Exploring magneto-electric nanoparticles (MENPs):a platform for implanted deep brain stimulation
Małgorzata Kujawska ,Ajeet Kaushik
Towards implanted deep brain stimulation(DBS):
The human brain is a complex network of 86 billion neurons and 85 billion nonneuronal cells and they are coordinated in a well-defined ratio (1:1) which is required for desired body functions.The connectivity among neuronal cells secretes neurotransmitters (e.g.,dopamine) to establish a perfect connection between the brain and a peripheral system i.e.,motor coordination.The secretion of neurotransmitters was found to be affected by aspects of lifestyle,age,and deteriorated health can damage the neurons’ connectivity,which can cause neurodegeneration leading to neurological disorders (Wang and Guo,2016;Kumar et al.,2021).Circuit disturbances resulting from changes within the synapses,cells’ intrinsic excitability,and impaired connectivity within local networks and between projection areas mediate neurodegenerative disease and are associated symptomatic features (Werner et al.,2019;McTeague et al.,2020;Vissani et al.,2020).Disruptions in neural circuitry have also been demonstrated to underly emotional processing across various psychiatric conditions (Werner et al.,2019).In this context,techniques for adequate circuit reconstruction offer therapeutic potential.The lack of effective treatment made this situation very complicated to manage and in need of alternative treatment strategies,which are always in high demand.As a result of this,introducing external stimulation to modulate electrical communication among neurons’circuitry is getting attention for understanding the neurobiology needed for diagnostics and treatment (Kozielski et al.,2021).DBS is a neurological procedure involving the implantation of electrodes into targeted brain regions to generate electrical fields stimulating neurons from an implantable pulse generator(Yue et al.,2012).DBS preferentially acts on large,myelinated axons and dendrites depolarizing them.By opening voltage-gated sodium channels,DBS causes action potentials and the release of neurotransmitters at the synapse.DBS targets both excitatory and inhibitory efferent and afferent neurons and thus affects circuit behavior and phenotype.DBS has been introduced at the clinical level,and the outcomes are satisfactory.This therapy is used to manage debilitating neurological symptoms in conditions such as Parkinson’s disease,essential tremor,and dystonia and to treat non-motor conditions such as pain,epilepsy,and neuropsychiatric disorders.DBS’standard of clinical application is,however,limited to a single target and mainly minimalizes symptoms as its long-term efficacy is restricted.Significantly,electrode implantation is associated with microhemorrhages,edema,and other responses changing the tissue impedance.Moreover,applying necessary external stimulation to achieve the desired neuronal activity is known to cause side-effects as well,because a high dose of externally applied heat,electric field,ultrasound,etc.is required for the mapping of the entire brain.DBS has been reported to exert a detrimental impact on cognition and speech (Wang and Guo,2016;Kumar et al.,2021).
Since DBS exerts complex electrical effects on single neurons and neuronal networks,changes neurotransmitter and receptor dynamics and protein expression,and shapes the microenvironment,including astrocytes,microglia,and endothelial cells,the circuitry,and biochemical effects exceed the temporal and spatial zone of the stimulation.Recent developments of DBS are towards novel clinical applications,stimulation targets,and methods of stimulation delivery.To achieve this,generating the desired stimulation (nano forces)inside the brain via implanting a nanosystem in the brain,i.e.,towards implanted DBS,is suggested by experts.
I n t h i s c o nt ex t,st i m u l i-re s p o n s i v e nanomaterials offer possibilities to cross biological barriers and induce multi-target effects.The magnetic-electro nanoparticles(MENPs) provide high flexibility in terms of stimulation parameters and patterns’ potential to modulate and dose therapeutic effects with high precision,even at the sub-molecular level,to effectively manage treatment for many different diseases (Yue et al.,2012;Guduru et al.,2015;Nguyen et al.,2021).
MENPs as suitable nano-systems for implanted DBS:
To manage neurological disorder in a personalized manner,neuronal stimulation has been recommended by biomedical experts.In this direction,the need for implanted DBS has been proven as an effective approach and this can bring a therapy in practice that is cellular functional modulation specific and can be controlled via optimizing several parameters.The implanted DBS involves an optimized and bio-acceptable external stimulation that can actuate neuron and other brain functions via inferencing and ending thermal,optical,electrical,and magnetic properties (Yue et al.,2012;Guduru et al.,2015;Nguyen et al.,2021).As state-of-the-art,various stimuli (light,magnetic,and ultrasonic)-responsive nano systems have been investigated for implanted neuronal stimulation (Wang and Guo,2016).This approach is getting attention because of cell-specific targeting,region-specific localization,and control of functional features via managing input parameters.MENPs conjugated with ligands demonstrate receptormediated targeted delivery across the bloodbrain barrier (BBB) and the ability to target specific neuronal cell populations (Kaushik et al.,2016,2019a,b).External stimulation can induce various properties in a nano-system at the interface of tissue and cell,which is required for stimulation.Nanostructures investigated for implanted DBS are singlefunctional i.e.,induced a particular property with reference to a stimulation.However,a multi-functional stimulation i.e.,generating many properties on a single stimulation,is suggested to manage brain stimulation more efficiently.Therefore,developing such a surface-functionalized stimuli-responsive nanosystem needs to be explored.
In this direction,MENPs have emerged as one of the potential stimuli-responsive nanoplatforms,designed as core(ferromagnetic)-shell (piezoelectric) nanosystems,known for generating electric field and acoustics on applying an ac-magnetic field.Such magneto-electro-elastic features of MENPs have been discussed theoretically and experimentally (Kaushik et al.,2017,2019a,b;Pandey et al.,2021).Kaushik et al.(2019b)have explored MENPs (BiTiO@CoFeO) for magnetically guided delivery of an anti-HIV drug(CRISPR-Cas9) across the BBB and release of the loaded drug on-allying an ac-magnetic field via customized electromagnetic coils.It has been proven that MENPs-based nanomedicine seems more effective as an applied ac-magnetic absorbed by the core of MENPs to produce strain deformation which is absorbed by the piezoelectric shell to produce acoustics (Kaushik et al.,2017;Pandey et al.,2021).Besides,a localized electric field produced by the MENPs,an ac-magnetic field stimulation on MEMPs can produce a localized thermal effect due to the ferro-electric nature of MENPs.Kaushik et al.(2019a,b) have demonstrated the nanoelectroporation (demonstrated by excessive cell-uptake) and controlled drug release due to surface charge which causes alteration in the length of MENPs-drug bonds.The outcomes of these recent research findings confirm that MENPs can produce a localized electric field,thermal effect,and acoustics at the same time on ac-magnetic field stimulation(pulse-to-pulse).These salient features have the capability to affect cellular functional performance,which can be manipulated to achieve therapeutics effects.
These stimuli-responsive features of MENPs can be optimized with reference to the need for neurons to achieve desired brain tissue and cellular stimulation.In this direction,the noninvasive and brain-cell compatible delivery of MENPs to the brain via the BBB (mince and baboon model) (Kaushik et al.,2016,2019a)and nasal route (mice) has been demonstrated successfully (Pardo et al.,2021).The results of the studies suggest that MENPs were distributed in the brain without deteriorating the elemental integrity of cells,affecting motor coordination,and causing toxicity with reference to blood profiling assessment(Kaushik et al.,2019a,b).
On applying an ac-magnetic field stimulation,MENPs can generate several manipulated features that may be managed for the implanted brain stimulation,as illustrated inFigure 1.
The external control over the induced feature,nano-invasive delivery to the brain,and bio-acceptability,projects MENPs as a suitable nano-platform to achieve a successful implanted DBD.To optimize the DBS,its parameters need to be precisely customized based on an individual’s neuro-physiopathology biomonitoring.The MENPs -based nanoplatform can combine BS and sensing biomarkers relevant for triggering,optimizing,and locking the stimulation.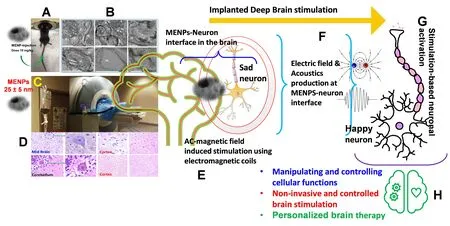
Figure 1 | Towards implanted brain stimulation.
Challenges and perspectives:
MENPs-response neuronal activation can be an efficient nextgeneration implanted DBS technique.This nano-system can be engineered to produce opto-electric,opto-thermal,magneto-electric,magneto-thermal,and acoustic-electric transduction to cover cellular and tissue functions of the brain present in the various regions.Such control over the stimulation and inducement features can be optimized according to the need of a targeted cell andtissues of the brain.However,these claims are in the early stage of medical research and more detailed studies are suggested for a better understanding of MENPs-cells and MENPs-Tissue interfacing in the correction of induced magnetic/electric/thermal/acoustics/or all together as a function of external stimulation.To achieve these tasks,material scientists,engineers,biomedical experts,and neurologists need to come together to perform multidisciplinary studies exploring MENPs-based implanted DBS in the case of a targeted brain diseases/disorder/or both for example epilepsy,Parkinson’s,Alzheimer’s diseases,spinal cord.Such MENPs-based implanted brain stimulation can be optimized for brain mapping and therapeutics needed for personalized health wellness management.The stimulus applied at nanoscale topography can boost exogenous signals that enable cell manipulation (He et al.,2021).Stimulation with the use of MENPs offers the capability of modulating axonal growth and guidance and multiple mechanisms,including neurotrophic factor release,synaptic remodeling,apoptosis inhibition,anti-inflammatory response,glutamate excitotoxicity decline,and enhancement of autophagy,which not only protects against neurodegeneration but also boost neurogenesis.
Altogether,the developments and technological innovations of MENPs application hold tremendous promise for improving the safety,clinical efficacy with disease-modifying effects,and diagnostic accuracy of brain stimulation.
Authors acknowledge respective affiliated institutions for providing facilities and support.
Małgorzata Kujawska,Ajeet Kaushik
Department of Toxicology,Poznan University of Medical Sciences,Poznań,Poland (Kujawska M)NanoBioTech Laboratory,Health Systems Engineering,Department of Natural Sciences,Florida Polytechnic University,Lakeland,FL,USA(Kaushik A)
Malgorzata Kujawska,PhD,kujawska@ump.edu.pl;Ajeet Kaushik,PhD,akaushik@floridapoly.edu.https://orcid.org/0000-0002-5306-9904(Malgorzata Kujawska);
https://orcid.org/0000-0003-4206-1541(Ajeet Kaushik)
Date of submission:
December 22,2021Date of decision:
January 10,2022Date of acceptance:
January 24,2022Date of web publication:
May 31,2022https://doi.org/10.4103/1673-5374.340411
Kujawska M,Kaushik A(2023) Exploring magneto-electric nanoparticles(MENPs):a platform for implanted deep brain stimulation.Neural Regen Res 18(1):129-130.
Open access statement:
This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Neuroaxonal and cellular damage/protection by prostanoid receptor ligands,fatty acid derivatives and associated enzyme inhibitors
- Extracellular vesicles in Alzheimer’s disease:from pathology to therapeutic approaches
- Molecular approaches for spinal cord injury treatment
- Sex-biased autophagy as a potential mechanism mediating sex differences in ischemic stroke outcome
- Adipose tissue,systematic inflammation,and neurodegenerative diseases
- Interleukin-1:an important target for perinatal neuroprotection?

