Protocadherin gamma C3:a new player in regulating vascular barrier function
Victoria Kaupp ,Kinga G.Blecharz-Lang ,Christina Dilling ,Patrick Meybohm ,Malgorzata Burek,
Abstract Defects in the endothelial cell barrier accompany diverse malfunctions of the central nervous system such as neurodegenerative diseases,stroke,traumatic brain injury,and systemic diseases such as sepsis,viral and bacterial infections,and cancer.Compromised endothelial sealing leads to leaking blood vessels,followed by vasogenic edema.Brain edema as the most common complication caused by stroke and traumatic brain injury is the leading cause of death.Brain microvascular endothelial cells,together with astrocytes,pericytes,microglia,and neurons form a selective barrier,the so-called blood-brain barrier,which regulates the movement of molecules inside and outside of the brain.Mechanisms that regulate blood-brain barrier permeability in health and disease are complex and not fully understood.Several newly discovered molecules that are involved in the regulation of cellular processes in brain microvascular endothelial cells have been described in the literature in recent years.One of these molecules that are highly expressed in brain microvascular endothelial cells is protocadherin gamma C3.In this review,we discuss recent evidence that protocadherin gamma C3 is a newly identified key player involved in the regulation of vascular barrier function.
Key Words:blood-brain barrier;brain microvascular endothelial cells;permeability;protein interaction;protocadherin gamma C3;protocadherins;tight junctions
Introduction
In the case of neuroinflammatory cerebrovascular diseases such as stroke,traumatic brain injury (TBI),subarachnoid hemorrhage (SAH),or others,the blood-brain barrier (BBB) is decisively influenced by the proinflammatory environment.In the acute stage,the changed dynamics and molecular composition of endothelial tight junction (TJ) proteins lead to a significant increase in capillary permeability.Brain edema is a common complication of capillary leakage.In most cases,acute swelling of the brain leads to a craniectomy and,from the point of view of the health system,requires costintensive care.Changes observed in delayed secondary brain injury after TBI,stroke,or SAH,such as increased brain edema,acute inflammation,and hyperexcitability in the injured part of the brain as a result of endothelial barrier leakage,have been attributed to the loss of claudin-5 at the molecular level.Claudin-5 is a TJ protein at the BBB with a molecular weight of 23 kDa and an important marker for barrier tightness (Greene et al.,2019;Winkler et al.,2021).After a stroke,TBI or SAH,its protein level first drops within 6 hours and then after 3 days,which leads to a biphasic opening of the BBB (Campbell et al.,2012;Knowland et al.,2014).In addition to TJs and adherens junctions (AJs),which are responsible for sealing the BBB,the latest scientific findings highlight another molecule,protocadherin gamma C3 (PCDHGC3),a protein with a molecular weight of 130 kDa,as an additional important molecular regulator of BBB permeability.This review discusses the role of protocadherins (PCDHs)and their major influence on the control of BBB tightness in brain microvascular endothelial cells (BMECs) and cerebrovascular diseases.
Search Strategy
Studies cited in this review were searched on PubMed using the following keywords:cadherin,protocadherin,protocadherin gamma C3,blood-brain barrier,brain microvascular endothelial cells,permeability,signaling pathways,tight-junctions.All years were chosen in the search.These searches were performed between September and December 2021.Not all studies could be cited due to page limitations.
Structure of Cell-Cell Junctions at the Blood-Brain Barrier
The special feature of BMECs is the formation of exceptionally dense intercellular junctions which enable a significantly lower permeability compared to the capillary endothelia in non-neuronal tissue.The paracellular transport route is closed by TJs and AJs,which are the major components of the physical barrier of the BBB.In the TJs lying on the lateral circumference,cell-cell adhesion is mainly mediated by the transmembrane proteins claudins,occludin,and representatives of the junctional adhesion molecules,which in turn are intracellularly linked to membrane-associated cytoplasmic proteins,such as the zonula occludens-1 protein (Greene et al.,2019).Claudin-5 alone,as the most important TJ protein,would be sufficient for the formation of cell-cell contact.Claudin-1,-3,and -12 as well as occludin,which also form perivascular zipper structures that close the intercellular gap,take on other important barrier functions (Castro Dias et al.,2019).Intracellularly,TJs bind to the zonula occludens proteins,which are connected to the cytoskeleton(Figure 1
).The connections via AJs are on the abluminal side and are mainly formed by the endothelial cell-specific vascular endothelial cadherin (VEcadherin) (Castro Dias et al.,2019).VE-cadherin binds intracellularly to the connecting molecules p120,β-catenin,and plakoglobin.These molecules are ultimately linked to the actin-binding proteins of the cytoskeleton.The interaction of cadherins with cytoplasmic catenins and the actin cytoskeleton is essential for strong cell-cell adhesion.Deletion or mutations of either cadherin or catenin result in morphological defects in the brain (Suzuki and Takeichi,2008).In addition to their barrier functions,TJs and AJs are involved in the intracellular signaling pathway of apoptosis as well as in processes of cell growth,angiogenesis,and gene expression.Various studies have also shown that cerebrovascular pathologies,such as TBI,stroke,or SAH,have a decisive influence on the entire neurovascular unit and its complex molecular structure (Andjelkovic et al.,2020;Winkler et al.,2021).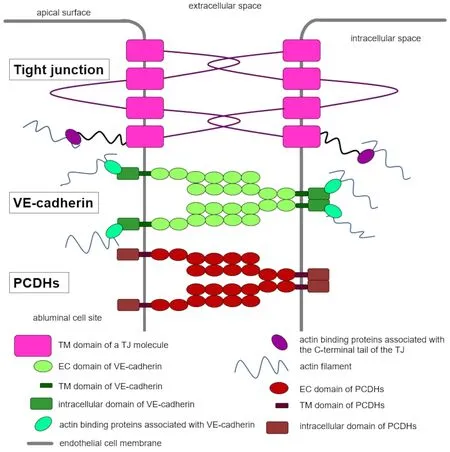
Figure 1 | Schematic representation of the organization of cell-cell contacts within an endothelial cell.
The expression of PCDHs in neuronal tissue has been intensively studied,which underscores their important neurobiological role (Schreiner and Weiner,2010).On the other hand,little information was available about the function of PCDHs at the BBB.Recently,our group focused in particular on the role of PCDHs in BMECs and described their previously unknown molecular function concerning BBB tightness (Dilling et al.,2017).
Protocadherin Family
PCDHs,a subgroup within a cadherin family of adhesion proteins,are highly expressed in the central nervous system (CNS) and play a role in embryogenesis (Sano et al.,1993;Wang et al.,2002).PCDHs are divided into clustered and non-clustered PCDHs.Clustered PCDHs are conserved across vertebrate species and are encoded by specific gene clusters α,β,and γ located on human chromosome 5q31 and mouse chromosome 18(Wu and Maniatis,1999).Mouse PCDH α cluster encodes 14 α-PCDHs,while the PCDH β and PCDH γ clusters encode 22 β-PCDHs and 22 γ-PCDHs,respectively (Garrett et al.,2019).All three PCDH clusters contribute to neural development.Knockout mice lacking the PCDH α and/or PCDH β clusters are viable and fertile (Hasegawa et al.,2016).The PCDH γ cluster is the only one required for postnatal viability.The knockout of the entire PCDH γ cluster or just the three C isoforms (PCDHGC3-C5) results in neonatal lethality and excessive cell death of neurons in the spinal cord and hypothalamus(Chen et al.,2012).Thus,the PCDH γ cluster is involved in neuronal survival,synaptogenesis,and the branching of dendrites (Garrett et al.,2012).Mice with mutated PCDH α cluster showed defects in the olfactory axons,reduced visual acuity due to changes in retinal ganglion cells,decreased dendrite arborization in hippocampal neurons,and reduced overall density of dendritic spine (Peek et al.,2017).No vascular defects were reported in these knockout mice.PCDHs consist of six or seven extracellular (EC) cadherin domains,a transmembrane domain,and the intracellular C-terminal domain (Figure 1
)(Morishita and Yagi,2007;Pancho et al.,2020).There are several similarities but also differences between PCDHs and classic cadherins.Some of these features are highlighted inFigure 1
.Compared with classic cadherins (as well as with TJs proteins),PCDHs have different,not fully characterized intracellular interaction partners (Pancho et al.,2020).Moreover,PCDH-mediated cellcell contacts are only partially dependent on calcium,when comparing i.e.with VE-cadherin.In analogy to classic cadherins,they are involved in signal transduction and PCDH-mediated cell-cell contacts (Haas et al.,2005;Dilling et al.,2017;Gabbert et al.,2020).As classic cadherins,PCDHs determine celltype-specific recognition showing homophilic trans interactions between two adjacent cells and cis interactions on the same cell membrane forming zipperlike structures between adjacent cells.The trans interactions are formed by EC1 (the most N-terminal cadherin domain) and EC2-4 (Figure 1
) while EC5-6 are involved in cis interactions,as experiments with mutant proteins have shown (Schreiner and Weiner,2010;Rubinstein et al.,2015).The crystal structures of the extracellular domains of α,β,and γ PCDHs confirmed these trans interactions of EC1-EC4 and a crucial role of EC6 in mediating cis interactions between PCDHs,revealing overall similar recognition dimer structures (Goodman et al.,2016a,b).Based on these results,a molecular mechanism for neuronal recognition has been proposed.However,how these interactions contribute to cell-cell contacts and the regulation of paracellular permeability in endothelial cells remains to be elucidated in detail.
Figure 2 | Protocadherin gamma C3 (PCDHGC3) knockout leads to an impaired barrier in brain microvascular endothelial cells (BMECs).
Protocadherin Gamma C3 as One Newly Discovered Regulator of Vascular Permeability
Vascular cells from different tissues (e.g.of the brain,heart,umbilical vein)all express various PCDH isoforms from the γ PCDH cluster (PCDHGs).RTPCR showed the expression of all 22 PCDHGs in the primary BMEC as well as in the BMEC cell lines cEND and cerebEND (Dilling et al.,2017).In cEND and cerebEND BMEC cell lines,knockout of PCDHGC3,one of the PCDH γ isoforms,leads to changes in the BBB endothelium,which are indicated by higher paracellular permeability and lower values of the transendothelial electrical resistance (Dilling et al.,2017;Gabbert et al.,2020;Figure 2
).Interestingly,increased protein levels of the TJs proteins claudin-5,claudin-3,and zonula occludens-1 and increased mRNA levels of VE-cadherin were measured in PCDHGC3 knockout cells,which indicates an activation of unknown molecular mechanisms by the knockout,leading to this unexpected result.However,occludin has been shown to be downregulated in these knockout cells on both mRNA and protein levels (Figure 2
),suggesting that PCDHGC3 and occludin both have a significant impact on BBB properties.In addition,further experiments by our group demonstrated an interaction of these two proteins by immunoprecipitation of overexpressed or endogenous proteins (Kaupp,2021).Further studies to refine the role of PCDHGC3 at the BBB and to break down the exact mechanisms including occludin involvement are ongoing.Concerning the higher proliferation rate of PCDHGC3 knockout compared to wild-type cells,we were able to draw a possible connection with the growthpromoting effect of PCDHGC3.Because of its tightness,the BBB prevents various substances from crossing the blood capillaries and entering the CNS.However,through active transport,some essential nutrients (e.g.glucose or amino acids) and other molecules can pass through the BBB and maintain brain homeostasis.In addition to the restricted paracellular and unspecific transcellular transport(diffusion,facilitated diffusion,ion channels),multiple substances can pass through the BBB via carrier-mediated transport (solute carriers,Slc),receptormediated transport,or transcytosis,which can be bidirectional (Wong et al.,2013).Under normal culture conditions,BMEC cell lines used to generate knockouts express high levels of various transporters and junctional proteins that respond to various drug treatments and conditions (Helms et al.,2016;Kaiser et al.,2018;Burek et al.,2020;Gerhartl et al.,2020;Rosing et al.,2020;Salvador et al.,2021;Schick et al.,2021).In this context,studies on the endothelial PCDHGC3 knockout showed differential mRNA and protein expression changes of molecules involved in transport through the BBB(Gabbert et al.,2020).Several genes for solute carrier (Slc) transporters(Slc2a1,Slc7a1,Slc7a5,and Slc16a1) were downregulated compared to wild-type cells (Figure 2
).This fact indicated a lower transcellular transport via solute carriers in knockout cells.However,Slc-mediated transport is essential for brain metabolism.In addition,the loss of solute carriers in the brain microvasculature is associated with the breakdown of the BBB and consecutive neuronal impairment,as shown e.g.for Glut1 (Zheng et al.,2010;Winkler et al.,2015;Veys et al.,2020).We also found that RAGE (receptor for advanced glycation end products) was significantly upregulated at the mRNA and protein level,while mRNA and protein levels of other receptor-mediated transporters (low-density lipoprotein receptor-regulated protein (Lrp1),transferrin receptor) were regulated in a reciprocal manner (Figure 2
).RAGE is known to play a crucial role in the deposition of amyloid-beta and BBB impairment in Alzheimer’s disease (Deane et al.,2003;Kook et al.,2012;Wan et al.,2015).It is still unclear whether PCDHGC3 can have a clinically relevant influence on RAGE expression.However,this interesting aspect needs further investigation.Active efflux pumps of the ATP-binding cassette superfamily are either up-or downregulated in PCDHGC3 knockout BMECs,whereby different regulatory mechanisms are assumed for these transporters (Figure 2
).The expression of ATP-binding cassette-pumps such as P-glycoprotein and breast cancer resistance protein (Bcrp) in BMECs is influenced by many different pathways (Miller,2015),and PCDHs,in turn,could be involved in this complex regulatory process.In CNS inflammation,the BBB is severely compromised.At the same time,BBB leakage triggers further neuroinflammation and thus contributes to the initiation and progression of numerous neurological and psychiatric diseases(Stolp and Dziegielewska,2009;Ortiz et al.,2014;Takeda et al.,2014;Hurtado-Alvarado et al.,2016;Sorby-Adams et al.,2017;Sonar and Lal,2018;Welcome,2020).In addition,peripheral and/or systemic inflammation can disrupt an intact BBB by upregulating inflammatory factors and increasing the migration of immune cells across the BBB (Varatharaj and Galea,2017;Curtaz et al.,2020a;Huang et al.,2021).Ourin vitro
studies have shown that wild type BMECs express various inflammatory molecules and can respond to treatment with diverse inflammatory factors (Burek and Forster,2009;Blecharz-Lang et al.,2018;Curtaz et al.,2020b;Gerhartl et al.,2020;Ittner et al.,2020).Contrary to our expectations,the PCDHGC3 knockout in BMECs led to decreased levels of variously known mediators that are involved in neuroinflammatory processes(Figure 2
).Decreased levels of inflammatory mediators appear to contradict a stronger response of PCDHGC3 knockout cells to stress conditions (tumor necrosis factor α and oxygen-glucose deprivation treatment);however,other pathways are likely to be implicated here and may affect the results.Hence,the role of PCDHs concerning inflammation at the BBB needs to be fully elucidated shortly.In conclusion,based on our experimental data,we propose a central regulatory role of PCDHs,especially PCDHGC3,in maintaining BBB homeostasis.Due to their clear influence on various signaling pathways,PCDHs may contribute in particular to the control of several BBB-specific properties such as permeability,active transport,and the induction of inflammatory mediators in BMECs.We expect to expand our incomplete knowledge of the importance of these molecules through further experiments.
Effects of Protocadherin Gamma C3 on Signaling Pathways Involved in Maintaining Permeability in Brain Microvascular Endothelial Cells
The mechanistic target of rapamycin (mTOR) is a serine-threonine protein kinase that is highly conserved from yeast,where it was originally identified,to mammals (Boutouja et al.,2019).Two mTOR complexes with partly overlapping,partly distinct components have been identified so far (Boutouja et al.,2019).mTOR complex 1 (mTORC1),which contains either mTOR1 or mTOR2,is rapamycin-sensitive and is activated,for example,via the PI3K-Akt-pathway through several mitogenic signals (e.g.growth factors,nutrients,and changes in cellular energy status).The activated mTORC1 then translates these signals into cellular reactions by activating downstream cascades and thus influences the cellular energy balance and metabolism(transcription,ribosome biogenesis,translation,nutrient uptake and storage,cell growth,autophagy regulation) and tissue proliferation (Fingar and Blenis,2004;Dobashi et al.,2011).mTOR complex 2 (mTORC2),which contains only mTOR2,is activated via association with ribosomes and has a positive effect on protein translation and cotranslational modifications (Oh et al.,2010;Zinzalla et al.,2011).It also processes signals for actin cytoskeleton polarisation and cell survival in a rapamycin-insensitive manner (Loewith et al.,2002;Wedaman et al.,2003;Jacinto et al.,2004;Sarbassov et al.,2004).
Qualitative and quantitative changes within the mTOR cascade were found in differential diseases such as cancer,neurodegenerative diseases,autoimmune and inflammatory reactions,and age-related diseases (Perl,2015;Lan et al.,2017;Boutouja et al.,2019;Murugan,2019;Suto and Karonitsch,2020;Chrienova et al.,2021).As a result,mTOR inhibitors (such as rapamycin=sirolimus,everolimus,and temsirolimus) have been developed to treat several types of cancer,to prevent in-stent restenosis after coronary angioplasty,and to manage organ transplant rejection (Costa and Simon,2005;Giordano and Romano,2011;Qi et al.,2013;Fine and Kushwaha,2016;Hua et al.,2019).Other drugs with influence on mTOR,such as metformin,are currently being investigated for their effects on mTOR-mediated diseases (Amin et al.,2019).
The role of mTOR at the BBB was also mostly reported in connection with diseases (van Vliet et al.,2016a,b;Van Skike et al.,2018;Zhang et al.,2020;Chi et al.,2021;Xiong et al.,2021;Yang et al.,2021).In cerebral ischemiareperfusion injury,Alzheimer’s disease,or epilepsy,BBB properties could be rescued by inhibiting mTOR signal transmission.
The first correlation between mTOR and PCDHs was made by Dallosso et al.(2012) who showed that PCDHGC3 has a tumor-suppressive effect on colon epithelia and that this effect is partly mediated by a suppression of mTOR and Wnt.PCDHGC3,the most highly expressed PCDH isoform in colon epithelia,is unmethylated (active) in normal colon and adenoma cells,and PCDHGC3 hypermethylation (inactivation) is a marker of adenoma-to-carcinoma transition.This role of PCDHGC3 as a tumor suppressor in colon epithelia can be attributed to the modification of the mTOR and Wnt signaling.PCDHGC3 overexpression leads to mTOR and Wnt inhibition,while the mTOR and Wnt cascades are stimulated by PCDHGC3 suppression (Dallosso et al.,2012).Several other studies have also found associations between the expression of certain PCDH isoforms and mTOR-mediated cell proliferation in cancer cells (Wu et al.,2015;Wang et al.,2016;Ye et al.,2021).In PCDHGC3 knockout BMECs,mTOR and p62/Seqestome-1,which is involved in the mTOR signaling pathway,were downregulated compared to wild-type BMECs (Dilling et al.,2017).
Wound healing assay showed a significantly higher migration rate of PCDHGC3 knockout cells compared to wild-type cells,which could be effectively suppressed by treatment with the mTOR inhibitor Torin-2.The faster migration rate of knockout cells correlated with increased proliferation,measured by 5-bromo-2′-deoxyuridine (BrdU) incorporation (Gabbert et al.,2020).
Mitogen-activated protein kinase (MAP kinase) pathways are involved in a variety of physiological processes and seven MAP kinase pathways have been identified to date.One of the best studied is the Ras-Raf-Mek-Erk cascade,which is stimulated by growth factors,mitogens,and cytokines (Chang et al.,2003;McCubrey et al.,2007) and consequently activates various substrates through phosphorylation (Courcelles et al.,2013) and downstream activation of MAP kinase-activated protein kinases (Gaestel,2015).The stimulation of this signaling pathway can influence numerous cellular functions,especially growth-related processes such as cell proliferation,differentiation,and survival (Cargnello and Roux,2011).The Ras-Raf-Mek-Erk cascade is changed in many diseases such as cancer,genetic syndromes,liver fibrosis,polycystic kidney disease,and vascular malformations (Niemeyer,2014;Foglia et al.,2019;Guo et al.,2020;Pang et al.,2020;Parker et al.,2020).Since dysregulation in the Ras-Raf-Mek-Erk pathways occurs particularly during malignant transformation,tumor growth,and resistance acquisition,kinase inhibitors have been developed that interfere at different levels of the Ras-Raf-Mek-Erk cascade and successfully have found their way into clinical cancer therapy (Asati et al.,2016;Degirmenci et al.,2020;Barbosa et al.,2021).A prominent example of the clinical use of such kinase inhibitors is the pharmacological treatment of malignant melanoma (Savoia et al.,2019).
Analyses of the role of MAP kinase pathways,in particular the Ras-Raf-Mek-Erk cascade,at the BBB have given different results.On the one hand,some studies indicate that cascade activation protects the BBB tightness either by TJs in endothelial cells (Uddin et al.,2012;Haupt et al.,2020) or by pericytes(Wu et al.,2020).On the other hand,numerous other studies showed that the upregulated elements of the Ras-Raf-Mek-Erk signaling pathway were associated with increased BBB permeability under different experimental conditions (Fischer et al.,2005;Maddahi and Edvinsson,2010;Stephan et al.,2013;Üllen et al.,2013;Walter et al.,2015;Fujimoto et al.,2016;Yang et al.,2016;Zhu et al.,2018;Lan et al.,2019).
The role of PCDHs in MAP kinase cascades has been described by Zhou et al.(2017b) who have shown that in non-small cell lung cancer overexpression of PCDH7 (a non-clustered PCDH) induces tumorigenesis by activating the MAP kinase signaling and thus leads to poor clinical outcome in patients.In PCDHGC3 knockout BMECs,the MAP kinase signaling pathway inhibitor SL327 inhibited an increased migration rate of knockout BMECs.In agreement with these results,a significantly higher phospho-Erk level was detected in knockout cells after serum starvation,which suggests highly active MAP kinase signaling under stress conditions in these cells (Gabbert et al.,2020).
The Wnt/β-catenin signaling pathway (so-called“canonical Wnt pathway”)is another well-studied cascade involved in many processes during embryogenesis,especially in neuronal development.In the absence of Wnt ligands,β-catenin phosphorylation,ubiquitination,and proteasome degradation,mediated by a β-catenin destruction complex,leads to low cytoplasmic β-catenin levels (Liebner and Plate,2010).In the presence of Wnt ligands,these ligands bind to a receptor complex containing receptors of the Frizzled family and Lrp5/6,which inactivates the β-catenin destruction complex with the help of the cytoplasmic protein Disheveled.This leads to a cytoplasmic β-catenin accumulation,a translocation to the nucleus,and a transcription factor-mediated activation of the Wnt/β-catenin -dependent gene expression (Liebner and Plate,2010).The Wnt/β-catenin signaling pathway is important for BBB development and is involved in the breakdown of the BBB under pathophysiological conditions (Laksitorini et al.,2019).BMECs express all known Wnt ligands and receptors,as shown for the hCMEC/D3 cell line (Laksitorini et al.,2019).The inhibition of Wnt/β-catenin signaling in hCMEC/D3 cells by a Wnt ligand (Wnt3a) improves the BBB phenotype,which leads to lower permeability and increased transporter activity (Laksitorini et al.,2019).
Colorectal cancer was the first human disease correlated with a dysregulation of the canonical Wnt/β-catenin cascade and remains the model disease for studies on the role of Wnt/β-catenin signaling in carcinogenesis (Segditsas and Tomlinson,2006).In the past twenty years,however,aberrant canonical Wnt/β-catenin signaling has been found in numerous other human cancer entities(Zhan et al.,2017).The specific properties of the canonical Wnt/β-catenin signaling pathway are decisive for CNS angiogenesis and the formation of the BBB (Liebner et al.,2008;Lim et al.,2008;Stenman et al.,2008;Daneman et al.,2009;Harati et al.,2013).However,the function and maintenance of the BBB in the adult brain also seem to depend on intact Wnt/β-catenin signaling(Wang et al.,2012;Tran et al.,2016;Corada et al.,2019).The dysfunction of this cascade is a pathogenetic process that is observed in many neurological diseases with impaired BBB,such as Alzheimer’s disease (Jia et al.,2019;Aghaizu et al.,2020),multiple sclerosis (Lengfeld et al.,2017),stroke (Jean LeBlanc et al.,2019;Jin et al.,2019),glioblastoma multiforme (McCord et al.,2017),vascular malformations (Kim,2016) and neuropsychiatric diseases(Gozal et al.,2021).
As described above,it could be shown that suppression of the Wnt/β-catenin signaling pathway is involved in the role of PCDHGC3 as a tumor suppressor in colon epithelia (Dallosso et al.,2012).Mah et al.(2016) showed through transient transfection of various protocadherin constructs that the PCDHGC3 isoform inhibits the Wnt/β-catenin pathway uniquely,while other isoforms upregulate it.The variable cytoplasmic domain of PCDHGC3 interacts directly with Axin1,a key component of the β-catenin destruction complex,to stabilize Axin1 on the plasma membrane and thus to inhibit Lrp6 phosphorylation,resulting in decreased Wnt/β-catenin signaling (Mah et al.,2016).Several other PCDHs also have tumor-suppressive effects in various cancer entities by negatively regulating Wnt/β-catenin signaling (Dallosso et al.,2009;Zhao et al.,2014;Chen et al.,2015;Lv et al.,2015;Xu et al.,2015;Yin et al.,2016;Zhou et al.,2017a;Zong et al.,2017;Weng et al.,2018;Wong et al.,2020).For individual PCDH isoforms in certain malignancies,however,contradicting results were found (Yang et al.,2005;Terry et al.,2006),which supports the idea that the effects of PCDHs on the Wnt/β-catenin pathway are context andtissue-specific.
In PCDHGC3 knockout cells,the inhibition of Wnt/β-catenin signaling by a selective inhibitor,XAV939,suppressed the higher migration rate of knockout cells in the wound healing assay.In addition,genes involved in this signaling pathway such as Axin-1,Gsk3b,Lrp5,and Pard3 were downregulated,while Axin-2,Ctnnb1,and Frizzled-1 were upregulated,which indicates differential changes in Wnt/β-catenin signaling in PCDHGC3 knockout BMECs (Dilling et al.,2017;Gabbert et al.,2020).
Conclusion and Perspectives
Changes in the expression status of only one protocadherin,as it has been shown for PCDHGC3,lead to multiple phenotypic changes in BMECs such as increased proliferation and migration rate,accompanied by lower barrier properties and therefore higher transcellular permeability and reduced transendothelial electrical resistance values.Under physiological conditions,a PCDHGC3 knockout in BMECs leads to the activation of mTOR,MAP kinase,and Wnt/β-catenin signaling cascades,and,conversely,responds to selective inhibitors,resulting in an inhibition of these signaling pathways(Dilling et al.,2017;Gabbert et al.,2020).Wnt/β-catenin plays a critical role in the development and management of BBB function.In addition,PCDHs are highly expressed in the developing brain,suggesting that the interaction of PCDHGC3 and Wnt/β-catenin signaling pathway activity in the development might be worth examination,too.Our results so far specified a strong activation of the signal cascades described above by the PCDHGC3 knockout and are consistent with the idea that PCDHGC3 acts as an antiproliferative factor,in accordance with recent studies by other authors (Dallosso et al.,2012;Mah et al.,2016).In terms of the involvement of mTOR,MAP kinase,and Wnt/β-catenin signaling pathway in numerous physiological and pathological processes,PCDHGC3 could play an important role in maintaining BBB homeostasis by regulating these signaling pathways in BMECs.Correspondingly,the further scientific effort is required to elucidate detailed knowledge about the mechanism of interaction between PCDHGC3 and intracellular signal cascades at the BBB.
Author contributions:
VK,KGBL,CD,PM,and MB wrote and revised the manuscript;KGBL and MB drew the illustrations.All authors approved the final manuscript.
Conflicts of interest:
The authors declare no conflicts of interest.
Availability of data and materials:
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement:
This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:
Panos Kouklis,University of Ioannina Faculty of Medicine,Greece.
Additional file:
Open peer review report 1.
- 中国神经再生研究(英文版)的其它文章
- Neuroaxonal and cellular damage/protection by prostanoid receptor ligands,fatty acid derivatives and associated enzyme inhibitors
- Extracellular vesicles in Alzheimer’s disease:from pathology to therapeutic approaches
- Molecular approaches for spinal cord injury treatment
- Sex-biased autophagy as a potential mechanism mediating sex differences in ischemic stroke outcome
- Adipose tissue,systematic inflammation,and neurodegenerative diseases
- Interleukin-1:an important target for perinatal neuroprotection?

