Gangliosides in nervous system development,regeneration,and pathologies
Juliana F.Vasques ,Renata Guedes de Jesus Gonçalves ,Almir Jordão da Silva-JuniorRobertta Silva MartinsFernanda GubertRosalia Mendez-Otero
Abstract Gangliosides,sialic acid-containing sphingolipids,are major constituents of neuronal membranes.According to the number of sialic acids and the structure of the oligosaccharide chain,gangliosides can be classified as simple or complex and grouped in different ganglio-series.Hundreds of gangliosides have been identified in vertebrate cells,with different expression patterns during development and related to several physiological processes,especially in the nervous system.While GD3 and its O-acetylated form,9acGD3,are highly expressed in early developmental stages,GM1,GD1a,GD1b,and GT1b are the most abundant ganglioside species in the mature nervous system.Mutations in enzymes involved in ganglioside metabolism can lead to the accumulation of specific species,a condition termed gangliosidosis and usually marked by severe neurological impairment.Changes in ganglioside levels have also been described in several neurodegenerative diseases,such as Alzheimer’s and Parkinson’s.In this review,we summarized recent information about the roles of GD3,9acGD3,GM1,GD1a,GD1b,GT1b,and other ganglioside species in nervous system development and regeneration,as well as clinical trials evaluating possible therapeutic applications of these molecules.
Key Words:9acGD3;gangliosides;GD1a;GD1b;GD3;glycolipids;GM1;GM2;GM3;GM4;GT1b
Introduction
Gangliosides are important components of biological membranes,consisting of amphipathic molecules with a negatively charged sialic acid-containing hydrophilic glycan headgroup linked to a hydrophobic ceramide tail.The many variations in the sugar composition and structure in the oligosaccharide chain,combined with variations in the length,saturation,and hydroxylation of the lipid moiety,generate a wide molecular diversity,with hundreds of gangliosides identified and described in all vertebrate cells and highly concentrated in the nervous system (Schnaar,2019).
Biosynthesis of gangliosides starts in the endoplasmic reticulum,with the formation of ceramide,which is then transferred to the Golgi apparatus,where it undergoes a gradual addition of monosaccharides by a series of specific glycosyltransferases.Lactosylceramide is the basis for most gangliosides,except for GM4,which is synthesized from galactosylceramide.Sequential addition of 1,2,or 3 sialic-acid molecules to the galactose residue of lactosylceramide by sialyltransferases forms the simple gangliosides GM3,GD3,and GT3,which serve as precursors for the synthesis of more-complex gangliosides of the a-,b-,and c-series,respectively,by elongation of the carbohydrate chain with additional galactose and N-acetylgalactosamine residues,which can also be sialylated.The O-series gangliosides have no sialic acid on the inner galactose residue of lactosylceramide (Figure 1
).For detailed information on ganglioside structure and biosynthesis,see Sandhoffet al.(2018).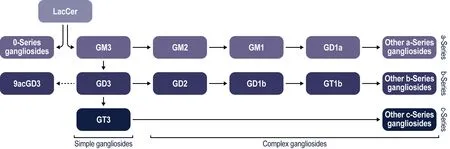
Figure 1 | Simplified schematic representation of ganglioside series and biosynthetic pathways.
Although they also traffic to subcellular compartments (Sano et al.,2009;Tsai et al.,2016;Ledeen and Wu,2018;Manganelli et al.,2021),gangliosides are mainly directed to the plasma membrane and inserted in the noncytosolic monolayer,with their glycans facing the extracellular space.Their molecular structure and biophysical properties favor cis interaction with other sphingolipids,cholesterol,and membrane proteins,contributing to the formation and stabilization of specialized membrane microdomains known as lipid rafts (Lunghi et al.,2021).In addition,spontaneous lateral self-association promotes ganglioside clustering,which facilitates trans-interaction with molecules located on the surface of juxtaposed cells or in the extracellular matrix.Either way,these arrangements promote approximation and interaction with specific signal transducers,adhesion receptors,or growthfactor receptors,leading to membrane assembly involved in carbohydratedependent functional cell adhesion and signaling,termed“glycosynapse”,which affect gene transcription and cellular phenotypes (Hakomori,2002).Gangliosides are widely expressed in mammalian tissues but are particularly abundant in the nervous system,where they were originally isolated and characterized (Klenk,1942).They occur primarily on the surface of neuronal cells and are enriched at the synaptic sites.The pattern of expression and the amount of gangliosides in the brain change consistently throughout development.While the simple gangliosides GM3 and GD3 predominate in earlier stages,more-complex species,particularly GM1,GD1a,GD1b,and GT1b,predominate in later phases and constitute more than 90% of the total ganglioside mass in adult life (Yu et al.,1988;Ngamukote et al.,2007).This change is related to developmental changes in the activity and gene expression of glycosyltransferases,which are under the stage-and celltype-specific regulation at both transcriptional and post-translational levels,including epigenetic regulation (Bieberich et al.,1998;Suzuki et al.,2011),and correlate with neurodevelopmental milestones,suggesting that gangliosides function in different cellular processes such as cell proliferation,migration,differentiation,neuritogenesis,synapse formation,and myelination.The importance of gangliosides in the development,integrity,and repair of the nervous system is evidenced by different pathological situations in which genetic mutations that compromise the synthesis or degradation of these molecules result in severe neurological disorders.Some of these disorders have a childhood onset,but altered ganglioside levels are also found in several neurodegenerative diseases such as Alzheimer’s disease (AD),Parkinson’s disease (PD),and amyotrophic lateral sclerosis,and in injury conditions,for example,stroke and spinal cord injury (see review by Sipione et al.,2020).In this review,we provided an overview of current knowledge of the expression of gangliosides in the nervous system and their functional roles in different aspects of neural development.We discussed the implications of gangliosides for neural repair and regeneration as well as therapeutic approaches,addressing how genetic deletion or exogenous administration of certain gangliosides has a neuroprotective effect in various injury or pathological conditions.
Search Strategy and Selection Criteria
We performed a literature search for original articles published in the last 10 years in the PubMed database,using the terms gangliosides,GM1,GD3,GD3,9-O-acetyl GD3,GD1a,GT1b,and GD1b,and selected the papers related to the nervous system.Although we focus on recent literature,we also mention classical and seminal studies that are critical to understanding the relationship between gangliosides and the development,function,and repair of the nervous system.
Roles of Gangliosides in Nervous System
Ganglioside GM1
The most abundant ganglioside in the adult central nervous system (CNS) is GM1,an a-series complex ganglioside derived from the simple ganglioside GM3.GM1 comprises 10–20% of the total ganglioside content in the mammalian CNS,is present in the plasma membrane of neurons and glial cells,and has been the most studied ganglioside species in recent decades(Chiricozzi et al.,2020;Liu S et al.,2021).In humans,GM1 is expressed at low levels at birth,increases slowly up to adulthood,and then progressively decreases with aging,which has been associated with cognitive decline and neurodegenerative diseases.The GM1 expression pattern suggests that it may be important in late-phase developmental and/or physiological processes.Interestingly,mice lacking GM1 and other complex gangliosides such as GD1a,GD1b,and GT1b showed motor and non-motor symptoms of PD,which were significantly attenuated by exogenous GM1 therapy (Wu et al.,2020).In another mouse strain lacking the same gangliosides (GM1,GD1a,GD1b,and GT1b),no significant differences were detected in dendritic morphology or spine density in motor-cortex neurons or the dentate gyrus,suggesting that the behavioral alterations seen in GM1-lacking animal models may be due to functional alterations in neural cells rather than to impairments in differentiation (Dobrović et al.,2011).GM1 also seems to be involved in the regeneration of the peripheral nervous system (PNS) in adults.An increase in the expression of Neu3 sialidase,the enzyme that converts GD1a,GT1b,and GD1b to GM1,was observed in axotomized neurons of the dorsal root ganglia,and inhibition of this enzyme reduced axonal regeneration.Interestingly,no alteration in Neu3 sialidase is detected in central neurons after lesion,but when this enzyme is exogenously applied to retinal explants,axonal growth is induced,indicating an important role of GM1 in regeneration (Kappagantula et al.,2014).
Overexpression of GM1 can be as deleterious to the CNS as its absence.GM1 is metabolized by β-galactosidase,and mutations in the β-galactosidaseencoding gene (GLB1
) induce GM1 accumulation in lysosomes,leading to GM1 gangliosidosis,a rare and extremely severe neurodegenerative condition,reinforcing the important relationship between GM1 levels and CNS development and function (for a detailed review of the different types of gangliosidosis,see Regier et al.,2016).Hinderer et al.(2020) recently demonstrated,using a murine model of GM1 gangliosidosis,that gene therapy using an adeno-associated viral vector carrying the correctGLB1
form injected directly into the cerebrospinal fluid reduced neurological abnormalities and increased lifespan.In addition,using patient-derived induced pluripotent stem cells with this mutation,Kajihara et al.(2020) showed that GM1 accumulates in neural stem cells (NSCs) and neurons derived from induced pluripotent stem cells,although no difference was detected in neurite outgrowth or in cell survival concerning control cells.However,the synaptic function was impaired in neurons derived from patients with GM1 gangliosidosis,and this alteration was due mainly to a reduction in neurotransmitter exocytosis.Several studies have found an important neurotrophic effect of GM1 in different cell types,through interaction with tropomyosin receptor kinase receptors in lipid rafts.Exogenous GM1 administration can affect the survival of CNS dopaminergic,glutamatergic,and cholinergic neurons,indicating a possible role for this molecule in the treatment of PD,AD,and other neurodegenerative disorders (see Chiricozzi et al.,2020,for review).In recent years,a series ofin vitro
studies have investigated the effects of the exogenous hydrophilic GM1 oligosaccharide chain (OligoGM1),the extracellular portion of GM1,which seems to be responsible for several bioactive properties.In a mouse neuroblastoma cell line,OligoGM1,by interacting with the tropomyosin receptor kinase-A,induced neuritogenesis (Chiricozzi et al.,2017),differentiation (Chiricozzi et al.,2019a),and neuroprotection (Chiricozzi et al.,2019b),and upregulated mitochondrial bioenergetics-related proteins(Fazzari et al.,2020).OligoGM1 also induced differentiation and maturationin vitro
of mouse cerebellar granule neurons (Di Biase et al.,2020a).The neuroprotective effects of GM1 have also been extensively studied in different preclinical models of neurologic damage and neurodegeneration.In vitro
,GM1 was able to attenuate cell death in NSCs exposed to propofol combined with remifentanil,general anesthetic drugs related to an increased risk of cognitive impairment in young children (Lu et al.,2017).Recent studies have demonstrated a beneficial effect of intraperitoneally administered GM1 on ketamine-induced neurotoxicity.For example,GM1 pre-treatment prevented cognitive deficits,reduced hippocampal apoptosis,and reversed the decrease in brain-derived neurotrophic factors induced by prolonged exposure to ketamine.Interestingly,these effects were abolished in the presence of a brain-derived neurotrophic factor-neutralizing antibody,suggesting that this neurotrophin is essential to GM1-induced neuroprotection (Meng et al.,2020).In a glaucoma model,GM1 injected into the vitreous body was able to inhibit the degeneration of retinal ganglion cells induced by axotomy via mitogen-activated protein kinase activation and cAMP response element-binding phosphorylation (Choi et al.,2003).Moreover,GM1 upregulates the levels of nerve growth factor and brain-derived neurotrophic factor,which act as key modulators for retinal cell survival and are usually decreased during retinal degeneration.Also,GM1 upregulation in astrocytes is induced during the degeneration of retinal ganglion cells (Pappenhagen and Inman,2018).In primary cultures of cortical astrocytes,GM1 treatment modulates glucose metabolism,facilitating astrocytic glycolysis,glycogen mobilization,and lactate secretion.In the presence of astrocytes,GM1 induces neuroprotection after exposure to excitotoxic doses of glutamate and enhances mitochondrial activity (Finsterwald et al.,2021).As mentioned above,mice lacking GM1 and other complex gangliosides are considered a preclinical model of PD (Wu et al.,2020),and Schneider et al.(2019) and Itokazu et al.(2021) recently demonstrated that intraperitoneal and intranasal infusion of GM1 reduced intracellular α-synuclein levels and increased the content of the dopaminergic marker tyrosine hydroxylase in the substantia nigra in α-synuclein-based rodent models of PD.Last,GM1 interacts with amyloid-β42,which is the most neurotoxic isoform of this peptide and is directly involved with AD pathology.This interaction may prevent amyloid-β oligomerization and protect against amyloid-β-induced neurotoxicity (Fatafta et al.,2021).Considering the benefits found in the preclinical models,several clinical trials have evaluated the responses to treatment with GM1 in patients with different pathologies.For example,Geisler et al.(1991) conducted a prospective,randomized,placebo-controlled,double-blind study with 34 patients with acute traumatic spinal cord injury,and found promising effects of intravenous injection of GM1 (trade name Sygen®) on the patients’ neurologic scores and motor recovery.The group conducted a second clinical trial with the same drug,at 28 centers in North America,including 760 patients with acute spinal cord injury,and found minimal and scattered adverse events(safety) and beneficial effects in patients with less-severe injuries (efficacy).However,Sygen therapy did not induce a significant improvement concerning a placebo in the most severely affected patients (Geisler et al.,2001).Trials with GM1-based therapies have been halted for several years due to reports that GM1 treatment might increase anti-ganglioside antibodies and result in Guillain-Barré syndrome in these patients (this hypothesis has proven to be equivocal,as it has been demonstrated that long-term intravenous and subcutaneous treatment with GM1 without adjuvants did not elicit anti-GM1 antibodies (Schneider et al.,1995));and especially due to the concern about bovine spongiform encephalopathy,as GM1 used in clinical trials was originally isolated from calf brains.In recent decades,clinical trials were conducted using porcine GM1.Nowadays,both bovine and porcine GM1 are considered safe and are used in clinical trials.For example,clinical trials have investigated a possible beneficial role of bovine GM1 in PD and the neurotoxicity and neuropathy generated by chemotherapy treatment.Regarding PD,a double-blind,randomized,controlled trial was carried out at a single center in the United States,to evaluate the effects of subcutaneous delayed-onset treatment with GM1 in patients with PD.The prolonged use of GM1 ganglioside proved to be safe,had an early symptomatic effect,and delayed the progression of symptoms for 2 years.These beneficial effects of GM1 were related to functional improvement in residual dopaminergic neurons (Schneider et al.,2013).For better comparison,a second pilot positron-emission tomography imaging study examined the effects of GM1 on dopamine transporter binding and on the integrity of dopaminergic terminals in the striatum,a parameter of disease progression.The symptomatic effects of GM1 were similar to those in the previous study,and treatment attenuated the loss of dopamine transporter binding in various regions of the striatum,which in some cases even increased (Schneider et al.,2015).
Clinical trials have also evaluated the effects of GM1 in preventing chemotherapy-induced neurotoxicity and neuropathy,which are the main causes of reduced treatment in cancer and therefore a limiting factor in treatment effectiveness.A case report of a patient with gastrointestinal tumors demonstrated that GM1 given intravenously once a day for 3 days reduced oxaliplatin-induced high-grade neurotoxicity (Zhu et al.,2013).A second study conducted at four academic hospitals in China evaluated oxaliplatin-induced peripheral neurotoxicity in colorectal cancer patients.The data from this trial supported the evidence that intravenous GM1 treatment could attenuate symptoms of acute neuropathy,although no evidence was found to support the use of GM1 to prevent cumulative neurotoxicity (Wang et al.,2020).Finally,the protective effect of GM1 on peripheral neurotoxicity induced by another chemotherapy agent,taxane,was recently evaluated.This clinical study analyzed the effect of intravenous administration of GM1 on the prevention of taxane-induced peripheral neuropathy,starting from a randomized,double-blind,placebo-controlled study,including 206 patients with early-stage breast cancer,and demonstrated that GM1 clinically reduced the severity and incidence of taxane-induced peripheral neuropathy (Su et al.,2020).Together,these studies revealed the broad range of functions related to GM1 signaling in the CNS and have reinforced it as a target in the treatment of different neurodegenerative diseases,mainly due to its neuroprotective effects.
Ganglioside GD3
GD3 is a b-series ganglioside produced by the enzyme St8sia1,also known as GD3-synthase (GD3S).GD3 is highly expressed in several regions of the nervous system during development,including the retina,hippocampus,and cerebellum,and has been related to important developmental processes such as cell migration and axonal extension.GD3 levels decrease progressively as the nervous system matures,and the synthesis of more-complex gangliosides such as GM1,GD1a,GD1b,and GT1b increases (Itokazu et al.,2017).GD3 is especially enriched in mouse NSCs and is present in approximately 80%of these cellsin vitro
;the reduction in GD3 expression correlates with a decrease in their proliferative capacity (Nakatani et al.,2010).In vivo
knockout of GD3S (GD3S-KO) induces a significant postnatal reduction in the number of NSCs in brain areas related to adult neurogenesis,such as the subventricular zone and dentate gyrus of the hippocampus,and results in depression-like behaviors (Wang et al.,2014).Interestingly,intracerebroventricular injection of GD3 in adult GD3S-KO mice restores the NSC population in both regions(Itokazu et al.,2019).Our group recently reported that GD3S-KO mice showed important alterations in the visual system.For example,adult GD3S-KO mice have a significant reduction in rod and retinal ganglion cell populations.Retinal ganglion cells are the only action potential-firing neurons in the retina,and their axons constitute the optic nerve,from which electrical signals are transmitted to visual regions in the brain.As expected,the number of axons in GD3S-KO mice was also significantly reduced concerning wild-type animals.Moreover,GD3S-KO mice displayed electrophysiological alterations in retinal ganglion cells,photoreceptors,bipolar and amacrine cells as detected by electroretinography,and reduced contrast sensitivity and visual acuity as seen in the optomotor response test (Abreu et al.,2021).GD3 also seems to be important in PNS development and regeneration.Adult GD3S-KO mice show reduced axonal density and myelination ratio in the sciatic nerve,as well as a lower pain threshold and impaired motor outcome at higher rotarod speeds.In vitro
approaches using dorsal root ganglia neurons demonstrated that neurite extension in GD3S-KO cells was significantly reduced compared to neurons from wild-type mice,but treatment with exogenous GD3 abolished this difference (Ribeiro-Resende et al.,2014).In a model of antibody-mediated injury to the motor nerve,aged GD3S-KO mice showed mild impairment of spontaneous regeneration of neuromuscular junctions compared to younger animals (Rupp et al.,2013).After sciatic-nerve crush,however,spontaneous axonal regeneration was greatly reduced in GD3S-KO mice compared to wild-type animals,a condition that was partially reversed by exogenous GD3 administration at the injury site(Ribeiro-Resende et al.,2014).Alterations in GD3 levels have also been described in different neurodegenerative diseases,such as Huntington disease,PD,and AD.GD3 is directly involved with apoptosis,triggering the opening of the mitochondrial permeability transition pore (Kristal and Brown,1999).In a mouse model of PD,knocking GD3S expression out or down protected about 50% of the dopaminergic neurons from degeneration and abolished the motor deficits induced by the parkinsonian toxin MPTP.The neuroprotective effects may be due to reduced GD3-mediated apoptosis,an increase in GM1 and GD1a levels,or both (Akkhawattanangkul et al.,2017;Dhanushkodi et al.,2019).GD3 and GD3S levels are upregulated in microglia after induction of global cerebral ischemia,and the loss of neurons in the hippocampus and the microglial phagocytic capacity and expression of the activation marker CD68 were significantly reduced in GD3S-KO animals (Wang et al.,2021).
Using a mouse model of AD,Bernardo et al.(2009) showed that GD3S-KO reduces the deposition of β-amyloid senile plaques and improves cognitive function.Conversely,intracerebroventricular injections of GD3 in another mouse model of AD restored the number of newly generated neurons in the hippocampus,therefore promoting neurogenesis (Itokazu et al.,2019).Alterations in GD3 levels have also been related to viral infections that affect the nervous system.For example,the Zika virus is a flavivirus associated with microcephaly and Guillain-Barré syndrome.Adult patients with no neurological alterations have increased serum levels of IgG autoantibodies against GD3 in the acute phase of Zika virus infection,which can be related to alterations in the NSC population and therefore to microcephaly (Nico et al.,2018).Recently,anti-GD3 IgM antibodies were detected in the serum of a patient with SARS-CoV-2 para-infectious myelitis,suggesting a possible role of this ganglioside in some of the neurological manifestations associated with COVID-19 (Rodríguez de Antonio et al.,2021).Finally,ganglioside biology is intimately related to cancer pathophysiology.GD3 is highly expressed in several types of tumors and participates in different pathological mechanisms,such as cancer cell proliferation and tumor invasion (Liu et al.,2018).Recently,Ohkawa et al.(2021) reported that GD3S-KO mice with glioma showed a reduction in cancer cell malignancy and prolonged survival,suggesting that GD3S may be an interesting therapeutic target for this disease.
9-O-acetyl GD3
Gangliosides,such as GD3,can be acetylated,and although it is not yet understood how acetylation impacts their function,this post-translational modification appears to occur in specific brain regions and at precise developmental times.O-acetylation of GD3 by the sialate O-acetyltransferase,encoded by theCASD1
gene,generates 9-O-acetyl GD3 (9acGD3).9acGD3 is abundant during nervous-system development,and its expression pattern is associated with cell migration and axonal extension in several regions.In the postnatal period,9acGD3 expression is drastically reduced,remaining detectable in a few CNS regions such as the subventricular zone (SVZ),rostral migratory stream,retina,and cerebellum (Mendez-Otero et al.,1988;Schlosshauer et al.,1988).9acGD3,as well as its precursor GD3,is preferentially expressed in immature cells,such as NSCs.9acGD3 is found in neuroepithelial,radial glia,and NSCs derived from embryonic stem cells(Azevedo-Pereira et al.,2015;Gubert et al.,2017).9acGD3-positive cells can be sorted from the developing or adult ventricular zone/SVZ to enrich a population of NSCs (Gubert et al.,2017).The SVZ is considered one of the few brain regions where neurogenesis persists in the adult,and the presence of 9acGD3 and its colocalization with other NSC markersin vivo
indicates that expression of this ganglioside may be related to the neurogenic potential of SVZ.Although this molecule is not exclusively expressed by NSC and progenitors,also being present in neuroblasts,it is not commonly found in mature neurons or stellate astrocytes (Mendez-Otero and Constantine-Paton,1990;Gubert et al.,2017).Several studies have shown the importance of 9acGD3 in neuronal migration during development.Santiago et al.(2004) found that bothin-vitro
andinvivo
9acGD3 inhibition impairs radial migration of cerebellar granule cells.A subsequent study demonstrated that 9acGD3 blockade or absence leads to internalization of the P2Y1 receptor and to a reduction in the frequency of spontaneous calcium oscillations mediated by this receptor,suggesting that 9acGD3 could act in neuronal migration,modulating P2Y1 function and localization (Santiago and Scemes,2012).Although the mechanisms are not fully understood,9acGD3 inhibition also impairs tangential migration,decreasing the number of neuroblasts that leave postnatal SVZ explants(Miyakoshi et al.,2012).Yang et al.(2007) showed that some 9acGD3 antibodies could also recognize β1-integrin receptors;however,other studies failed to find this epitope in protein extracts from SNC or in GD3S-KO mice(Constantine-Paton et al.,1986;Schlosshauer et al.,1988;Kawai et al.,2001;Santiago and Scemes,2012).Santos-Silva et al.(2019) found that in cultures of olfactory ensheathing cells,9acGD3 plays a role in the migration of both neuronal and glial cells.In olfactory ensheathing cells,9acGD3 colocalizes with β1-Integrin,probably in lipid rafts,membrane microdomains associated with cellular functions such as cell-cell or cell-matrix contact (Campos et al.,2021).The presence of 9acGD3 in growth cones and its colocalization with integrin indicate that this molecule is also important in axonal growth(Negreiros et al.,2003).The role of 9acGD3 in nervous-system lesions and diseases has been little studied.The expression of this ganglioside in adult rats is restricted to the above-mentioned regions,but after sciatic nerve crush,9acGD3 is reexpressed in this nerve during axonal regeneration During this process,9acGD3 is re-expressed not only in regenerating neurons but also in the migratory Schwann cells that support this process (Ribeiro-Resende et al.,2007).Considering that GD3S-KO animals do not express 9acGD3 and have reduced regenerative potential of peripheral nerves,as described above,it is possible to question whether the presence of 9acGD3 is important for the normal regeneration capacity seen in this system.
Another interesting point regarding 9acGD3 is its anti-apoptotic function,in contrast to its precursor,GD3,which seems to have pro-apoptotic potential.Birks et al.(2011) observed that in tumors,such as glioblastomas,9acGD3 is highly expressed,and that cleaving acetyl groups restored GD3 expression and decreased tumor viability.Interestingly,unlike its putative membrane expression during development and in the adult SVZ (Gubert et al.,2017),in glioblastomas,9acGD3 seems to be expressed intracellularly (Birks et al.,2011).The anti-apoptotic function of 9acGD3 has been described in other cancer cells,such as lymphoblasts of patients with childhood acute lymphoblastic leukemia.The multiple functions associated with 9acGD3 and its high expression during nervous-system development indicate that this molecule is important during this period.9acGD3 persistence in specific CNS areas in the adult,however,is little explored and could provide important insights about SVZ or retina physiology,for instance.
Other gangliosides
Other gangliosides are highly expressed and related to the development,maintenance,and/or degeneration of the nervous system.The most predominant gangliosides at later stages of CNS development are GD1a,GD1b,and GT1b.Their levels,however,decrease naturally during aging,as reported in mice (Vorwerk,2001;Ngamukote et al.,2007).GD1a and GT1b are the most abundant gangliosides in axonal membranes (De Vries and Zmachinski,1980).The a-series ganglioside GD1a is one of the major ligands for myelin-associated glycoprotein.GD1a,as well as GM1b,can bind to myelin-associated glycoprotein with high affinity and compensate for the effects on peripheral axonal integrity seen in transgenic mouse models expressing only GM3,but not in mouse strains lacking a-and b-series gangliosides (Lopez and Baez,2018,for review).Exogenous addition of GD1ain vitro
andin vivo
in cuprizone-induced demyelination models stimulates oligodendrocyte progenitor cells to proliferate,mature,and promote remyelination (Qin et al.,2017).GD1b,a b-series ganglioside,is expressed in both white and gray matter of the spinal cord (Vajn et al.,2013).A recent case report suggested a relationship between serum anti-GD1b and loss of myelin and axon integrity in a patient with chronic sensory ataxic neuropathy and chronic lymphocytic leukemia,suggesting a role of GD1b in axon-myelin stabilization (Tagliapietra et al.,2020).GT1b is moderately expressed in all Rexed’s laminae of gray matter (Vajn et al.,2013).Immunohistochemistry revealed that 90% and 18% of small and medium-sized neurons,respectively,and only 11% of large neurons in dorsal root ganglia were stained with antibodies against GD1a (Gong et al.,2002).Along with GD1a,GT1b can participate in axon outgrowth and regeneration inhibition by its association with a multimeric inhibitory signaling complex that includes Nogo receptor and myelin-associated glycoprotein (Sipione et al.,2020).Interestingly,as mentioned above,in the PNS,GD1a,and GT1b are converted to GM1 after axotomy,which could destabilize their interaction with myelin and favor axon growth by preventing the inhibitory action of myelin (Kappagantula et al.,2014).The inhibitory activity of GD1a and GT1b in axon growth could be blocked,as suggested by Xu and coworkers (Xu et al.,2015),after treatment with anti-GD1a and GT1b antibodies.In their study,this treatment resulted in neurite outgrowthin vitro
and preservation of spinal-cord axons and neurons,as well as increased lifespan in in-vivo models of amyotrophic lateral sclerosis.Additionally,reduced levels of GT1b and GD1a have been detected in patients with PD (Huebecker et al.,2019) and a model of Huntington’s disease (Maglione et al.,2010).GT1b has been related to glutamatergic stimuli,and its interaction with the AMPAR-trafficking complex may contribute to the internalization of this receptor (Prendergast et al.,2014).Intraplantar injection of GT1b produced nociceptive responses and enhanced formalin-induced nociception,related to NMDA and metabotropic glutamate receptors (Watanabe et al.,2011).Alternatively,GT1b may act as a protective molecule,since intraventricular injection of GT1b inhibited DNA damage and seizures in animals with kainic acid injected into the striatum(Yamamoto and Mohanan,2003).In addition,Lim et al.(2020) showed that GT1b acts as a TLR2 agonist and induces proinflammatory microglia activation and central sensitization in a spinal cord injury model.GM2,a monosialic ganglioside,comprises close to 5% of total ganglioside mass in the CNS but was suggested as one of the main gangliosides expressed in mouse spinal cord motor neurons (Matsumoto et al.,1995;Vorwerk,2001;Leal et al.,2020).In GM2 synthase knockout mice,the lack of GM2 as well as several other complex gangliosides,such as GD2,GM1,GD1a,GD1b,and GT1b,leads to progressive demyelination and axonal degeneration in the CNS and PNS with age (Sheikh et al.,1999);while GM2 accumulation induces cytotoxic effects,mainly in neurons,leading to neuronal death(Leal et al.,2020).Therefore,large variations in the levels of GM2 can lead to different deleterious processes.GM3,another monosialic ganglioside,is expressed mainly in non-neural tissues,but can also be found in astrocytes(Prokazova et al.,2009).Evidence supports the hypothesis that GM3 regulates insulin signaling:a decrease in insulin-receptor signaling can be correlated with the increase in GM3 levels in AD brains since GM3 was shown to suppress IGF-1 receptor signaling (Chan et al.,2012;Talbot et al.,2012;Dam et al.,2017).Kabayama et al.(2007) found that GM3 is increased in the adipocyte plasma membrane and binds to the extracellular domain of the insulin receptor,resulting in receptor internalization and inhibition of insulin signaling.Furthermore,GM3-synthase knockouts show enhanced insulin signaling (Yamashita et al.,2003).Menichella et al.(2016) showed that GM3 depletion prevented denervation in mouse footpad skin and fully reversed neuropathic pain in diet-induced obese diabetic mice.Regarding the nervous system,mutations in both GM2 and GM3 synthases impair the production of the ganglio-series of glycosphingolipids,leading to axon degeneration in the CNS and perturbing axon-glia interactions (Yamashita et al.,2005).Nevertheless,GM3 has been related to neuroprotective effects,acting as a low-affinity receptor for soluble a-Klotho,an endocrine factor with important neuroprotective,cognitive-enhancing,and antiaging properties(Dalton et al.,2017).A recent study showed that the intracerebroventricular administration of exogenous GM3 in an amyotrophic lateral sclerosis model delayed the onset of the disease and prolonged animal survival (Dodge et al.,2015).GM4,a less-discussed monosialic ganglioside,is found in high amounts in myelin and to a lesser extent in astrocytes (Yu and Iqbal,1979).Nonetheless,a recent study showed that the addition of fluorinated GM4 to oligodendrocyte progenitor cellsin vitro
increased the differentiation of mature oligodendrocytes,although common GM4 had no effect (Kieser et al.,2018).Therefore,manipulation of GM4 could influence myelination in therapeutic strategies.Finally,as mentioned above,several gangliosides have been associated with cancer.GD2 is usually expressed in low levels only in the nervous system and a few other cell types such as melanocytes,lymphocytes,and mesenchymal stem cells.However,this ganglioside is highly expressed in several types of solid tumors,including (but not limited to) neuroblastomas,retinoblastomas,and melanomas,which makes it an interesting tumor-cell marker and target for therapeutic agents (Nazha et al.,2020,for review).Prapa et al.(2021)demonstrated that glioblastomas also express high levels of GD2 and that this ganglioside is a valuable antigen target for chimeric antigen receptor-T cell therapy,a promising therapeutic approach for different types of cancers,not only glioblastomas.
Conclusion
Over the years,many efforts have been made to unravel the mechanisms underlying degeneration and regeneration in the CNS and PNS,especially in protein cellular components,gene expression,and signaling pathways.Yet,little is known about the function of lipids,especially gangliosides,in regenerative processes.Gangliosides are important membrane components of the CNS and are present in glial and neuronal cells,regulating physiological and pathological processes,including neurogenesis,inflammation,and axon regeneration (Figure 2
).In the CNS,regenerative processes require the recapitulation of certain developmental programs.After lesion,while the portion distal to the injury site degenerates,growth cones are formed in the proximal stump of the injured axon and developmental genes are expressed.For instance,mature neurons upregulate the expression of genes that are mostly expressed during development,such as growth-associated protein-43 and activating transcription factor 3,to enter a regenerative state (Chauhan et al.,2020).Some gangliosides participate in neural development,and while their role in regeneration is not fully understood,they could be modulated to favor regeneration in the mature CNS.9acGD3,for example,is important for development,participating in neuron migration and neurogenesis,and returning to be expressed in regenerating mature neurons.Also,it is important to identify the molecules with which gangliosides interact to cause their effects.Hakomori (2010) reported that some gangliosides may interact with growth-factor receptors or kinases via tetraspanins,forming a complex called glycosynapse.The implication of these structures for regeneration and development is still unknown.There are interesting animal models in which ganglioside synthases are knocked down or knocked out.In these models,not only is a specific ganglioside absent,but several gangliosides downstream from the silenced enzyme fail to be synthesized.Knockout animals also accumulate upstream gangliosides.Therefore,knockout models can help to clarify the role of a group or series of gangliosides or the importance of the enzyme itself,although it is difficult to investigate the role of a single ganglioside in models.In double-knockout models,where two enzymes are absent and consequently more gangliosides are lacking,this difficulty is increased.Furthermore,gangliosides may undergo post-translational modifications,such as acetylation,that directly impact their function,as exemplified here by GD3 and 9acGD3.In terms of regeneration,evidence provided in this review suggests that some gangliosides,such as GD1a and GT1b,are involved in the stabilization of the myelin-axon interaction.Although the myelin-axon interaction may be inhibitory for axon regeneration,remyelination is required to recover axon function,and certain gangliosides are important for this process.It would be interesting to replace specific gangliosides in combination with regenerative strategies in an appropriatetime window,to promote remyelination and prevent myelin-related inhibition of growth.In therapy with gangliosides,systemic administration can be difficult because of their limited capacity to cross the blood-brain barrier.Less than 0.4% of a radiofluorinated ganglioside was found in a non-human primate brain after intravenous injection (Revunov et al.,2020).On the other hand,GM1 micelles crossed the blood-brain barrier when injected intracardially in zebrafish and intravenously in rats,and increased the delivery of doxorubicin to brain tumors (Zou et al.,2017).Interestingly,Di Biase et al.(2020b) reported,using anin-vitro
model,that the oligosaccharide portion of GM1 can cross the human blood-brain barrier.Therefore,differences among species must be considered.Intracerebroventricular administration most efficiently delivers gangliosides into the brain,although it is an invasive technique.
Figure 2 | Schematic representation of ganglioside functions in nervous system development,function,and repair.
Some gangliosides,such as GM1,GM3,or GT1b,have been shown to act as neuroprotective molecules.Therefore,if proven effective,gangliosides could be combined with an approach promoting axon regeneration,since axon regeneration is not necessarily related to neuroprotection;this would be a more complete and efficient therapy.Great care is needed to guarantee the purity of gangliosides used in therapy.Some preparations of gangliosides extracted from nerve tissues can be contaminated with proteins,since some gangliosides are associated with them,such as myelin basic protein;therefore,preparation of these gangliosides requires special steps for purification.This being the case,evaluation of studies that did not provide the source or purity of gangliosides must take into account the possibility of alterations resulting from contaminants,especially in terms of therapy.Gangliosides are prevalent in the nervous system and are crucial for several aspects of neural development,but because they are technically more difficult to extract and study than proteins,for example,many of their properties remain little understood.Despite the technical difficulties and limitations in isolating gangliosides and evaluating their clinical use,the evidence reviewed here demonstrates that ganglioside-based therapies may be a promising alternative for the treatment of different types of injuries or diseases,promoting neuroprotection,repair,and regeneration.
Acknowledgments:
We thank Janet W.Reid (JWR Associates,New York,NY,USA) for revising the manuscript,Manuela de Lima Corrêa Magalhães (no affiliation) and Vladmir Pedro Peralva Borges Martins (Universidade Federal Fluminense,Niteroi,Brazil) for assistance with the figures.
Author contributions:
JFV,RGJG,AJSJ,RSM and FG performed data search and manuscript writing;JFV compiled and revised the manuscript;RMO discussed,reviewed and corrected the text.All authors approved the final version.
Conflicts of interest:
The authors declare no conflicts of interest.
Open access statement:
This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Neuroaxonal and cellular damage/protection by prostanoid receptor ligands,fatty acid derivatives and associated enzyme inhibitors
- Extracellular vesicles in Alzheimer’s disease:from pathology to therapeutic approaches
- Molecular approaches for spinal cord injury treatment
- Sex-biased autophagy as a potential mechanism mediating sex differences in ischemic stroke outcome
- Adipose tissue,systematic inflammation,and neurodegenerative diseases
- Interleukin-1:an important target for perinatal neuroprotection?

