann et al.,2021).We introduced a resampled circumpapillary B-scan averaging technique to improve the inter-layer contrast,enabling the evaluation of the retinal layer thickness right after eye opening at postnatal day 13 (P13) (Beckmann et al.,2021).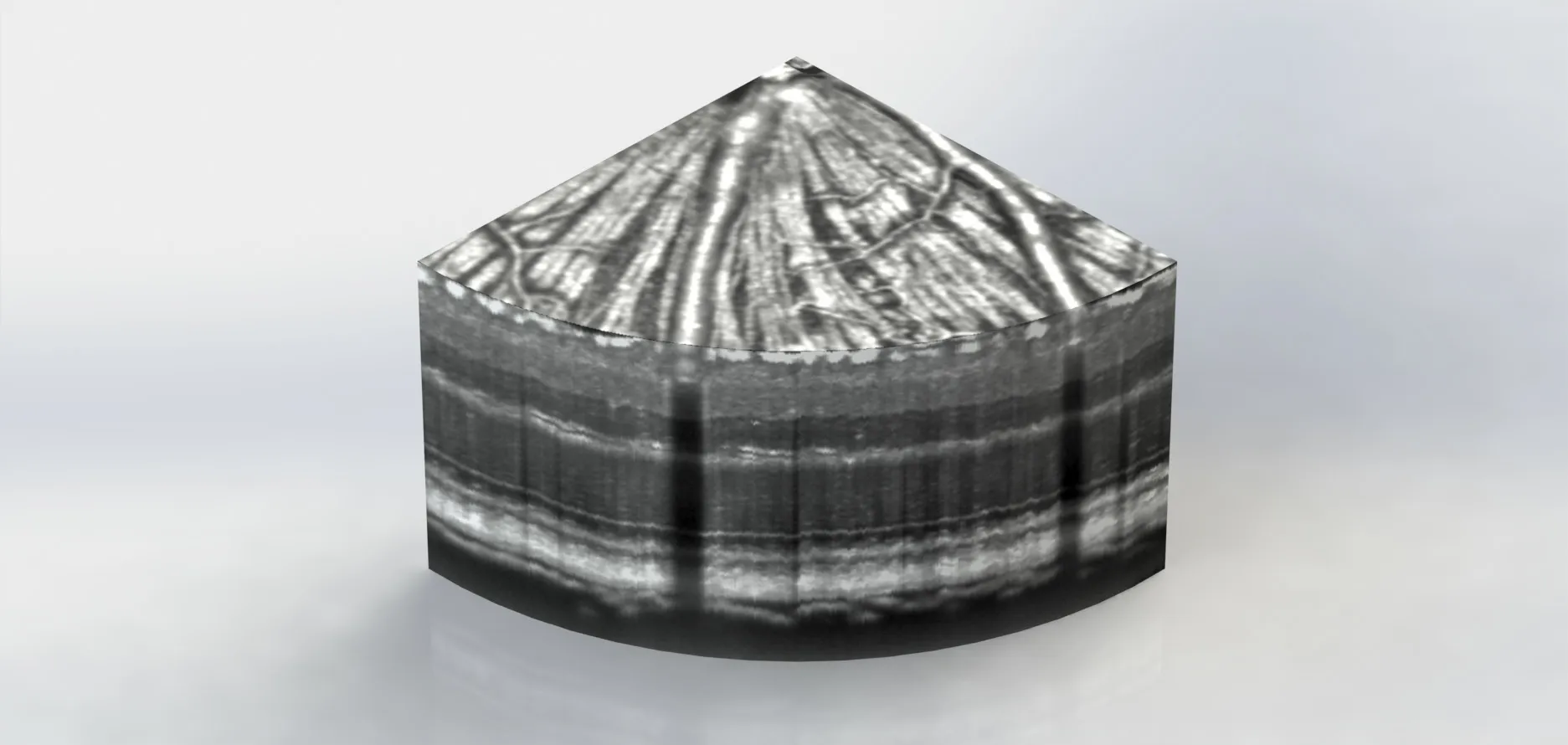
Figure 1 | This slice of the mouse retina was captured by vis-OCTF.
With the newly developed vis-OCTF imaging system and data analytic tools,we tracked RGC damage following optic nerve crush(ONC) injury in mice (Grannonico et al.,2021).The acute ONC injury was used to study the rapid degeneration and loss of RGCs and their axons within a short time frame,which is particularly beneficial for technical validation.We quantified the RGC axon bundle damage using three parameters:the lateral width,the axial thickness of RGC axon bundles,and the overall patterning of axon bundles from the peripheral to the optic nerve head (Grannonico et al.,2021).At 12 days following the ONC injury,the RGC axon bundles showed 10% and 25% reduction in thickness and width,respectively.Using semi-log Sholl analysis,we also found an overall fast decrease in RGC axon bundle density moving outward from the optic nerve head,suggesting the peripheral loss of RGCs (Grannonico et al.,2021).Interestingly,at 5 days following the ONC injury,we found uneven changes in axon bundles in different retinal regions (Grannonico et al.,2021).For example,we found a 12–13% reduction in bundle width in both central and peripheral retina.By contrast,we found a 2% increase in RGC axon bundle thickness in the central retina and a 5% increase in the peripheral retina (Grannonico et al.,2021).Taken together,at 3–5 days following the ONC injury,we found about 38% of RGC axon loss (Yi et al.,2016),and the axon bundles became elongated or swollen along the axial axis (Grannonico et al.,2021).However,at the same time,no apparent changes were observed in the overall RNFL thickness (Yi et al.,2016).
Our findings may suggest a characteristic retinal response to the ONC damage.As the RNFL thickness is not reduced right after the insult,the RNFL thinning is not a good indicator of early RGC damage.In fact,Wollstein and colleagues proposed the“broken stick”model,which shows that after the tipping point,the visual field (VF) damage became correlated with the RNFL thinning,but before the tipping point,the RNFL thickness measurements showed poor association with the VF values(Wollstein et al.,2012).The tipping point is the mean average RNFL thickness of 75µm in glaucomatous eyes (95% confidence interval,68.9–81.8),compared with 90µm from the age-matched healthy group(Schuman et al.,2020).In other words,there was a 16.7% reduction in RNFL thickness at the time of development of the earliest VF defect.Another study suggested about 30%of RGC axon loss before a functional deficit can be detected by the standard automated perimetry (Quigley et al.,1989).Moreover,individuals may exhibit very different patterns of disease progression,emphasizing the need to monitor patients longitudinally.For example,Shin and colleagues followed 292 eyes of 192 patients with primary openangle glaucoma for 6 years.They found that 72 eyes (24.7%) showed progressive GCIPL thinning,and among the 72 eyes,41 eyes showed VF progression (Shin et al.,2018).Again,all these studies were limited by abilities to detect functional abnormalities and structural changes in early glaucoma.
In summary,detecting RGC damages at the earliest stages and identifying glaucoma progression are vital for the timely management of glaucoma to preserve longterm vision.With our newly developed vis-OCTF and associated analytic methods,it is possible that we could follow RGC damage at the single axon bundle level,which may offer an accurate and specific indicator of RGC damage.Our work on the clinically translatable vis-OCTF using animal models will help to establish an objective evaluation of neural damage in glaucoma,which may have a profound impact on patients’ quality of life and drug development in the future.
This work was supported by National Institute of Health (NIH) grants R01EY029121(to XL and HFZ) and U01EY033001 (to HFZ).
Xiaorong Liu,Hao F.Zhang
Department of Biology,Department of Psychology,Program in Fundamental Neuroscience,University of Virginia,Charlottesville,VA,USA (Liu X)Department of Biomedical Engineering,Northwestern University,Evanston,IL,USA(Zhang HF)
Xiaorong Liu,PhD,xl8n@virginia.edu.https://orcid.org/0000-0002-7655-6342(Xiaorong Liu)
Date of submission:
January 6,2022Date of decision:
January 27,2022Date of acceptance:
February 8,2022Date of web publication:
May 31,2022https://doi.org/10.4103/1673-5374.343906
Liu X,Zhang HF (2023)Characterization of retinal ganglion cell damage at single axon bundle level in mice by visible-light optical coherence tomography fibergraphy.Neural Regen Res 18(1):135-136.
Open access statement:
This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Neuroaxonal and cellular damage/protection by prostanoid receptor ligands,fatty acid derivatives and associated enzyme inhibitors
- Extracellular vesicles in Alzheimer’s disease:from pathology to therapeutic approaches
- Molecular approaches for spinal cord injury treatment
- Sex-biased autophagy as a potential mechanism mediating sex differences in ischemic stroke outcome
- Adipose tissue,systematic inflammation,and neurodegenerative diseases
- Interleukin-1:an important target for perinatal neuroprotection?

