Brain-derived neurotrophic factor rs6265 (Val66Met)single nucleotide polymorphism as a master modifier of human pathophysiology
Van Thuan Nguyen ,Braxton Hill ,Naiya Sims,Aaron Heck,Marcus Negron,Claire Lusk,Cristi L.Galindo
Abstract Brain-derived neurotrophic factor is the most prevalent member of the nerve growth factor family.Since its discovery in 1978,this enigmatic molecule has spawned more than 27,000 publications,most of which are focused on neurological disorders.Brain-derived neurotrophic factor is indispensable during embryogenesis and postnatally for the normal development and function of both the central and peripheral nervous systems.It is becoming increasingly clear,however,that brain-derived neurotrophic factor likewise plays crucial roles in a variety of other biological functions independently of sympathetic or parasympathetic involvement.Brain-derived neurotrophic factor is also increasingly recognized as a sophisticated environmental sensor and master coordinator of whole organismal physiology.To that point,we recently found that a common nonsynonymous (Val66→Met)single nucleotide polymorphism in the brain-derived neurotrophic factor gene (rs6265) not only substantially alters basal cardiac transcriptomics in mice but subtly influences heart gene expression and function differentially in males and females.In addition to a short description of recent results from associative neuropsychiatric studies,this review provides an eclectic assortment of research reports that support a modulatory role for rs6265 including and beyond the central nervous system.
Key Words:brain-derived neurotrophic factor;neuropsychiatric disorders;rs6265;Val66Met
Introduction
Brain-derived neurotrophic factor (BDNF) is a member of the neurotrophin family of nerve growth factors (NGF),which in addition to BDNF include NGF itself,neurotrophin 3 (NT3),and NT4/5.NTs,including BDNF,are initially produced as pro-proteins (pro-NTs),which upon cleavage release an N-terminal pro-domain region and mature C-terminus of roughly equal sizes.All pro-NTs,including pro-BDNF,bind with relatively low affinity to a common tyrosine kinase receptor,p75 neurotrophin receptor (p75).Mature NTs selectively bind with high affinity to one of three tropomyosin-related kinases(TrkA,TrkB,and TrkC),which subsequently dimerize and transduce signaling.BDNF elicits context-dependent signaling events that can lead to a myriad of biological functions primarily via its“specific receptor,TrkB.In addition to playing pivotal roles in both the central and peripheral nervous systems,BDNF has pleiotropic effects on whole organismal physiology,including,for example,sympathetic and parasympathetic regulation of cardiovascular functions (Fujitani et al.,2021),immunomodulation,and regulation of the body’s energy balance via influencing cellular responses to glucose and insulin,mitochondrial functions,thermogenic tissue differentiation,and exercise-mediated cognition (Di Rosa et al.,2021).
BDNF is arguably one of the most complex neuronal proteins,with nuanced differences at every level of its processing leading to vastly different outcomes.Full-length pro-BDNF can be actively or passively secreted to elicit antegrade or retrograde signaling.BDNF can be secreted into the bloodstream or act locally in an autocrine or paracrine fashion.The binding of pro-BDNF to p75leads to the recruitment of a co-receptor and subsequent signaling that differs depending on cell type and condition.Pro-BDNF can be cleaved before,during,or after secretion releasing the carboxy-terminal mature domain (mBDNF) and the pro-domain portion that participates in the processing and secretion of mBDNF.Binding of mature BDNF to TrkB leads to activation of a myriad of signaling pathways,including mitogen-activated protein kinase,phosphoinositol-3 kinase,phospholipase C,protein kinase A/cAMP,Janus kinase,nuclear factor kappa-light-chain-enhancer of activated B cells,and others.The prodomain may also function as a third independent ligand,although precisely how this occurs is not fully characterized.
It makes sense that the mechanisms of action of neurotrophic factors,such as BDNF might be highly context-dependent.On the other hand,BDNF’s coordinated effects on whole organismal physiology,including the central nervous system,parasympathetic,sympathetic,and localized organ functions,is incredible given the inability of BDNF to cross the blood-brain barrier.An excellent example of BDNF’s inter-cellular and inter-systemic functionality is at the neuromuscular junction where BDNF acts locally in response to the brain,environmental input,and whole-body metabolism.Despite the coordinated nature of these physiologically connected events,BDNF transcription and secretion in the neurological,muscular,and circulatory systems are apparently localized.BDNF is constitutively expressed in myofibers,satellite cells,Schwann cells,and endothelial cells and is upregulated in muscles in response to exercise and intermittent fasting.BDNF is important for normal muscle mass and functional maintenance and mediates exercise-induced enhancement of cognitive function and energy metabolism,but BDNF is not released into the circulation from contracting muscle.This revelation has fueled dozens of studies focused on BDNF-mediated retrograde signaling and subsequent neuromuscular function (Stuardo et al.,2020).The few studies of muscle cells themselves have shown that BDNF is required for maintaining myoblast progenitors and for the early phases of myogenic differentiation(Clow and Jasmin,2010).More recently,muscle-derived BDNF was shown to induce AMPK-mediated fat oxidation in an autocrine fashion (Ahuja et al.,2021).Additionally,BDNF plays a direct role in skeletal muscle fibertype specification and locomotion,as demonstrated using conditional BDNF knockout mice (Delezie et al.,2019).
Among the hundreds of polymorphisms in the BDNF gene,BDNF rs6265(G196A or Val66Met) in exon XI within the prodomain region of BDNF is one of the most common and consequential.Mechanistically,this non-conservative single nucleotide substitution alters BDNF expression,localization,and signal transduction contributing to nuanced alterations in phenotype.The rs6265 variant disrupts a translin-binding region required for targeting of BDNF mRNA to dendrites,and the G→A transition subsequently affects hippocampal function and episodic memory (Chiaruttini et al.,2009).In the BDNF protein,the methionine substitution interrupts a sortilin binding site,subsequently disrupting activity-dependent secretion of BDNF at the synapse.Moreover,replacement of the“G”allele with an“A”in the BDNF gene eliminates a CpG methylation site,allowing for epigenetic and genotypic interaction that could partially account for environmentally influenced phenotypes,like depressive disorders for example (Ursini et al.,2016;Nociti et al.,2018,2019;Nassan et al.,2020).The Val→Met change also elicits divergent signal transduction events and functional outcomes among the three alternative BDNF peptides.The amino acid substitution directly alters the~28 kDa proBDNF precursor isoform and subsequent signaling and also confers an acquired binding ability of the cleaved prodomain to sortilin related VPS10 domain-containing receptor 2 (Zanin et al.,2017).The polymorphism even affects downstream signaling of the otherwise identical“mature”portion,albeit indirectly via aberrant compartmentalization and dysregulation of the balance of pro-BDNF/p75NTR and mBDNF/TrkB signaling complexes.
A multitude of human genetic studies has demonstrated the critical structural and functional roles of BDNF rs6265 in the brain,such as synaptic plasticity,hippocampal volume,hippocampal activation,and memory performance(Additional Table 1
).Herein,we emphasize discussing the results of the latest studies that have reported novel functions of BDNF rs6265 and association with a variety of complex pathophysiologies (Figure 1
).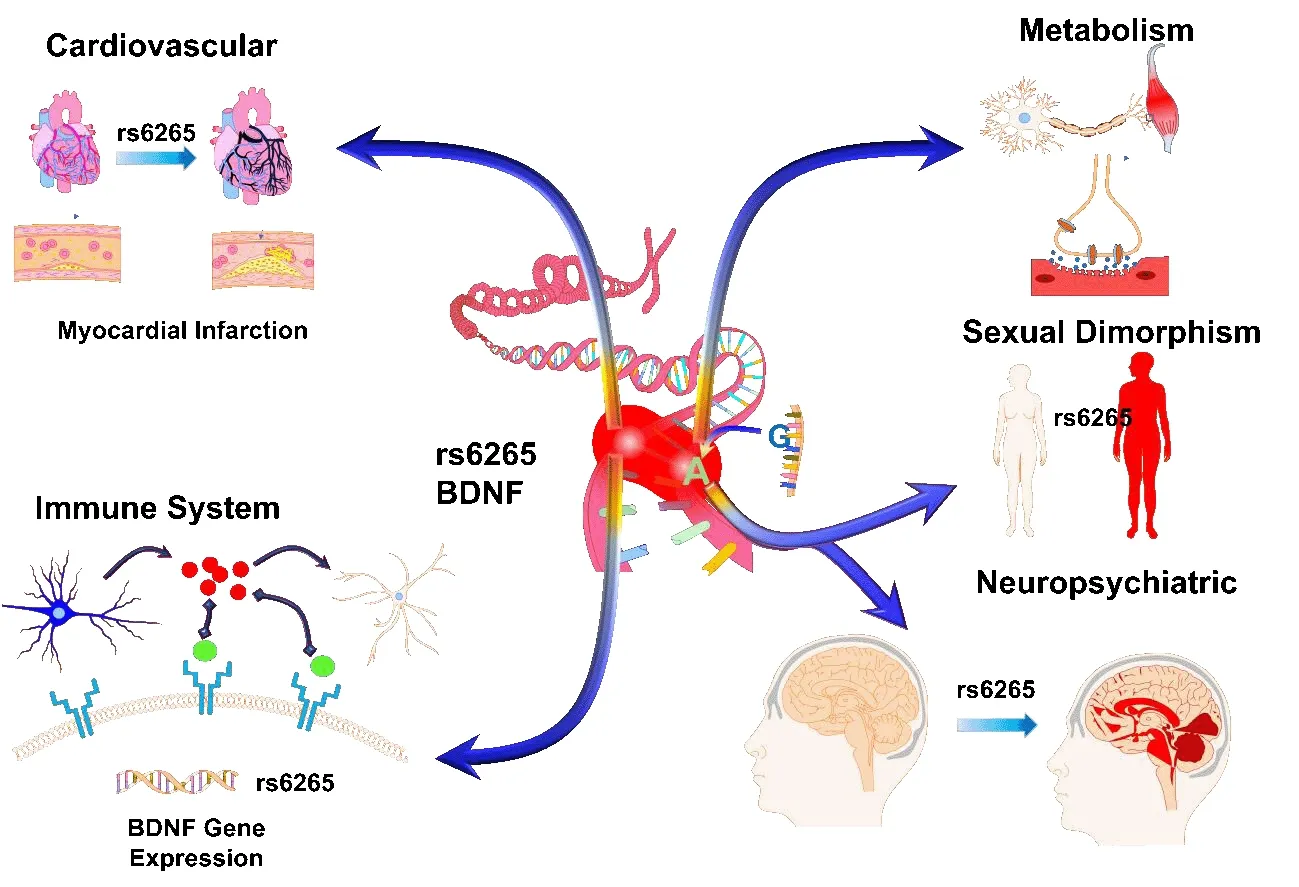
Figure 1 | Schematic showing effects of the brain-derived neurotrophic factor (BDNF)rs6265 single nucleotide polymorphism Val66Met on various body compartments.
Search Strategy and Criterion
PubMed literature searches were performed from August 2021 to January 2022 using the following search words:rs6265,[Val66Met NOT rs6265],[G196A NOT rs6265 NOT Val66Met],restricted to humans,and then articles were manually compiled by disease/condition/disorder.All years were chosen in the search.Only pathological conditions were included,with all presented in theAdditional Table 1
.For obtaining animal studies,the following terms were used in various combinations:BDNF,rs6265,Val66Met,proBDNF,BDNF prodomain– with obesity,skeletal muscle,Cre-recombinase,knockout,animal models,flox,TrkB,p75,neurotrophins,NGF,signaling pathways.Neuropsychiatric
There is a correlation between Met allele carrier status and BDNF disbalance in the brain that likely undergirds at least some of the associative studies linking BDNF rs6265 with various neuropsychiatric disorders,including posttraumatic stress disorder,depression,anxiety disorder,bipolar disorder,suicidal behavior,and schizophrenia (Additional Table 1
).On the other hand,relatively small cohorts and differences in outcome measures have provided conflicting results,suggesting that these conclusions may be influenced by gender,age,ethnicity,and epigenetics (Faris et al.,2020).For example,BDNF rs6265 was shown to be associated with risk of major depressive disorder in Chinese and Caucasians,whereas other studies of Korean or Japanese subjects found no association of BDNF rs6265 with major depressive disorder(Yan et al.,2014).Similarly,the BDNF rs6265 (Met66Met) genotype was associated with a higher risk of developing dyskinesia earlier during treatment with dopaminergic agents and with older age of onset (Foltynie et al.,2009);whereas subsequent studies were unable to find any association using similar clinical measurements (Gao et al.,2010;Karakasis et al.,2011).Association of BDNF with various other psychiatric disorders,including suicidal behavior and attention deficit hyperactivity disorder,have likewise yielded conflicting results (Additional Table 1
).Some of these discrepancies might be explained by relatively small numbers of patients leading to insufficient statistical power.Thus,obtaining replicated results would minimally require consideration of cross-sectional,environmental,and possibly epigenetic effects,for associative investigations of BDNF rs6265 and neuropsychiatric disorders at the haplotype and genotype levels (Abdolhosseinzadeh et al.,2020).A recent study of a large cohort of 392 Parkinson’s disease patients,including 227 BDNF Val/Val subjects and 152 BDNF Val66Met or Met66Met patients,reported a significant association between carrier status and various cognitive functions (van der Kolk et al.,2015).Met allelic carriers showed a significantly smaller decline in set-shifting during follow-up,suggesting a role for BDNF rs6265 in modulating mental flexibility in Parkinson’s disease patients.Interestingly,BDNF rs6265 (Met66) carriers had faster memory decline (4×),hippocampal volume loss,along with increased cerebrospinal fluid tau and ptau181 (6×) than Val66 homozygotes (Lim et al.,2018).An even larger study of 1024 older patients at baseline and 12-year follow-up showed a correlation between the BDNF rs6265 allele and the development of chronic late-life depression (Januar et al.,2015).This observation is consistent with other findings that the BDNF rs6265 polymorphism is related to the severity of depressive symptoms,independently of neurotransmitter levels (Czira et al.,2012).Human associative studies of BDNF rs6265 are especially difficult to interpret,in part due to influences of co-morbidities.A case in point is the correlation between BDNF rs6265 and cancer-related cognitive impairment,which can be further subdivided by chemotherapy regimens,nicotine usages,and genetic predisposition for anxiety or depression-related disorders(Additional Table 1).
Metabolic
BDNF regulates food intake and subsequently body weight (Vanevski and Xu,2013),and the rs6265 allele has been associated with eating disorders and obesity in humans (Additional Table 1
).There is evidence to suggest that the rs6265 allele might modulate metabolic homeostasis systemically.For example,increased depression was associated with Han Chinese subjects with Type 2 diabetes,purportedly by virtue of decreased serum levels of BDNF in rs6265 carriers (Zhou et al.,2013). A connection between the Met allele,lower serum BDNF,and West Ukrainian patients with Hashimoto’s thyroiditis has also been reported.Further,in an elderly Chinese population with exceptional longevity,the rs6265 allele was correlated with unfavorable metabolic profiles,including higher weight and blood pressure,along with lower high-density lipoprotein cholesterol levels (Peng et al.,2017).On the other hand,no association was found between rs6265 and metabolic disturbances in a study of veterans with post-traumatic stress disorder(Tudor et al.,2018).A significant impediment to ascertaining BDNF’s role in metabolism is the lack of knowledge related to BDNF-mediated regulation of food intake via the brain centers associated with hunger and satiation versus those linked to depression or other psychological confounders.Immune System
BDNF contributes to the neuroimmune axis as a bidirectional mediator between nerves and immune cells,which produce BDNF and express TrkB and p75receptors.BDNF also promotes the proliferation of central innate immune cells,and microglial cells secrete BDNF in response to inflammatory cytokines (Kozlov et al.,2020).In the context of immunity,BDNF has antinociceptive actions that may blunt neuropathic inflammatoryassociated pain.BDNF-mediated analgesic effects may be affected by the rs6265 mutation since G196A carrier and methylation status were found to be associated with chronic post-surgical pain (Tian et al.,2018) and pain catastrophizing in persons with fibromyalgia (da Silveira Alves et al.,2020;Polli et al.,2020).BDNF rs6265 also affects several neurodegenerative diseases with strong inflammatory components,including Alzheimer’s disease,amyotrophic lateral sclerosis,multiple sclerosis (MS),and bronchial asthma.BDNF’s role in non-neurogenic inflammation is less clear,but several isolated studies have suggested a wider involvement for BDNF in the immune system more generally.An association of the BDNF rs6265 allele was suggested for allergic rhinitis,chronic periodontitis,Hepatitis B-induced cirrhosis,and Crohn’s disease,for example (Additional Table 1
).These seemingly unrelated connections underscore the pleiotropic nature of BDNF’s inter-systemic influence on whole organismal physiology.As a chronic autoimmune neurodegenerative disease,MS provides perhaps the strongest support for the involvement of the BDNF rs6265 polymorphism in the neuroimmune axis.However,the results of human studies have been largely enigmatic due to conflicting results likely arising from interactions among multiple genetic factors (Shen et al.,2018),including epigenetic modifications (Nociti et al.,2019).A lower volume of cerebral gray matter and hippocampal subfields were reported for MS patients who are Met allele carriers (De Meo et al.,2021),and there might be sexual differences,as suggested by the association of the Met allele with increased MS susceptibility in females and earlier onset of disease in males (Mirowska-Guzel et al.,2008).This latter possibility is supported by genetic imaging studies that found differences in parieto-prefrontal network activation and hippocampal disengagement in healthy subjects,but not in MS patients(Cerasa et al.,2010).On the other hand,the Met allele has also been proposed as protective for MS (Portaccio et al.,2021),as others reported that MS Met allele carriers had preserved grey matter volumes (Zivadinov et al.,2007;Dinacci et al.,2011;Ramasamy et al.,2011),increased hippocampus posterior cingulate cortex connectivity (Fera et al.,2013) and higher circulating BDNF levels,relative to healthy control subjects (Liguori et al.,2009).Still,others have reported a lack of association of BDNF rs6265 with MS susceptibility or clinical course (Lindquist et al.,2005;Blanco et al.,2006;Mero et al.,2012).Larger and longitudinal studies,with patients segregated by gender and ethnic status,would perhaps provide clarity for some of these discrepancies.Moreover,an interaction between methylation status and MS disease progression was recently reported (Nociti et al.,2018,2019),suggesting epigenetic influences may additionally contribute to some of these discrepancies.
Cardiovascular
BDNF and its receptor play an important role in heart development,microvasculature development,and regulation of heart rate via brainstem cholinergic parasympathetic neurons.Nonetheless,the focus on BDNF’s role in the cardiovascular system is relatively recent,recently reviewed (Fujitani et al.,2021).An increase in circulating BDNF is thought to promote the survival of cardiomyocytes and is associated with increased expression of pro-angiogenic factors (Trombetta et al.,2020).These findings demonstrate that a higher level of cardiorespiratory fitness is associated with a higher level of circulating BDNF,which in turn is related to lower cardiovascular risk (Kermani and Hempstead,2019).However,the mechanisms involved in BDNF-mediated regulation of cardiovascular functions are poorly understood.Several studies investigated the potential association between BDNF rs6265 and cardiovascular disease,although the outcomes were unclear (Sustar et al.,2016;Amadio et al.,2017;Jiang et al.,2017).Insights are provided by some studies of healthy individuals,showing Val→Met mediated alterations in sympathovagal balance (Yang et al.,2010).Recent studies from our laboratory showed that the Met allele was associated with worse skeletal muscle function but better cardiac function in Duchenne muscular dystrophy patients(Raucci et al.,2020).Additional human studies are required,informed by animal models of cardiovascular diseases in controlled experimental settings(discussed in a separate section).
Sexual Dimorphism
Several studies have reported sex-linkage of rs6265 with a variety of psychiatric disorders,including patients with panic disorder (Konishi et al.,2014),as well as the age of onset of psychosis in cannabis users (Lodhi et al.,2019),which might result from differences in cerebral blood flow and functional connectivity between brain regions with high versus low BDNF expression (Wei et al.,2012).Moreover,estrogen affects expression levels of BDNF,and platelet levels of BDNF are altered during menstruation.Men and women also displayed differential reactivity to stress depending on carrier status,as measured by saliva levels of cortisol (Shalev et al.,2009).The male-specific linkage between rs6265 and cognitive benefits of physical activity (Watts et al.,2018),as well as cognition in older adult subjects,were likewise previously reported (Hupfeld et al.,2018;Barha et al.,2019).Carrier status in males was also shown to correlate with major depressive disorder(Foltynie et al.,2005;Verhagen et al.,2010),but replicative studies did not find a gender-specific association (Cho et al.,2010;Ozan et al.,2010).On the other hand,female-specific linkages were reported for methamphetamine use,(Heinzerling and Shoptaw,2012),attention-deficit/hyperactivity disorder,attention deficit hyperactivity disorder (Li et al.,2014),functional outcomes with post-stroke rehabilitation,and rate of long-term open-angle glaucoma progression (Shen et al.,2019).These findings provide yet another layer of complexity that could be related to the effects of BDNF on the cardiovascular system and metabolism,especially in light of reported sex-dependent effects of the BDNF rs6265 allele on sympathovagal balance (Chang et al.,2014).
Insights from Animal Models
Homozygous BDNF knockout mice die shortly after birth,due primarily to intramyocardial hemorrhage (Donovan et al.,2000),but heterozygous BDNFknockdown mice provide some insights.BDNFmice exhibit increased appetite and obesity,reduced locomotor activity,and mild but variable alterations in learning,memory,and psychological stress responses.Conditional deletion of BDNF has likewise provided valuable information regarding systemic versus tissue-specific effects of BDNF/TrkB signaling.Specific deletion of BDNF from microglial cells using BDNFmice crossed with mice expressing Cre-recombinase under control of the Cx3Cr1 (C-X3-C motif chemokine receptor 1) promoter,for instance,revealed that BDNF is protective against nerve injury-induced synapse remodeling and neuropathic pain (Huang et al.,2021).These findings also have implications for multiple sclerosis and other neuroinflammatory diseases.
The BDNF rs6265 mutation is human-specific and while human studies of the polymorphism are mainly limited to associative studies,the development of a transgenic knock-in mouse that expresses the human BDNF gene with G196A mutation (Chen et al.,2006) has provided valuable insights into the underlying mechanisms that can be related back to neurological and psychiatric disorders,energy use and obesity,and sexual differences.BDNF rs6265 involvement in neuropsychology is overwhelmingly represented in the literature (Additional Table 1
),for instance,because neuronal signaling is dramatically affected by the reduction in activity-dependent (but not constitutive) release of BDNF consequent to the Val→Met alteration.Lower bioavailability of BDNF impairs hippocampal function,the area of the brain that is important for learning and memory and is dysregulated in a variety of neurological and psychiatric disorders.Hippocampal BDNF expression is increased in response to exercise and is also thought to act by increasing muscle-specific production and release of FNDC5,a circulating adipokine with thermogenic properties (Ieraci et al.,2016).The rs6265 allele,however,was shown to abrogate exercise-induced up-regulation of BDNF expression,weight loss,and neurogenesis in mice after 4 weeks of voluntary wheel running (Ieraci et al.,2016).These mice have also been used as a model for diet-induced obesity,because when fed a high-fat diet they develop extreme obesity and metabolic dysfunctions (Yang et al.,2018),presumably better representing the physio-psychological complexity of disease in humans.However,whereas association studies in humans have linked the Met allele with lower BMI,mice with homozygous expression of the human BDNF gene with Met allele are considerably overweight,even when fed a normal ad lib diet,when compared to Val/Val littermate controls.Skeletal muscle-specific knockout of the gene encoding the TrkB receptor(Ntrk2
) using muscle creatine kinase (MCK)-promoter-driven Cre recombinase,on the other hand,showed that the BDNF/TrkB signaling nexus is essential for mitochondrial energy regulation and metabolic balance in diet-induced obese mice (Chan et al.,2015).Disentangling these discrepancies is complicated by BDNF’s pleiotropic regulation of multiple neurobiological processes,and in humans,additional modulatory environmental,psychological,and pharmacological interactions.In mice,BDNF rs6265 Met homozygosity was associated with arterial thrombosis (Amadio et al.,2017) and with increased pro-inflammatory and migratory macrophages after experimental myocardial infarction (Sandrini et al.,2020).Recent studies from our laboratory showed that cardiomyocytes isolated from transgenic Val66Met mice exhibit reduced myocyte contractility,which could help to explain observed alterations in cardiac function (Raucci et al.,2020;Negron et al.,2021).The BDNF rs6265 polymorphism possibly exerts subtle molecular signaling modifications that might exacerbate overt cardiac comorbidities.Recently reported alterations in basal cardiac gene expression profiles in Val66Met suggest that the BDNF rs6265 Met allele influences the development of diastolic dysfunction,perhaps in the context of comorbidities that exacerbate dysfunctional myocardial relaxation (Negron et al.,2021).On the other hand,a stronger inflammatory profile was observed specifically in male Met/Met mice,which might confer protection from cardiac remodeling and histopathology associated with the BDNF rs6265 polymorphism.These were serendipitous findings that ironically surfaced on the heels of a study aimed at defining the role of BDNF in Duchenne Muscular Dystrophy cardiomyopathy (Galindo et al.,2016),an X-linked disease affecting primarily the male population.Additional studies are required,perhaps utilizing alternative models in addition to Val66Met mice,such as recently developed mice (Bird et al.,2019) and rat models (Mercado et al.,2021)which express the rodent Val68Met equivalent mutation of the human BDNF Val66Met rs6265 polymorphism.
Brain-Derived Neurotrophic Factor-Based Therapies
BDNF’s generally positive effects across multiple organs coupled with its lower expression in a variety of disorders make it an attractive therapeutic target(Miranda-Lourenco et al.,2020).As the BDNF protein has a very short half-life and is also impermeable to the blood-brain barrier,strategies that modulate endogenously produced levels are desirable.Some herbal extracts,such as ginseng,for instance,were found to harbor BDNF-inducing compounds,identified by a high throughput screening strategy (Fukuchi et al.,2019).BDNF gene delivery using viral vectors or BDNF-producing cells,intranasal delivery,and conjugating BDNF to carrier molecules have been tested for circumventing the brain-blood barrier,with variable successes.BDNF gene transfer using an adenoviral vector is an especially promising strategy for the treatment of obesity and metabolic syndrome disorder,as demonstrated across several animal models of both genetic and diet-induced adiposity(Siu et al.,2017).Strategies aimed at TrkB receptor activation are another major area of focus,including small molecule agonists,BDNF mimetics,and receptor transactivators or facilitators.The small molecule flavonoidderivative,7,8-dihydroxyflavone,for instance,has been widely demonstrated in pre-clinical studies to mimic many of the positive effects of BDNF,such as efficacy for treating diet-induced obesity (Chan et al.,2015).So far,clinical trials aimed at testing neurotrophins therapeutics have largely failed,due to undesirable side effects and/or minimal efficacy as measured by magnitude and consistency of a variety of clinical outcomes (Miranda-Lourenco et al.,2020).These failures,however,only underscore the importance of increased study and the necessity of breaking organ-based boundaries to incorporate more systemic-based study using the valuable technical tools available and by developing new ones more reflective of human complexities and the highly contextual nature of BDNF.
Conclusion
Despite an astounding number of studies aimed at unraveling the physiological mystery of how BDNF modulates the human condition,there remain perhaps as many questions as there are answers.Mechanisms of action of BDNF are a conglomeration of genetic,somatic,and environmental cues that vary by organ and chronology.While the best scientific experiment is simple– a null hypothesis with a single variable– BDNF is perhaps a study in complex crosstalk that is more likely the reality for many,if not most signaling and regulatory proteins.Human studies of BDNF reflect this assertion,as comorbidities are increasingly considered and roles of BDNF outside of classical neuropsychological disorders are on the rise (Figure 2
andAdditional
Table 1
).Pushing the boundaries of BDNF research going forward will require cross-disciplinary and novel approaches,along with continued fortitude and patience for those of us who have chosen to study this enigmatic protein.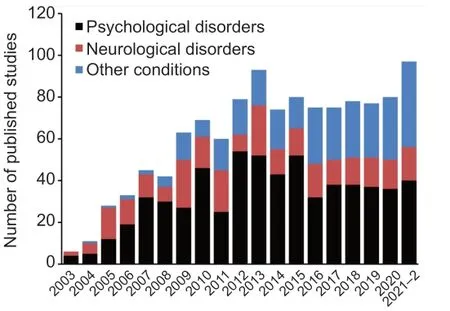
Figure 2 | The proportion of published studies of the brain-derived neurotrophic factor (BDNF) rs6265 polymorphism in association with non-neurological human disorders is increasing.
Author contributions:
Conceptualization,writing– original draft:CLG and VTN.Writing– review &editing:BH,NS,AH,MN,and CL.Funding acquisition:CLG.All authors approved the final version of the manuscript.
Conflicts of interest:
The authors declare no conflicts of interest.
Availability of data and materials:
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement:
This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional file:
Comprehensive list of published studies of the BDNF rs6265 polymorphism subdivided by disease or disorder.
- 中国神经再生研究(英文版)的其它文章
- Neuroaxonal and cellular damage/protection by prostanoid receptor ligands,fatty acid derivatives and associated enzyme inhibitors
- Extracellular vesicles in Alzheimer’s disease:from pathology to therapeutic approaches
- Molecular approaches for spinal cord injury treatment
- Sex-biased autophagy as a potential mechanism mediating sex differences in ischemic stroke outcome
- Adipose tissue,systematic inflammation,and neurodegenerative diseases
- Interleukin-1:an important target for perinatal neuroprotection?

