Astrocyte evolution and human specificity
Carmen Falcone,Verónica Martínez-Cerdeño
The cerebral cortex is one of the most complex structures of the mammalian central nervous system and accounts for the extraordinary cognitive abilities in primates and humans.Since the 19century,neur ons have been believed to be the main players in the building of the brain,yet astrocytes also play a crucial role as fundamental building blocks of the cerebral cortex complexity.Currently,astrocytes are recognized as pivotal players in the central nervous system exerting a myriad of functions,such as water homeostasis and exchange of nutrients with the bloodbrain barrier.Astrocytes also play a crucial role in the development and regulation of connectivity including synapse formation and elimination,and synaptic function and plasticity (Verkhratsky and Nedergaard,2018).Furthermore,injury or stress induces reactive astrogliosis that provides antioxidant defense and scar formation.While astrocytes have been mostly studied in mice,we are becoming aware of astrocyte heterogeneity and increased complexity in mammals and primates (Oberheim et al.,2009;Falcone et al.,2019,2020).Astrocytes likely co-evolved with neurons,becoming more specialized in mammals versus other vertebrates.Only recently attention has been put towards specific types of astrocytes in the primate brain.Understanding how astrocytes develop,evolve across mammalian species,and regulate neuronal development and function is crucial to understand the cerebral cortex complexity characteristic of human and nonhuman primates.
Radial glia represents the main type of neuroglia in the central nervous system of many early vertebrates,which are almost completely devoid of other types of parenchymal glia.While many vertebrates contain proto-astrocytes,reptiles are the first with“typical”astrocytes in the central nervous system.Astrocytes in geckos show an immature phenotype that resembles rat embryonic astrocytes (Du et al.,2021).Caimans,instead,present glial fibrillary acidic protein-immunoreactive astrocytes intermingled with radial glia.Moreover,astrocyte-like cells are limited to some brain regions in lizards and snakes but absent in turtles (Clinton et al.,2014;Lõrincz and Kálmán,2020).Evolution increased the complexity of morphology and function and rendered different types of astrocytes.Human astrocytes are much larger,more complex,and more heterogeneous than astrocytes in non-primates (Oberheim et al.,2009;Falcone et al.,2019).Two major types of astrocytes have been broadly described in mammals,the protoplasmic and fibrous astrocytes.Protoplasmic astrocytes have numerous short-branched processes and are abundant in the grey matter,while fibrous astrocytes have long unbranched processes and are predominantly present in the white matter.Two other astrocyte types with distinct morphology are known to localize to specific layers of the cerebral cortex,interlaminar astrocytes (ILAs) and varicose-projection astrocytes (VP-As) (Oberheim et al.,2009),(Figure 1
).While protoplasmic astrocytes are confined to an individual cortical layer and have a spherical domain of action,ILAs processes transverse several cortical layers and have a radial site of action,and VP-As have processes extending long distances within a single layer and have a tangential site of action.ILAs possess a cell body in cortical layer I,and long processes traveling perpendicular to the pia towards deeper layers of the cortex.We recently described two types of ILAs,pial and subpial ILAs(Falcone et al.,2019).Pial ILAs soma is in contact with the pia surface,while subpial astrocytes soma is located in the upper layer I with processes contacting the pial surface.While pial ILAs were thought to be specific to primates,we showed that they are present in many other mammalian orders,albeit with lower density and lower degree of complexity.Typical subpial ILAs are only present in some primates,are less abundant than pial ILAs (Falcone et al.,2019).Recording from several long-process astrocytes in the human cortex showed that they displayed passive electrophysiological properties similar to those of protoplasmic astrocytes,with no significant differences in resting membrane potential,input resistance,and membrane capacitance (Sosunov et al.,2014).This suggests that ILAs share many functions with protoplasmic astrocytes in the cerebral cortex and the brain.Protoplasmic astrocyte processes cover a spherical area of~35–75 µm of cortical tissue within a single layer and the processes of one astrocyte intermingle with processes from neighboring astrocytes (Vasile et al.,2017).In contrast,typical ILAs have long interlaminar processes that cover a single mini-columnar area that spans several layers.In other words,they cover a vertical,rather than spherical area,in which they could potentially interact with protoplasmic astrocytes,neurons,and other cortical structures throughout many layers of the cortex.While ILA may share functions with protoplasmic astrocytes,they are distinct because they probably manage functions in different territories.Accordingly,it has been proposed that interlaminar processes may be responsible for radiallyorganized glial-mediated influences on the ionic microenvironment in the cortex(Colombo et al.,1998).In agreement with this hypothesis,species with a more organized columnar structure present a greater number of longer interlaminar processes,as in great apes (Falcone et al.,2019).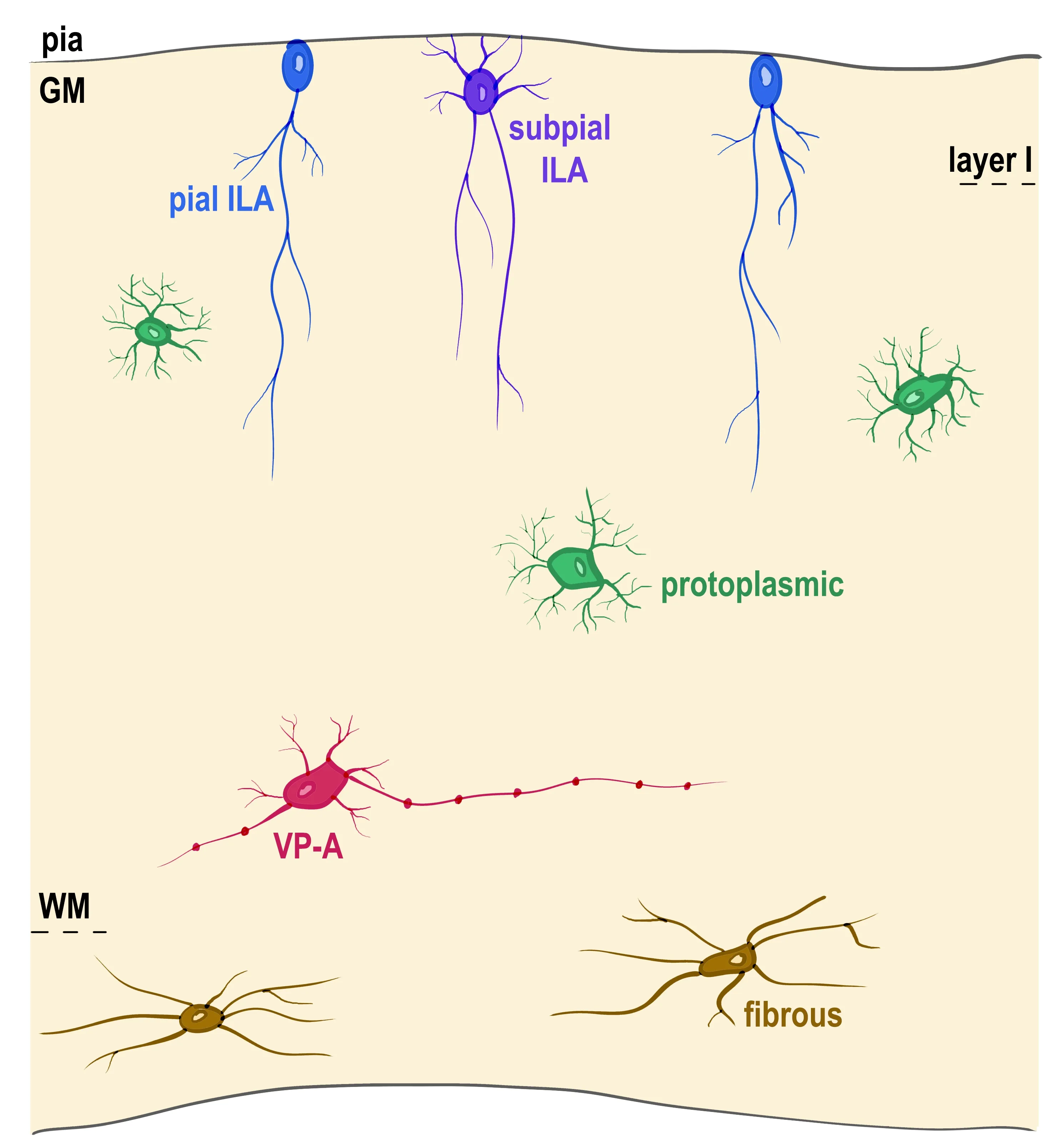
Figure 1 | Astrocyte subtypes in the human cerebral cortex.
VP-As are present in the cortical layers V,VI,and white matter and contain one to five long processes with evenly spaced varicosities and some shorter processes.VP-As had been previously described to be specific to humans and chimpanzees(Oberheim et al.,2009).We recently performed a thorough analysis VP-As in species ranging from ancient primates(prosimians) to primates evolutionary closer to human (apes) and found that VP-As are exclusively present in human and other apes(hominoids),while being absent in other primates.Little is known about VP-A function.We found that VP-A presence is individualspecific,meaning that they are only present in a few individuals from the same species(Falcone et al.,2021).We also found that VP-As are only present in individuals whose ILAs also present with varicosities.This made us hypothesize that VP-As are not a specific type of astrocyte,and because they share both the location within the cortex and the presence of long-processes observed in fibrous astrocytes,we hypothesize that VPAs are a modified form of fibrous astrocytes that undergo a morphological change in response to specific conditions.Potential conditions that could be associated with the appearance of varicosities in the processes of VP-As and ILAs include stress,aging,and specific pathological conditions (Falcone et al.,2021).
Human astrocytes are more diverse and also possess more special anatomical features such as bigger soma,more complex and longer processes,a specialized transcriptomic profile,and a more diverse pool of functions (Oberheim et al.,2009;Falcone et al.,2019;Li et al.,2021).Human astrocytes contain many conserved genes but also thousands of genes differentially expressed when compared to mice (Li et al.,2021).Specifically,mouse astrocytes show a higher expression of genes involved in metabolism and mitochondrial respiration,while human astrocytes show higher expression of genes involved in defense response and genes associated with extracellular space and secreted factors (Li et al.,2021).Furthermore,RNA sequencing experiments performed in induced pluripotent stem cells-derived astrocytes showed significant interspecies differential gene expression in astrocytes,with specific differences in cellular respiration,glucose and lactate transmembrane transport,and pyruvate utilization,suggesting a higher metabolic capacity in humans compared to chimpanzees (Zintel et al.,2020).Accordingly,our data indicate that primate ILAs show an overlapping expression of some genes with mouse ILAs,such as Sox2,Pax6,Nestin,Aqp4,and Glast,but in addition,they express specific genes,such as S100b,Cryab,and Hopx (Falcone et al.,2021).In addition,to the astrocyte function in homeostasis,plasticity,and response to injury widely known in rodents,astrocytes have also been suggested to have specific roles in primate behavior and cognition (Han et al.,2013).Accordingly,forebrain engraftment of human glial progenitor cells enhances learning in adult mice,and human astrocytes can enhance learning,memory,and social skills in mice,thus providing them with cognitive traits more similar to primates than rodents(Han et al.,2013).
Understanding how astrocytes co-evolve with neurons across mammals,and specifically in primates,is crucial to understand the appearance of particular cognitive abilities in this phylogenetic group and to provide insights on astrocyte-specific human pathologies.
This work was supported by Shriners Hospitals.
Carmen Falcone,Verónica Martínez-Cerdeño
Department of Pathology and Laboratory Medicine,UC Davis School of Medicine;Institute for Pediatric Regenerative Medicine and Shriners Hospitals for Children of Northern California,Sacramento,CA,USA (Falcone C,Martinez-Cerdeño V)MIND Institute,UC Davis Medical Center,Sacramento,CA,USA (Martinez-Cerdeño V)
Verónica Martínez-Cerdeño,PhD,vmartinezcerdeno@ucdavis.edu.https://orcid.org/0000-0002-9613-3603(Verónica Martínez-Cerdeño)
Date of submission:
November 11,2021Date of decision:
December 18,2021Date of acceptance:
January 12,2022Date of web publication:
May 31,2022https://doi.org/10.4103/1673-5374.340405
Falcone C,Martinez-Cerdeño V (2023) Astrocyte evolution and human specificity.Neural Regen Res 18(1):131-132.
Availability of data and materials:
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement:
This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:
Janosch P.Heller,Dublin City University,Ireland.
Additional file:
Open peer review report 1.
- 中国神经再生研究(英文版)的其它文章
- Neuroaxonal and cellular damage/protection by prostanoid receptor ligands,fatty acid derivatives and associated enzyme inhibitors
- Extracellular vesicles in Alzheimer’s disease:from pathology to therapeutic approaches
- Molecular approaches for spinal cord injury treatment
- Sex-biased autophagy as a potential mechanism mediating sex differences in ischemic stroke outcome
- Adipose tissue,systematic inflammation,and neurodegenerative diseases
- Interleukin-1:an important target for perinatal neuroprotection?

