A potential new tool to enhance translational success rate in stroke research by backcrossing techniques in transgenic mice
Takayuki Nakagomi,Hideaki Nishie,Toshinori Sawano,Akiko Nakano-Doi
Ischemic stroke is a leading disease of the central nervous system,frequently coupled to severe damage and dysfunction in patients.Animal models mimicking human stroke provide useful tools for studying the pathomechanisms (e.g.,inflammation,neuroprotection,and neural regeneration),the treatment efficiency of various materials (e.g.,bioactive molecules or drugs),and transplantation usefulness by various cell types[e.g.,neural stem/progenitor cells (NSPCs),and mesenchymal or hematopoietic stem cells] under ischemic stroke.
In addition to conventional mouse models,transgenic mice overexpressing or lacking specific genes have been widely used to understand how specific molecules and factors (e.g.,trophic factors) influence ischemic stroke pathogenesis.Moreover,transgenic mice have been used as alternative objects in central nervous system research.For example,to understand the neural development and regeneration mechanisms through NSPCs,NSPC fate has been studied using transgenic mice that allow trailing NSPC markers(e.g.,Nestin or Sox2) labeled with specific proteins[e.g.,green fluorescent protein (GFP)],such as Nestin-GFP or Sox2-GFP mice.
However,to successfully translate preclinical stroke research into clinical applications using transgenic mice,developing appropriate models in stroke research is essential.In this article,we introduced a potential novel tool to promote translational success rate in stroke research using backcrossing techniques in transgenic mice.
Difficulties in translational stroke research from animal experiments to the bedside treatment of patients with stroke:
Previously,several studies displayed targeting material (e.g.,bioactive molecules,drugs,and cells) efficiency in stroke research using both conventional (Wang et al.,2018) and transgenic (Im et al.,2020) mice.However,preclinical stroke research could be very rarely translated successfully into clinical applications in patients with stroke (Herson and Traystman,2014).Such lack of translational research between the animal experiments and treatment of patients with stroke casts doubt on the usefulness of animal studies (Ringelstein et al.,2013).Several factors could be responsible for such failure.One possible reason is that the pathogenesis of patients with stroke is very complex compared with that of animal stroke models.For example,in the case of patients with stroke,several factors(e.g.,age,sex,infarcted area size,ischemic stroke regions,treatment time after stroke onset,or complications,such as hypertension and diabetes)would vary among the individuals and certain reportedly affects the prognosis of patients with stroke (Herson and Traystman,2014).
Mouse stroke model-related problems potentially associated with the lack of clinical applications of translational research:
Several factors could be related to the low translational success rate from the aspect of mouse models.In 1999,the Stroke Therapy and Academic Industry Roundtable has stressed the importance of preclinical study quality,including appropriate rodent models (e.g.,focal cerebral infarction models that allow extended recovery,performance of studies in a blinded-randomized fashion,behavioral outcome measurement combined with histopathology,replication of studies by at least two independent laboratories,investigations using permanent and transient ischemic models,studies using both male and female animals).Supporting this aspect,differences in stroke modes (e.g.,permanent versus transient ischemic models)would become important factors as these two stroke models differ not only in cellular responses(e.g.,endogenous stem cell activation,neural regeneration,and vasculogenesis promotion,or vascular cell death prevention) but also in prognosis (e.g.,neurological function loss or improvement) following an ischemic insult (Tanaka et al.,2020).Despise the first description of the Stroke Therapy and Academic Industry Roundtable in 1999,several present studies still do not follow the recommendation (Herson and Traystman,2014).Therefore,poor preclinical study quality might be associated with failure.However,most principal reasons that result in the lack of clinical applications of translational research might be attributed to ischemic area size variations in stroke mouse models.Although C57BL/6 mice have been commonly used in stroke research,they possess anatomical cerebral blood variations,including multiple arterial branches and collateral vessels (Qian et al.,2018).Therefore,even after occluding the main middle cerebral artery (MCA) trunk,a widely used approach in mouse stroke models,ischemic areas in these mice vary among the individuals (Taguchi et al.,2010).Importantly,C57BL/6 mice frequently exhibit widespread brain damage following ischemic stroke,including not only the cortex but also the striatum,while they occasionally display very small infarction areas (Taguchi et al.,2010).The precise underlying induction mechanisms of ischemic area size variations in C57BL/6 mice remain unclear.However,certain branching vessels could possibly originally dominate additional brain territories,thereby leading to the large size of widespread infarction following stroke.In contrast,several collateral vessels might occasionally rescue the brain from the ischemic/hypoxic insult,thereby reducing the ischemic area size.
Akin to ischemic area size variations,C57BL/6 mouse survival rates also vary significantly.Indeed,C57BL/6 mice frequently died during the acute phase (within a week following ischemic stroke)(Zhang et al.,2012) and only a small portion of them could survive up to the chronic phase (Nishie et al.,2021).Therefore,not only histological characteristics but also neurological functions are usually assessed within a limited time window such as acute periods.Although the relationship between ischemic area size and survival rate in C57BL/6 mice should be clarified in future studies,larger cerebral infarction size might be associated with a higher mortality rate by causing additional brain damage,such as brain herniation and hemorrhagic infarction.
Moreover,C57BL/6 mice have been used to develop most transgenic mouse models(Yamaguchi et al.,2000).Traditionally,transgenic C57BL/6 mice have been widely used in central nervous system research targeting various disease types,such as ischemic stroke.However,our recent study (Nishie et al.,2021) clearly described the presence of the aforementioned defects of C57BL/6 wild-type mice,possessing heterogeneous infarcted area size and short-time survival (Taguchi et al.,2010;Zhang et al.,2012).Therefore,evaluating the precise role of specific elements (e.g.,genes,bioactive molecules,or trophic factors) and the certain cell dynamics(e.g.,cell differentiation or cell migration) during ischemic stroke using mice of this background would be visibly difficult,although several researchers still use them for stroke research.
Backcrossing techniques to establish a reproducible ischemic stroke model in transgenic mice:
In contrast to the findings in C57BL/6 wildtype mice,we previously showed that CB-17 wild-type mice (CB-17/Icr-+/+Jcl mice) displayed fewer cerebral arteries branching from the MCA compared with C57BL/6 wild-type mice (Taguchi et al.,2010).Therefore,even after occluding the main MCA branch,CB-17 wild-type mice displayed a high reproducibility rate of the ischemic area,limited within the ipsilateral side of the cortex.In addition,CB-17 wild-type mice could survive for longer following MCA occlusion (MCAO) with very low mortality rates (Taguchi et al.,2010;Tanaka et al.,2020).These findings indicate that CB-17 mice are very useful to evaluate ischemic stroke pathogenesis exactly up to chronic periods.Backcrossing is useful to transfer a desirable trait by initially generating hybrid mice [F1 (first filial hybrid)] between two inbred strains (Mazzoli et al.,2020).By crossing F1 mice with a parental inherent strain and repeating this step,the counterpart phenotypes gradually increase with the proceeding number of generations (N) and theoretically reach the following values [e.g.,F1(50%),N2 (second generation;75%),N3 (third generation;87.5%),N4 (fourth generation;93.75%),N5 (fifth generation;96.88%),N6 (sixth generation;98.44%),N7 (seventh generation;99.22%),N8 (eighth generation;99.61%),N9 (ninth generation;99.80%),N10 (tenth generation;99.90%),N11 (eleventh generation;99.95%),and N12 (twelfth generation;99.98%)] (Figure 1
).Therefore,to overcome the drawbacks of C57BL/6 background mice using backcrossing,we recently crossed transgenic C57BL/6 mice with CB-17 wildtype animals and tried to create transgenic CB-17 mice (Nishie et al.,2021).
Proof of reproducibility and high survival rates in transgenic mice upon backcrossing:
In this study,we used Nestin-GFP mice to understand endogenous NSPC fate and expression patterns.As described previously (Nishie et al.,2021),we first crossed male Nestin-GFP mice (C57BL/6 background) with female CB-17/Icr-+/+Jcl mice(CB-17 wild-type mice).Next,the hybrid male mice were serially backcrossed with CB-17 wildtype females.In the aforementioned study (Nishie et al.,2021),we examined the traits of the developed Nestin-GFP mice (with a CB-17 background) obtained by backcrossing more than six generations (≥N6) (Figure 1
).Upon MCAO,we observed that the developed mice (of the CB-17 background)displayed fewer blood vessels around the MCA,highly reproducible infarcted volume based on 2,3,5-triphenylteterazolium staining,and an increased survival rate of 28 days following MCAO.2,3,5-Triphenylteterazolium and histological analysis showed that the ischemic areas in the developed CB-17 Nestin-GFP mice were restricted within the ipsilateral cortical side,while the ischemic areas in the C57BL/6 Nestin-GFP mice varied,consistent with the findings in C57BL/6 wild-type mice.Notably,all CB-17 Nestin-GFP mice could survive for 28 days post-MCAO,while 60%of the C57BL/6 Nestin-GFP mice died only within a week.In addition,GFPcells could be mainly observed in the ischemic,as well as conventional neurogenic areas,such as the subventricular zone,in CB-17 Nestin-GFP mice.However,microarray analyses indicated that GFPcells with NSPC activities obtained from the ischemic areas and the subventricular zone exhibited diverse features.Overall,the developed CB-17 Nestin-GFP mice could be used for precise NSPC activity-displaying GFPcell expression pattern and dynamics studies over long periods,which is impossible in C57BL/6 Nestin-GFP mice.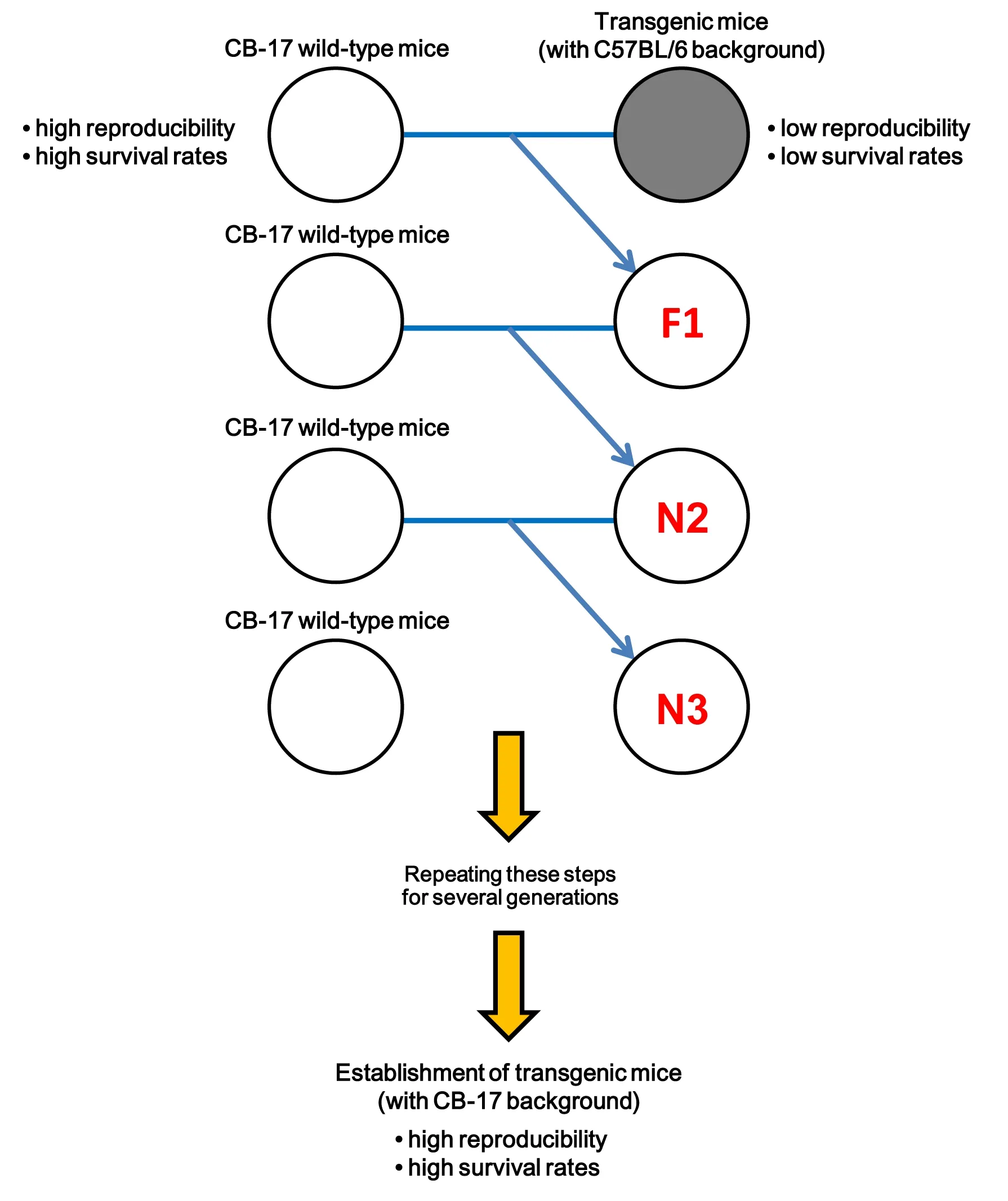
Figure 1 | Schematic representation of backcrossing techniques to establish a reproducible ischemic stroke model in transgenic mice.
Therefore,the mice developed in this study represent a potential new tool for investigating neural regeneration mechanisms under pathological conditions such as ischemic stroke.Certainly,similar backcrossing techniques using CB-17 wild-type mice could be applied for transgenic C57BL/6 mice overexpressing or lacking specific genes (Sawano et al.,2015).Therefore,using transgenic CB-17 mice,researchers can evaluate more in detail how specific elements could affect ischemic stroke-related pathogenesis.
and future remarks:
In conclusion,our recent study indicated that a reproducible mouse stroke model could be successfully established in transgenic mice with high survival rates through backcrossing with CB-17 mice (Nishie et al.,2021).Therefore,these methods would be potentially very useful in transgenic mice to investigate the exact roles of specific elements as well as the pathogenesis mechanism of ischemic conditions.Several aspects should still be clarified in future studies.For example,previous studies in rodents showed that pathophysiology following ischemia displays age-and sex-related differences (Herson and Traystman,2014).Therefore,although we used Nestin-GFP mice (of a CB-17 background)ranging age of 8–16 weeks,additional studies using younger or older mice would be required.Moreover,in this study (Nishie et al.,2021),we performed investigations using both male and female Nestin-GFP mice (with a CB-17 background)obtained from backcrossing more than six generations (≥ N6).Therefore,whether the sexdependent differences could be observed should be gender-specifically investigated in the future.
In addition,considering the heterogeneity of the infarcted area size and regions in patients with stroke,a C57BL/6 mouse stroke model might reflect closer human pathophysiology compared with those of CB-17 background mice.Therefore,C57BL/6 stroke model mice might be useful for“confirmative”preclinical studies at late stages,while CB-17 stroke model mice might be useful for“exploratory”preclinical studies at early stages,in which stroke model reproducibility is important to discern the efficiency after treatment as an initial screening.
In general,>N10–12 is required to establish a complete congenic mouse line.Thus,a certain amount of time is needed until establishing newly generated congenic mice after backcrossing.However,rapid congenic techniques are currently under development,potentially resulting up to 99.8% more offspring than six generations (>N6) if these methods are used (Andrews et al.,2021).Therefore,it is possible to shorten the time required for creating a given congenic mouse using backcrossing.Therefore,using such mice in future studies,researchers eventually might not only omit futile efforts and costs during preclinical studies but also enhance basic research translation into clinical applications in stroke research.
This work was partially supported by Japan Society for the Promotion of Science (JSPS)KAKENHI (JP19K16934;to AN-D),Grant-in-Aid for researchers,Hyogo College of Medicine (2018;to AN-D),Hyogo College of Medicine Diversity Grant for Research Promotion under MEXT Funds for the Development of Human Resources in Science and Technology,Initiative for Realizing Diversity in the Research Environment (Characteristic-Compatible Type) (2020,2021;to AN-D),and Grant-in-Aid for Graduate Students,Hyogo College of Medicine(2021;to HN).
Takayuki Nakagomi,Hideaki Nishie,Toshinori Sawano,Akiko Nakano-Doi
Institute for Advanced Medical Sciences,Hyogo College of Medicine,Hyogo,Japan (Nakagomi T,Nishie H,Nakano-Doi A)
Department of Therapeutic Progress in Brain Diseases,Hyogo College of Medicine,Hyogo,Japan(Nakagomi T,Nakano-Doi A)
Department of Biomedical Sciences,Ritsumeikan University,Shiga,Japan (Sawano T)
Takayuki Nakagomi,MD,PhD,nakagomi@hyo-med.ac.jp.https://orcid.org/0000-0003-2274-410X(Takayuki Nakagomi)
Date of submission:
December 20,2021Date of decision:
January 27,2022Date of acceptance:
February 14,2022Date of web publication:
May 31,2022https://doi.org/10.4103/1673-5374.343899
Nakagomi T,Nishie H,Sawano T,Nakano-Doi A (2023) A potential new tool to enhance translational success rate in stroke research by backcrossing techniques in transgenic mice.Neural Regen Res 18(1):107-108.
Open access statement:
This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Neuroaxonal and cellular damage/protection by prostanoid receptor ligands,fatty acid derivatives and associated enzyme inhibitors
- Extracellular vesicles in Alzheimer’s disease:from pathology to therapeutic approaches
- Molecular approaches for spinal cord injury treatment
- Sex-biased autophagy as a potential mechanism mediating sex differences in ischemic stroke outcome
- Adipose tissue,systematic inflammation,and neurodegenerative diseases
- Interleukin-1:an important target for perinatal neuroprotection?

