Brain network modulation in Alzheimer’s disease:clinical phenotypes and windows of opportunity
Lorenzo Pini
Dementia,for which there is no cure or effective treatment,is the leading cause of disability and death worldwide.Due to the high global prevalence and economic impact on families,caregivers,and communities,this condition represents one of the most significant public health challenges of our time.Dementia is an umbrella term describing a range of progressive neurodegenerative diseases,including Alzheimer’s disease (AD),which is the most common cause of cognitive and functional impairment among older adults.The presence of misfolded protein aggregates characterizes neurodegenerative disorders (e.g.,amyloid-beta (Aβ) and tau in AD).Stemming from this,advancements in the molecular imaging field paved the way to new experimental AD treatments targeting Aβ.However,the results have been disappointing so far,and there is an ongoing debate about the emerging role and efficacy of anti-Aβ monoclonal antibodies (Musiek and Bennett,2021),stressing the need for alternative biomarkers to guide new,effective preventive,and therapeutic interventions.Here,we report recent advancements in the field of functional connectivity in AD,underscoring the link with the underlying molecular pathology.We then discuss the meaning of the interplay between AD phenotypes,disease stage,and brain stimulation interventions.
In the last decades,functional imaging has been an important tool for evaluating circuits/networks within the brain,which represent an emerging biomarker for neurodegenerative diseases.Studies evaluating brain function often use a technique called functional magnetic resonance imaging.This methodology investigates changes in the blood oxygenation level dependent (BOLD) signal during task execution.For each brain voxel (a 3-dimensional unit embedding signals in brain scans -analogous to the 2D pixel of computers screens),changes in the BOLD time-series are determined by fluctuations in arterial partial pressure of both Oand CO.Fluctuations in BOLD,while vascular in nature,can be considered a reliable proxy of neural activity.Functional activation is then analyzed by correlation of the observed signal changes in each voxel with the given stimulus protocol.Notably,oscillations in neural signals have also been observed during mind wandering,that is when the brain is not actively processing stimuli,or engaged in a specific task but lies in a non-sleeping resting state.Brain regions showing temporal synchronization in these resting oscillatory patterns are considered functionally connected in resting-state neural networks.Thus,activity in one area could be modulated through the activity of other brain regions.From this perspective,a neural system completes complex behaviors through multiple interactions between brain regions (top-down).Additionally,the behavior of these neural oscillators is influenced by external stimulus(Doelling and Assaneo,2021).Accordingly,neural networks sustain different domains of cognition,such as memory,attentional processes,visualspatial functions,language abilities,social cognition,and emotion recognition.However,the biological underpinnings of this functional resting scaffold are still largely unknown and debated.
There is preliminary evidence suggesting that resting-state networks play a role in intrinsic signaling in synaptic homeostasis and in predicting and responding to tasks and events (reviewed in Pezzulo et al.,2021).This is in stark contrast to the assumption that activity in the resting brain represents nothing more than random noise.These studies have shifted the focus from a local approach,where cognitive functions are strictly confined within the anatomical borders of brain regions,to a functional view,characterized by complex long-range interactions among brain areas supporting cognitive and sensory functions (Pezzulo et al.,2021).This framework is also supported by a growing body of literature reporting consistent alterations of the functional connectome linked with the severity of cognitive deficits in neurological and psychiatric disorders(van den Heuvel and Sporns,2019).Notably,functional connectivity breakdown is evident in the earliest stages of the cascade of pathophysiological events leading to neurodegeneration (Pievani et al.,2014).Recently,we showed that patients in the prodromal stage of AD (referred as mild cognitive impairment) exhibit lower functional connectivity strength in brain networks and connectivity reduction was associated with the degree of cognitive impairment (Pini et al.,2020).Other studies have shown that brain network alterations might occur even earlier in the AD process.In an earlier seminal study among children carrying AD mutation,Quiroz et al.(2015)reported connectivity alterations in the default mode network (DMN),a set of inter-connected temporo-parieto-frontal regions that includes the hippocampus,which sustains memory function.These results support the hypothesis that AD is fueled by network disintegration early in the disease course.
Although misfolded protein staging distribution in neurodegenerative diseases was mapped long ago,the mechanisms by which these proteins spread throughout the brain have remained controversial.The hypothesis that proteins might spread via large-scale resting-state networks was first put forward by Warren et al.(2013).In AD,aggregation of Aβ peptides preferentially affects DMN regions,in line with the finding that the DMN is impaired in the prodromal disease stage (Pini et al.,2020).The AD molecular-network coupling distribution seems to follow different stages,which can be conceptualized in a“two-stage”model.These two stages of AD pathology have differential effects on functional connectivity,with the early Aβ accumulation (first) phase characterized by significantly increased functional connectivity.This hyper-connectivity may subsequently hasten the spread of tau (second phase),due to activitydependent modulation of tau release.This then results in the progressive decline of functional connectivity with increased neocortical tau pathology (Schultz et al.,2017).According to this model,large-scale neural networks are marked by tau,while high connectivity represents a loadshifting process transiently serving a potential compensatory role (Graff-Radford et al.,2021).This functional brain framework provides new insights about the where (i.e.,the spatial topology of brain functional networks) and when(i.e.,hyper-connectivity vs hypo-connectivity dysfunctional patterns) in disease development.It could also guide new brain stimulation interventions aimed at normalizing/restoring brain function,prior to irreversible neurodegeneration(Pini et al.,2021).
Recent studies suggested that brain connectivity can be modulated (both enhanced and diminished) through non-invasive brain stimulation techniques.Transcranial magnetic stimulation(TMS) is one of the most common techniques applied to perturbate network connectivity.TMS induces electric currents through the cortical surface,triggering neural action potentials.Although preliminary findings showed improved cognitive performance in AD patients after TMS(reviewed in Pini et al.,2018),there is a lack of clear evidence concerning the clinical efficacy of multisession stimulation.To wit,the Food and Drug Administration recently rejected a commercial TMS system request for treatment of AD,identifying several issues,including uncertainty about clinical benefit.Another brain stimulation method,transcranial direct current stimulation,has been increasingly used in patients with neurodegeneration.However,the evidence of beneficial effects in dementia is still insufficient(Pini et al.,2018).The lack of clear effectiveness may depend,at least partially,on several factors,such as where and when to stimulate.Regarding the where,most of the previous stimulation protocols adopted a“local”brain perspective,assuming a 1:1 relationship between a specific cognitive deficit (e.g.,memory) and a specific anatomical region (e.g.,temporoparietal) (Pini et al.,2018).In contrast,network imaging studies might help to identify“functional gate”stimulation targets aimed at modulating longrange connections in dysfunctional networks(e.g.,DMN).This approach may be more effective because stimulating network cortical hubs can create downstream effects along a functional gradient,modulating both cortical and subcortical regions and,maximizing potential benefits.We have recently explored this assumption in a randomized,double-blind pilot study in AD and frontotemporal dementia patients.We reported clinical and cognitive improvement following brain network stimulation with a specific clinicalcognitive pattern depending on the target network(Pini et al.,2022).
However,network target selection represents a critical choice,that should be informed by several factors.We have recently pointed out that a“one size fits all”assumption for brain network stimulation will not work for AD treatment (Pini et al.,2021).Broadly,AD can be divided into typical and atypical phenotypes,and although these patients have similar Aβ distribution,the clinical and network phenotypes differ.Typical AD affects about 95% of patients and is characterized by symptom onset– usually beginning with memory impairment– after age 65.The other 5% of patients develop atypical AD and tend to exhibit their first symptoms at younger ages.Atypical AD patients,including posterior cortical atrophy,primary progressive aphasia,and behavioral/dysexecutive (B/D),show less pronounced deficits in the memory domain.Posterior cortical atrophy patients present with visual difficulties,including simultanagnosia,oculomotor apraxia(Balint syndrome),and finger agnosia (Gerstmann syndrome).Primary progressive aphasia patients show focal language difficulties,years before impairments in other cognitive domains,and in B/D behavioral or executive dysfunctions represent early clinical signs (Graff-Radford et al.,2021).Accordingly,these AD phenotypes exhibit functional abnormalities in different brain networks.Although typical AD involves mainly memory networks (e.g.,DMN),atypical variants are characterized by early aberrant connectivity in networks linked with their respective cognitive deficits (e.g.,language network in primary progressive aphasia,visual and dorsal-attention networks in posterior cortical atrophy,and attentional networks in B/D) (Pini et al.,2021).Based on these assumptions,target choice for brain stimulation might follow this cognitivenetwork gradient (Figure 1
).Future studies should investigate possible additional clinical benefits of a phenotype-personalized brain network stimulation design,compared to a molecularnetwork perspective based on misfolded protein distribution.Similarly,different temporal windows (i.e.,when),could influence the efficacy of neuromodulation(Figure 1
).It has been established that measurable changes in several AD biomarkers occur decades before symptom onset and clinical diagnosis (Quiroz et al.,2015).Focusing on this long prodromal stage of AD could provide new insights into a window of opportunity for brain network stimulation interventions to potentially restore brain dynamics before irreversible neurodegeneration occurs.It may be that the most effective therapeutic window for such treatment is early in the disease during the hyper-connectivity stage (first stage).However,future studies should evaluate whether this hyper-connectivity plays a compensatory role.In this case,enhancing hypo-connected networks(second stage) might represent a more effective therapeutic option.These putative critical turning points should be methodically and systematically investigated.Additionally,new light should be shed on the possibility that both aging and disease duration (i.e.,the timing of biomarker level alterations,such as Aβ or tau accumulation)dimmish network response to brain stimulation.This begs the question of whether a limited window of responsivity to network modulation exists,or whether an optimal therapeutic window of opportunity can maximize clinical benefits.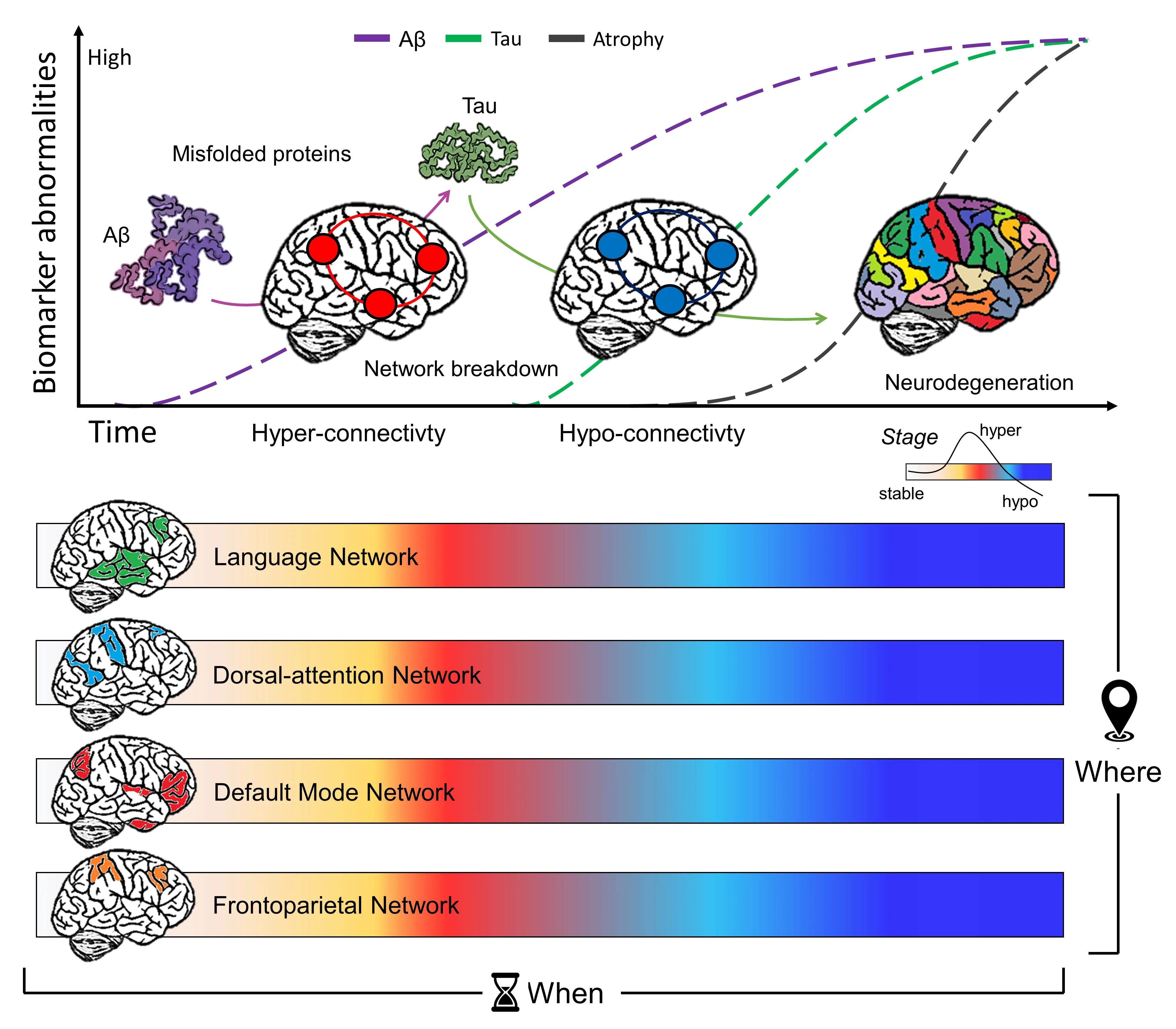
Figure 1 | Functional connectivity can help to guide the selection of the most appropriate parameters for non-invasive brain stimulation protocol based on spatial and temporal patterns.
Addressing these issues represents a major challenge to further develop effective interventions.Recently,a new methodology to assess dysconnectivity following brain lesions,referred as“lesion network mapping”(LNM),has been developed.LNM measures brain network dysconnectivity by embedding the lesion into a normative connectome and using the average BOLD signal from that volume to assess signal synchronization with all other brain regions (Fox,2018).LNM has been widely applied to stroke and brain lesion patients.This approach was recently adapted to investigate brain dysconnectivity convergence from heterogeneous patterns at the group level in atrophied regions in individual patients (Tetreault et al.,2020).Moving beyond this,LNM has the potential to assess brain disconnection at the subject level,paving the way to new tailored and personalized network stimulation interventions.We have shown that peak atrophy in patients with different phenotypes is linked with specific dysconnectivity patterns,informing on cortical hubs expressing the earliest vulnerability to the individual pathophysiological trajectory (Pini et al.,2021).However,this model,although simple and beautiful in its application,does not capture the inter-individual variability of brain connections,which might critically influence response to stimulation.These assumptions remain speculative,since,to date,there have been no studies applying LNM to identify brain stimulation targets in the AD population.
In summary,a better comprehension of brain connectivity in AD might pave the way to new tailored-network interventions,guided by an understanding of networks critical in different clinical phenotypes and windows of opportunity for change.Moreover,new insights about the relationship between macro-scale systems and micro-scale molecular processes might help to identify which individuals would benefit most.Finally,considerable work should be done to better unravel and characterize all the modulatory factors that might influence the relationship between dementia and brain stimulation response.
Lorenzo Pini
Padova Neuroscience Center,University of Padova,Padova,Italy
Lorenzo Pini,PhD,lorenzo.pini@unipd.it.https://orcid.org/0000-0002-9305-3376(Lorenzo Pini)
Date of submission:
December 7,2021Date of decision:
January 20,2022Date of acceptance:
January 28,2022Date of web publication:
May 31,2022https://doi.org/10.4103/1673-5374.340410
Pini L (2023) Brain network modulation in Alzheimer’s disease:clinical phenotypes and windows of opportunity.Neural Regen Res 18(1):115-116.
Open access statement:
This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Neuroaxonal and cellular damage/protection by prostanoid receptor ligands,fatty acid derivatives and associated enzyme inhibitors
- Extracellular vesicles in Alzheimer’s disease:from pathology to therapeutic approaches
- Molecular approaches for spinal cord injury treatment
- Sex-biased autophagy as a potential mechanism mediating sex differences in ischemic stroke outcome
- Adipose tissue,systematic inflammation,and neurodegenerative diseases
- Interleukin-1:an important target for perinatal neuroprotection?

